Pharmaceutical Cocrystal Formation of Pyrazinamide with 3-Hydroxybenzoic Acid: A Terahertz and Raman Vibrational Spectroscopies Study
Abstract
:1. Introduction
2. Results and Discussion
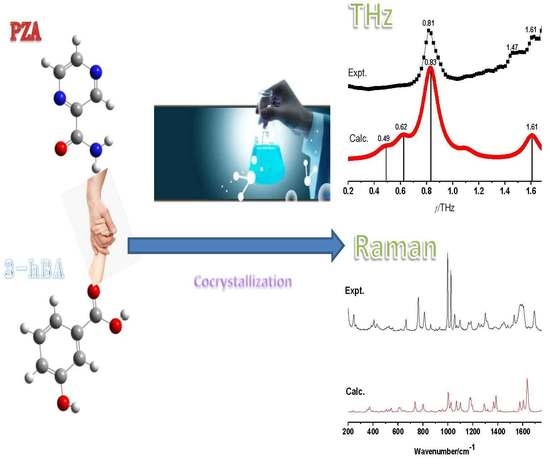
2.1. THz Absorption Spectral Characterization and Analysis of PZA, 3-hBA, Physical Mixture, and Their Cocrystals
2.2. Raman Spectral Characterization and Analysis of PZA, 3-hBA, Physical Mixture, and Their Cocrystals
3. Materials and Methods
3.1. Chemicals and Sample Preparation
3.2. Apparatus and Procedure
3.3. Theoretical Calculations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–716. [Google Scholar] [CrossRef]
- McNamara, D.P.; Childs, S.L.; Giordano, J.; Iarriccio, A.; Cassidy, J.; Shet, M.S.; Mannion, R.; O’donnell, E.; Park, A. Use of a glutaric acid cocrystal to improve oral bioavailability of a low solubility API. Pharm. Res. 2006, 23, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fang, H.X.; Zhang, Q.; Zhang, H.L.; Hong, Z. Spectroscopic investigation on cocrystal formation between adenine and fumaric acid based on infrared and Raman techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Cheney, M.L.; Shan, N.; Healey, E.R.; Hanna, M.; Wojtas, L.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Effects of crystal form on solubility and pharmacokinetics: A crystal engineering case study of lamotrigine. Cryst. Growth Des. 2010, 10, 394–405. [Google Scholar] [CrossRef]
- Roy, S.; Quiñones, R.; Matzger, A.J. Structural and physicochemical aspects of dasatinib hydrate and anhydrate phases. Cryst. Growth Des. 2012, 12, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Frøstrup, B.; Bond, A.D. Polymorphs of pridopidine hydrochloride. Cryst. Growth Des. 2012, 12, 2961–2968. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, H.; Xue, J.; Tang, W.; Fang, H.; Zhang, Q.; Li, Y.; Hong, Z. Vibrational spectroscopic study of polymorphism and polymorphic transformation of the anti-viral drug lamivudine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 1158–1163. [Google Scholar] [CrossRef]
- Wang, J.R.; Ye, C.; Zhu, B.; Zhou, C.; Mei, X. Pharmaceutical cocrystals of the anti-tuberculosis drug pyrazinamide with dicarboxylic and tricarboxylic acids. CrystEngComm 2015, 17, 747–752. [Google Scholar] [CrossRef]
- Kwiatkowska, S.; Piasecka, G.; Zieba, M.; Piotrowski, W.; Nowak, D. Increased serum concentrations of conjugated diens and malondialdehyde in patients with pulmonary tuberculosis. Respir. Med. 1999, 93, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Walubo, A.; Smith, P.J. Oxidative stress during antituberculous therapy in young and elderly patients. Biomed. Environ. Sci. 1995, 8, 106–113. [Google Scholar]
- Strausz, J.; Müller-Quernheim, J.; Steppling, H.; Nagel, M.; Ferlinz, R. Oxygen radical production by alveolar macrophages in sarcoidosis in relation to activity status of bronchoalveolar lavage lymphocytes. Pneumologie 1990, 44 (Suppl. 1), 222–223. [Google Scholar] [PubMed]
- Mashhadi, S.M.; Yunus, U.; Bhatti, M.H.; Tahir, M.N. Isoniazid cocrystals with anti-oxidant hydroxy benzoic acids. J. Mol. Struct. 2014, 1076, 446–452. [Google Scholar] [CrossRef]
- McMahon, J.A.; Bis, J.A.; Vishweshwar, P.; Shattock, T.R.; McLaughlin, O.L.; Zaworotko, M.J. Crystal engineering of the composition of pharmaceutical phases. 3. Primary amide supramolecular heterosynthons and their role in the design of pharmaceutical co-crystals. Z. Für Krist. 2005, 220, 340–350. [Google Scholar] [CrossRef]
- Abourahma, H.; Cocuzza, D.S.; Melendez, J.; Urban, J.M. Pyrazinamide cocrystals and the search for polymorphs. CrystEngcomm 2011, 13, 6442–6450. [Google Scholar] [CrossRef]
- Lou, M.; Mao, S.H.; Luo, Y.H.; Zhao, P.; Sun, B.W. Synthesis, co-crystal structure and characterization of pyrazinamide with m-hydroxybenzoic acid, p-hydroxybenzoic acid and 3,4-dihydroxy benzoic acid. Res. Chem. Intermed. 2015, 41, 2939–2951. [Google Scholar] [CrossRef]
- Cherukuvada, S.; Row TN, G. Comprehending the formation of eutectics and cocrystals in terms of design and their structural interrelationships. Cryst. Growth Des. 2014, 14, 4187–4198. [Google Scholar] [CrossRef]
- Hennigan, M.C.; Ryder, A.G. Quantitative polymorph contaminant analysis in tablets using Raman and near infra-red spectroscopies. J. Pharm. Biomed. Anal. 2013, 72, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Xue, J.; Wang, Q.; Du, Y. Investigation into structure and dehydration dynamic of gallic acid monohydrate: A Raman spectroscopic study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 128–133. [Google Scholar] [CrossRef]
- Shi, L.; Duan, X.H.; Zhu, L.G.; Liu, X.; Pei, C.H. Directly insight into the inter- and intramolecular interactions of CL-20/TNT energetic cocrystal through the theoretical simulations of THz spectroscopy. J. Phys. Chem. A 2016, 120, 1160–1167. [Google Scholar] [CrossRef]
- Du, Y.; Cai, Q.; Xue, J.; Zhang, Q.; Qin, D. Structural investigation of the cocrystal formed between 5-fluorocytosine and fumaric acid based on vibrational spectroscopic technique. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 251–257. [Google Scholar] [CrossRef]
- Cheballah, Y.; Ziane, A.; Bouarab, S.; Vega, A. Density functional study of the optical response of FeN and CoN nitrides with zinc-blend and rock-salt structures. J. Phys. Chem. Solids 2017, 100, 148–153. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Q.; Li, Z.; Yin, X.; Hu, F. Experimental and theoretical investigations of tartaric acid isomers by terahertz spectroscopy and density functional theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xue, J.; Wang, Y.; Jin, S.; Zhang, Q.; Du, Y. Investigation into tautomeric polymorphism of 2-thiobarbituric acid using experimental vibrational spectroscopy combined with DFT theoretical simulation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hakey, P.M.; Allis, D.G.; Hudson, M.R.; Ouellette, W.; Korter, T.M. Terahertz spectroscopic investigation of S-(+)-ketamine hydrochloride and vibrational assignment by density functional theory. J. Phys. Chem. A 2010, 114, 4364–4374. [Google Scholar] [CrossRef] [PubMed]
- Hakey, P.M.; Allis, D.G.; Ouellette, W.; Korter, T.M. Cryogenic terahertz spectrum of (+)-methamphetamine hydrochloride and assignment using solid-state density functional theory. J. Phys. Chem. A 2009, 113, 5119–5127. [Google Scholar] [CrossRef]
- Erxleben, A. Application of vibrational spectroscopy to study solid-state transformations of pharmaceuticals. Curr. Pharm. Des. 2016, 22, 4883–4911. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hong, Z.; He, J.; Chen, Y. Precisely optical material parameter determination by time domain waveform rebuilding with THz time-domain spectroscopy. Opt. Commun. 2010, 283, 4701–4706. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Komjáti, B.; Urai, Á.; Hosztafi, S.; Kökösi, J.; Kováts, B.; Nagy, J.; Horváth, P. Systematic study on the TD-DFT calculated electronic circular dichroism spectra of chiral aromatic nitro compounds: A comparison of B3LYP and CAM-B3LYP. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 155, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Rohani, S. Polymorphism and crystallization of active pharmaceutical ingredients (APIs). Curr. Med. Chem. 2009, 16, 884–905. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Reed, C.; Oyama, S.T. Variability in the structure of supported MoO3 catalysts: Studies using Raman and X-ray absorption spectroscopy with ab initio calculations. J. Phys. Chem. B 2001, 105, 8519–8530. [Google Scholar] [CrossRef]
- Wong, M.W. Vibrational frequency prediction using density functional theory. Chem. Phys. Lett. 1996, 256, 391–399. [Google Scholar] [CrossRef]
- Chempath, S.; Yihua Zhang, A.; Bell, A.T. DFT studies of the structure and vibrational spectra of isolated molybdena species supported on silica. J. Phys. Chem. C 2007, 111, 1291–1298. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (pyrazinamide, PZA; 3-hydroxybenzoic acid, 3-hBA) are available from the authors. |







| Mode | Experimental Result/THz | Theoretical Calculation/THz | Vibrational Mode Assignment |
|---|---|---|---|
| a | — | 0.40 | PZA and 3-hBA molecular out of plane bending vibration |
| b | 0.76 | 0.81 | R1, R2, H12–N11–H13 out of plane bending vibration |
| c | 1.47 | 1.47 | PZA and 3-hBA molecular in plane bending vibration |
| d | 1.66 | 1.61 | PZA and 3-hBA molecular out of plane bending vibration |
| Mode | Theoretical Wavenumber/cm−1 | Experimental Wavenumber/cm−1 | Mode Assignment |
|---|---|---|---|
| ν1 | 237 | 246 | ω (C18–H19, C14–H25, C16–H21, O22–H23) |
| ν2 | 260 | 265 | Def (R2), ρ (H12–N11–H13, C9=O10) |
| ν3 | 344 | - | ρ (O22–H23) |
| ν4 | 374 | 386 | ρ (H29–O28–C27=O30, H12–N11–H13, C15–O22–H23) |
| ν5 | 405 | 405 | ρ (C16–H21, C15–O22–H23, H12–N11–H13) |
| ν6 | 427 | 431/450 | ω (C17–H20, C14–H25, C16–H21) |
| ν7 | 511 | 513 | ρ (H12–N11–H13, C9=O10, C16–H21, C15–O22–H23) |
| ν8 | 526 | 526 | Def (R1), ρ (12H–N11–H13, C15–O22–H23, C9=O18) |
| ν9 | 560 | 552 | ω (H12–N11–H13) |
| ν10 | 625 | 625 | Def (R2), ρ (H12–N11–H13, C9=O10) |
| ν11 | 666 | 663 | Def (R1, R2), ρ (H12–N11–H13, O30=C27–O28–H29) |
| ν12 | 770 | 762 | Def R1, δ (O30=C27–O28–H29) |
| ν13 | 819 | 813 | ω (N12–H11–H13) |
| ν14 | 890 | 864/908 | ω (C14–H25, C16–H21, C4–H7, C5–H8) |
| ν15 | 940 | 934 | ω (O28–H29) |
| ν16 | 998 | 998 | Def (R1) |
| ν17 | 1024 | 1026 | Def (R2) |
| ν18 | 1054 | 1055 | Def (R2) |
| ν19 | 1079 | 1071 | ρ (C18–H19, C16–H21, C14–H25, C17–H20, C22–H23) |
| ν20 | 1119 | 1096 | δ (H12–N11–H13) |
| ν21 | 1179 | 1166/1185 | δ (H21–C16=C17–H20), ρ (C18–H19, O22–H23, C14–H25) |
| ν22 | 1293 | 1300 | ρ (O28–H29, C4–H7, C10–H19, C14–H29, C17–H20)) |
| ν23 | 1324 | 1316 | ρ (C14–H25, C17–H20, C18–H19, C16–H21, O22–H23, O28–H29) |
| ν24 | 1388 | 1386 | ρ (N11–H13, C4–H7, C5–H8, C2–H24) |
| ν25 | 1433 | 1433 | δ (H13–N11–C9, H29–O28–C27=O30), τ (-NH2), ρ (C5–H8) |
| ν26 | 1453 | 1450 | δ (H13–N11–C9), ρ (O28–H29, C16–H21, C18–H19, C5–H8) |
| ν27 | 1468 | 1478 | δ (H30=C27–O28–H29), ρ (H13–N11, C16–H21, C4–H7, C5–H8, C17–H20) θ (C26–C27) |
| ν28 | 1555 | 1530 | Def(R2) |
| ν29 | 1575 | 1580 | δ (H12–N11–H13) |
| ν30 | 1590 | 1593 | Def (R2) |
| ν31 | 1623 | 1610 | Def (R1) |
| ν32 | 1693 | 1693 | θ (C27=O30, C9=H10), δ (H12–N11–H13, C27–O28–H29) |
| Chemical Bond | Bond Length/Å | ||
|---|---|---|---|
| PZA | 3-hBA | Cocrystal | |
| C27=O30 | - | 1.225 | 1.258 |
| C27–O30 | - | 1.431 | 1.323 |
| O28–H29 | 0.963 | 1.000 | |
| N11–H13 | 0.998 | 1.022 | |
| N11–C9 | 1.467 | 1.334 | |
| C9=O10 | 1.238 | 1.259 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Xue, J.; Hong, Z.; Du, Y. Pharmaceutical Cocrystal Formation of Pyrazinamide with 3-Hydroxybenzoic Acid: A Terahertz and Raman Vibrational Spectroscopies Study. Molecules 2019, 24, 488. https://doi.org/10.3390/molecules24030488
Wang Q, Xue J, Hong Z, Du Y. Pharmaceutical Cocrystal Formation of Pyrazinamide with 3-Hydroxybenzoic Acid: A Terahertz and Raman Vibrational Spectroscopies Study. Molecules. 2019; 24(3):488. https://doi.org/10.3390/molecules24030488
Chicago/Turabian StyleWang, Qiqi, Jiadan Xue, Zhi Hong, and Yong Du. 2019. "Pharmaceutical Cocrystal Formation of Pyrazinamide with 3-Hydroxybenzoic Acid: A Terahertz and Raman Vibrational Spectroscopies Study" Molecules 24, no. 3: 488. https://doi.org/10.3390/molecules24030488