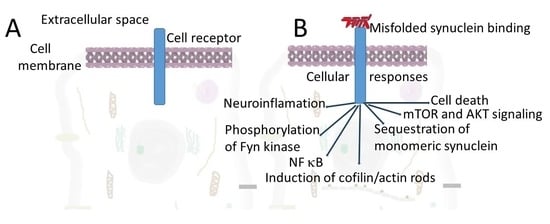
Cell Responses to Extracellular α-Synuclein
Abstract
:Author Contributions
Funding
Acknowledgments
References
- Surguchov, A. Intracellular dynamics of synucleins: Here, there and everywhere. Int. Rev. Cell Mol. Biol. 2015, 320, 103–169. [Google Scholar] [PubMed]
- Lee, S.J.; Desplats, P.; Sigurdson, C.; Tsigelny, I.; Masliah, E. Cell-to-cell transmission of non-prion protein aggregates. Nat. Rev. Neurol. 2010, 6, 702–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmanouilidou, E.; Melachroinou, K.; Roumeliotis, T.; Garbis, S.D.; Ntzouni, M.; Margaritis, L.H.; Stefanis, L.; Vekrellis, K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 2010, 30, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- Desplats, P.; Lee, H.J.; Bae, E.J.; Patrick, C.; Rockenstein, E.; Crews, L.; Spencer, B.; Masliah, E.; Lee, S.J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha- synuclein. Proc. Natl. Acad. Sci. USA 2009, 106, 13010–13015. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Desplats, H.J.; Lee, B.; Spencer, E.; Masliah, E. Cell-to cell transmission of α-synuclein aggregates. Methods Mol. Biol. 2012, 849, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Abounit, S.; Wu, J.W.; Duff, K.; Victoria, G.S.; Zurzolo, C. Tunneling nanotubes: A possible highway in the spreading of tau and other prion-like proteins in neurodegenerative diseases. Prion 2016, 10, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, A.; Kacprzak, M.; Kaźmierczak, A. Alpha-synuclein decreases arachidonic acid incorporation into rat striatal synaptoneurosomes. Folia Neuropathol. 2007, 45, 230–235. [Google Scholar] [PubMed]
- Dehay, B.; Vila, M.; Bezard, E.; Brundin, P.; Kordower, J.H. Alpha-synuclein propagation: New insights from animal models. Mov. Disord. 2016, 31, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y. Regulation of protein homeostasis by unconventional protein secretion in mammalian cells. Semin. Cell Dev. Biol. 2018, 83, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Abdelmotilib, H.; Maltbie, T.; Delic, V.; Liu, Z.; Hu, X.; Fraser, K.B.; Moehle, M.S.; Stoyka, L.; Anabtawi, N.; Krendelchtchikova, V.; et al. α-Synuclein fibril-induced inclusion spread in rats and mice correlates with dopaminergic Neurodegeneration. Neurobiol. Dis. 2017, 105, 84–98. [Google Scholar] [CrossRef]
- Aulić, S.; Masperone, L.; Narkiewicz, J.; Isopi, E.; Bistaffa, E.; Ambrosetti, E.; Pastore, B.; De Cecco, E.; Scaini, D.; Zago, P.; et al. α-Synuclein Amyloids Hijack Prion Protein to Gain Cell Entry, Facilitate Cell-to-Cell Spreading and Block Prion Replication. Sci. Rep. 2017, 7, 10050. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.G.; Temido-Ferreira, M.; Vicente Miranda, H.; Batalha, V.L.; Coelho, J.E.; Szegö, É.M.; Marques-Morgado, I.; Vaz, S.H.; Rhee, J.S.; Schmitz, M.; et al. α-Synuclein interacts with PrPC to induce cognitive impairment through mGluR5 and NMDAR2B. Nat. Neurosci. 2017, 20, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, E.; Legname, G. The role of the prion protein in the internalization of α-synuclein amyloids. Prion 2018, 12, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Brás, I.C.; Lopes, L.V.; Outeiro, T.F. Sensing α-Synuclein From the Outside via the Prion Protein: Implications for Neurodegeneration. Mov. Disord. 2018, 33, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Czapski, G.A.; Gąssowska, M.; Wilkaniec, A.; Cieślik, M.; Adamczyk, A. Extracellular alpha-synuclein induces calpain-dependent overactivation of cyclin-dependent kinase 5 in vitro. FEBS Lett. 2013, 587, 3135–3141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, L.B.; Reimer, L.; Ferreira, N.; Betzer, C.; Jensen, P.H. Protein Partners of α-Synuclein in Health and Disease. Brain Pathol. 2016, 26, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Tyson, T.; Steiner, J.A.; Brundin, P. Sorting out release, uptake and processing of alpha-synuclein during prion-like spread of pathology. J. Neurochem. 2015, 139, 275–289. [Google Scholar] [CrossRef]
- Olanow, C.W.; Prusiner, S.B. Is Parkinson’s disease a prion disorder? Proc. Natl. Acad. Sci. USA 2009, 6, 12571–12572. [Google Scholar] [CrossRef]
- Minamide, L.S.; Maiti, S.; Boyle, J.A.; Davis, R.C.; Coppinger, J.A.; Bao, Y.; Huang, T.Y.; Yates, J.; Bokoch, G.M.; Bamburg, J.R. Isolation and characterization of cytoplasmic cofilin-actin rods. J. Biol. Chem. 2010, 285, 5450–5460. [Google Scholar] [CrossRef]
- Surgucheva, I.; He, S.; Sharma, R.; Rich, M.; Ninkina, N.N.; Stahel, P.; Surguchov, A. Role of synucleins in traumatic brain injury. Mol. Cell. Neurosci. 2014, 63, 114–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.F.; Fleming, M.R.; Amiri, A.; El-Hassar, L.; Surguchev, A.A.; Hyland, C.; Jenkins, D.P.; Desai, R.; Brown, M.R.; Gazula, V.R.; et al. Kv3.3 Channels Bind Hax-1 and Arp2/3 to Assemble a Stable Local Actin Network that Regulates Channel Gating. Cell 2016, 165, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Gąssowska, M.; Czapski, G.A.; Pająk, B.; Cieślik, M.; Lenkiewicz, A.M.; Adamczyk, A. Extracellular α-synuclein leads to microtubule destabilization via GSK-3β-dependent Tau phosphorylation in PC12 cells. PLoS ONE 2014, 9, e94259. [Google Scholar] [CrossRef]
- Kim, C.; Spencer, B.; Rockenstein, E.; Yamakado, H.; Mante, M.; Adame, A.; Fields, J.A.; Masliah, D.; Iba, M.; Lee, H.J.; et al. Immunotherapy targeting toll-like receptor 2 alleviates neurodegeneration in models of synucleinopathy by modulating α-synuclein transmission and neuroinflammation. Mol. Neurodegener. 2018, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Cha, S.H.; Kang, S.J.; Kim, J.B.; Jou, I.; Park, S.M. Prion-like Propagation of alpha-Synuclein Is Regulated by the FcgammaRIIB-SHP-1/2 Signaling Pathway in Neurons. Cell Rep. 2018, 22, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.M.; Okada, T.; Kajimoto, T.; Hirase, M.; Matovelo, S.A.; Nakamura, S.; Yoshida, D.; Ijuin, T.; Nakamura, S.I. Extracellular α-synuclein drives sphingosine 1-phosphate receptor subtype 1 out of lipid rafts, leading to impaired inhibitory G-protein signaling. J. Biol. Chem. 2018, 293, 8208–8216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.H.; et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353, aah3374. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Dos-Santos-Pereira, M.; Acuña, L.; Hamadat, S.; Rocca, J.; González-Lizárraga, F.; Chehín, R.; Sepulveda-Diaz, J.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Microglial glutamate release evoked by α-synuclein aggregates is prevented by dopamine. Glia 2018, 66, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Bao, X.; Zang, C.; Yang, H.; Sun, F.; Che, Y.; Wu, X.; Li, S.; Zhang, D.; Wang, Q. Integrin CD11b mediates α-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2017, 14, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Hinz, S.; Navarro, G.; Franco, R.; Müller, C.E.; Fuxe, K. Understanding the Role of Adenosine A2AR Heteroreceptor Complexes in Neurodegeneration and Neuroinflammation. Front. Neurosci. 2018, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.A.; Romero-Ramos, M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell. Neurosci. 2018, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Wilkaniec, A.; Gąssowska, M.; Czapski, G.A.; Cieślik, M.; Sulkowski, G.; Adamczyk, A. P2X7 receptor-pannexin 1 interaction mediates extracellular alpha-synuclein-induced ATP release in neuroblastoma SH-SY5Y cells. Purinergic Signal. 2017, 13, 347–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, A.A.; Wördehoff, M.M.; Hoyer, W.; Tamamis, P. Uncovering the Binding and Specificity of β-Wrapins for Amyloid-β and α-Synuclein. J. Phys. Chem. B 2016, 120, 12781–12794. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.E.; Tang, B.L. Rabs, SNAREs and α-synuclein--membrane trafficking defects in synucleinopathies. Brain Res. Rev. 2011, 67, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Dalfó, E.; Gómez-Isla, T.; Rosa, J.L.; Nieto Bodelón, M.; Cuadrado Tejedor, M.; Barrachina, M.; Ambrosio, S.; Ferrer, I. Abnormal alpha-synuclein interactions with Rab proteins in alpha-synuclein A30P transgenic mice. J. Neuropathol. Exp. Neurol. 2004, 63, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Masaracchia, C.; Hnida, M.; Gerhardt, E.; Lopes da Fonseca, T.; Villar-Pique, A.; Branco, T.; Stahlberg, M.A.; Dean, C.; Fernández, C.O.; Milosevic, I.; et al. Membrane binding, internalization, and sorting of alpha-synuclein in the cell. Acta Neuropathol. Commun. 2018, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Cho, E.D.; Kim, H.K.; You, S.; Lee, H.J.; Hwang, D.; Lee, S.J. β1-integrin-dependent migration of microglia in response to neuron-released α-synuclein. Exp. Mol. Med. 2014, 46, e91. [Google Scholar] [CrossRef]
- Surguchev, A.A.; Surguchov, A. Integrins—A missing link in synuclein’s pathogenic mechanism. J. Neurosci. Res. 2018, 1–3. [Google Scholar] [CrossRef]
- Bhasne, K.; Mukhopadhyay, S. Formation of Heterotypic Amyloids: α-Synuclein in Co-Aggregation. Proteomics 2018, 18, e1800059. [Google Scholar] [CrossRef]
- Orr, A.A.; Shaykhalishah, H.; Mirecka, E.A.; Jonnalagadda, S.V.R.; Hoyer, W.; Tamamis, P. Elucidating the multi-targeted anti-amyloid activity and enhanced islet amyloid polypeptide binding of β-wrapins. Comput. Chem. Eng. 2018, 116, 322–332. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, S.; Nikkhah, M.; Hosseinkhani, S. Investigation of the effects of carbon-based nanomaterials on A53T alpha-synuclein aggregation using a whole-cell recombinant biosensor. Int. J. Nanomed. 2017, 12, 8831–8840. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]

| Name of the Receptor | Properties | References |
|---|---|---|
| N-methyl-D-aspartate receptor | NMDAR—Glutamate ionotropic receptor and ion channel in nerve cells. | [12] |
| Lymphocyte-activation gene 3 (LAG3) (CD223) | LAG3—immune checkpoint receptor with diverse biologic effects on T cell function. | [26,27] |
| TLR2 receptors | TLR2—toll-like receptor 2—a membrane receptor expressed on the cell surface binding extracellular molecules and transmitting signals to the cells of the immune system. | [28] |
| CD11b integrin (the α-chain of integrin αMβ2) | CD11b—transmembrane receptor facilitating cell-extracellular matrix adhesion. | [29] |
| Adenosine A2AR heteroreceptor complex | Adenosine receptor, G protein-coupled receptor (GPCR) family which possess seven transmembrane alpha helices, as well as an extracellular N-terminus and an intracellular C-terminus. | [30] |
| PrPC | PrPC—a cellular prion protein. α-Synuclein directly interacts with PrPC [10,11,12]. This cooperation facilitates the transfer of α-synuclein between cells [10] and induces cofilin/actin rods formation. | [11,12,13] |
| Neurexin-α | Neurexin-α is a presynaptic protein connecting neurons at the synapse. Located mostly on the presynaptic membrane, contains a single transmembrane domain. | [17] |
| P2X7 | PDX7—purinoceptors for ATP serves as a pattern recognition receptor for extracellular ATP-mediated apoptotic cell death. | [28,31,32] |
| mGluR5 | mGluR5—metabotropic glutamate receptor 5 is a member of the family of G protein-coupled receptors | [12] |
| Fc gamma receptor IIb | FCGR2B is a low affinity receptor for IgG. Mutation in the gene leads to a lupus phenotype | [24] |
| Gangliosides in the lipid rafts | Gangliosides in the lipid rafts acts as receptors for extracellular α-synuclein [33] | [25] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surguchev, A.A.; Emamzadeh, F.N.; Surguchov, A. Cell Responses to Extracellular α-Synuclein. Molecules 2019, 24, 305. https://doi.org/10.3390/molecules24020305
Surguchev AA, Emamzadeh FN, Surguchov A. Cell Responses to Extracellular α-Synuclein. Molecules. 2019; 24(2):305. https://doi.org/10.3390/molecules24020305
Chicago/Turabian StyleSurguchev, Alexei A., Fatemeh Nouri Emamzadeh, and Andrei Surguchov. 2019. "Cell Responses to Extracellular α-Synuclein" Molecules 24, no. 2: 305. https://doi.org/10.3390/molecules24020305
APA StyleSurguchev, A. A., Emamzadeh, F. N., & Surguchov, A. (2019). Cell Responses to Extracellular α-Synuclein. Molecules, 24(2), 305. https://doi.org/10.3390/molecules24020305