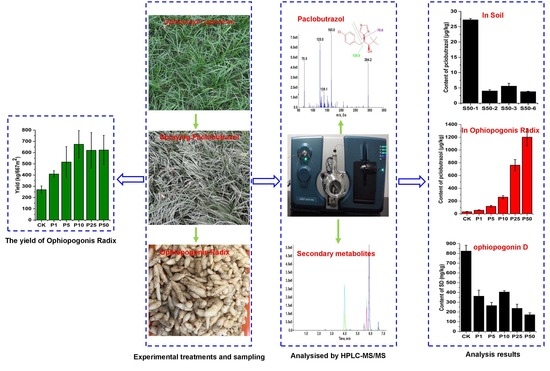
Residue of Paclobutrazol and Its Regulatory Effects on the Secondary Metabolites of Ophiopogon japonicas
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC-MS/MS Conditions
2.2. Sample Pretreatment
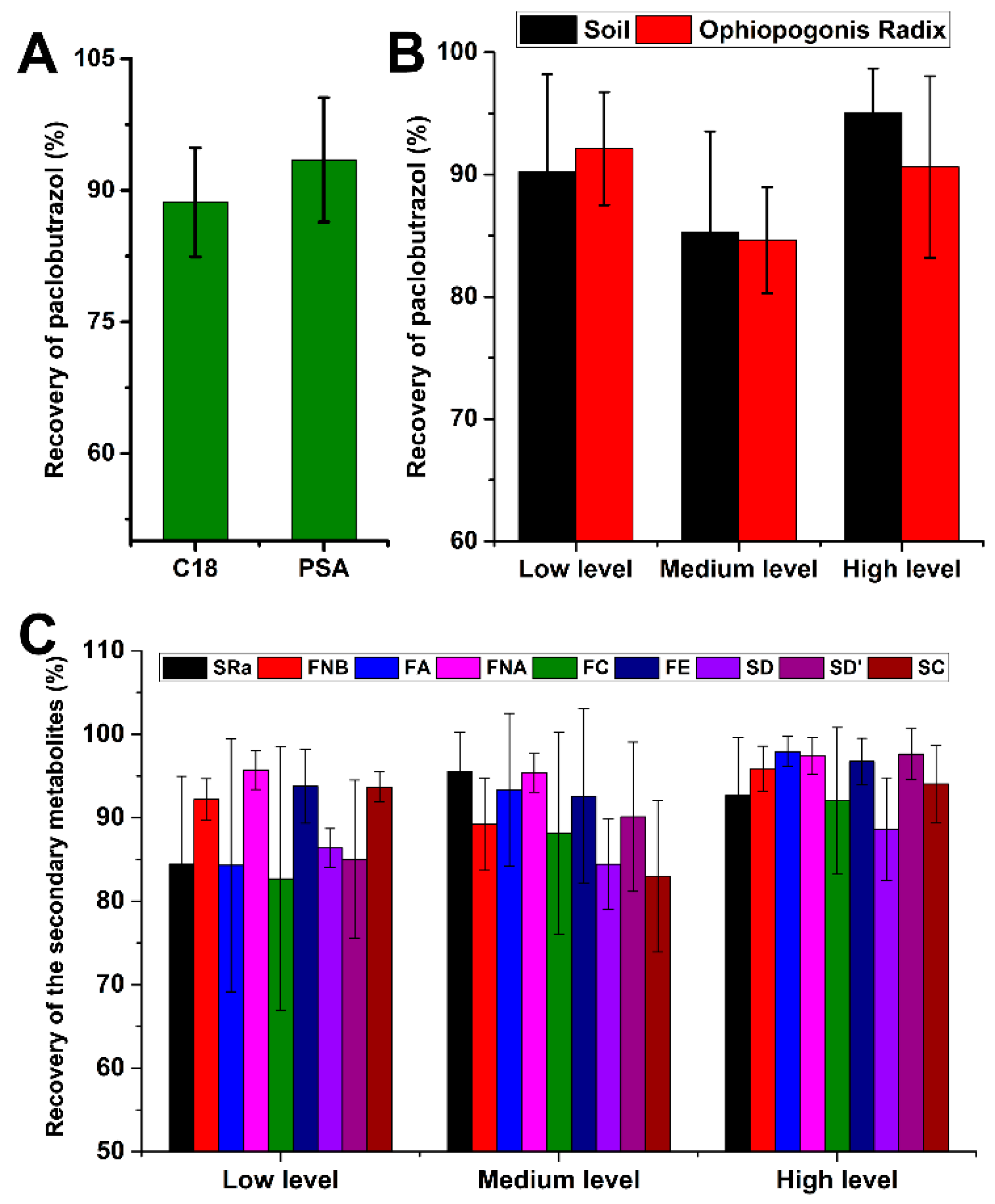
2.3. Method Validation
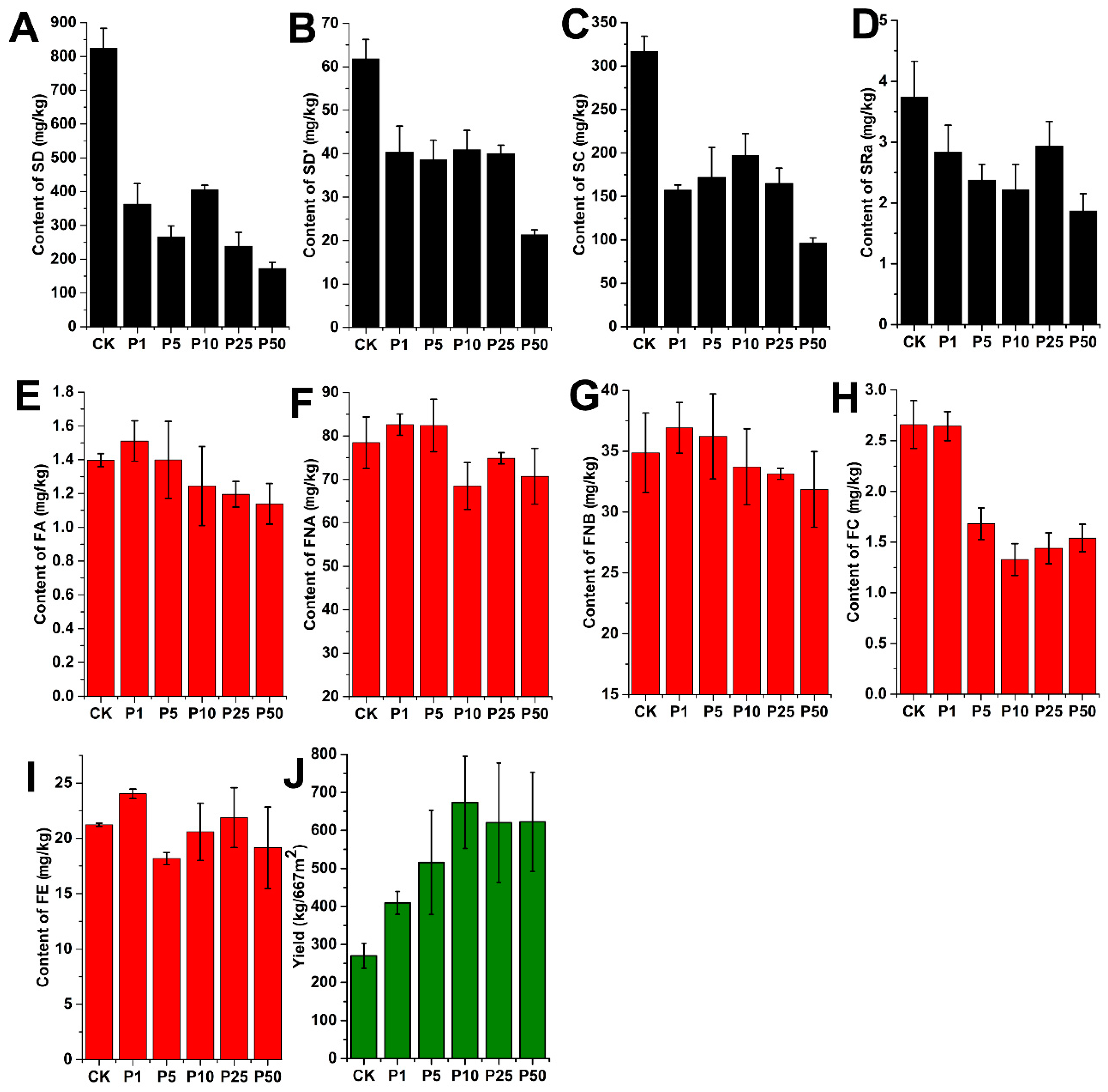
2.4. Sample Analysis
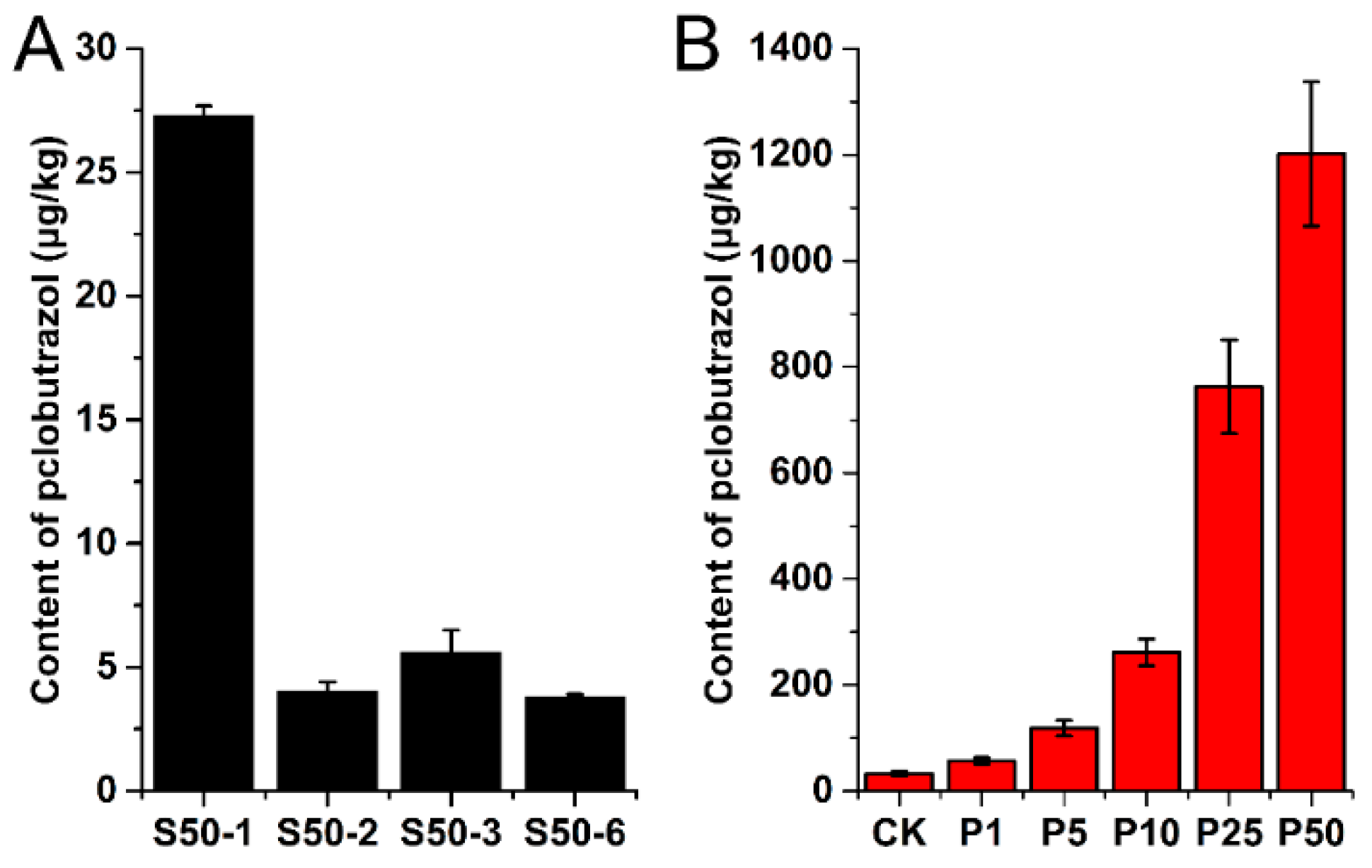
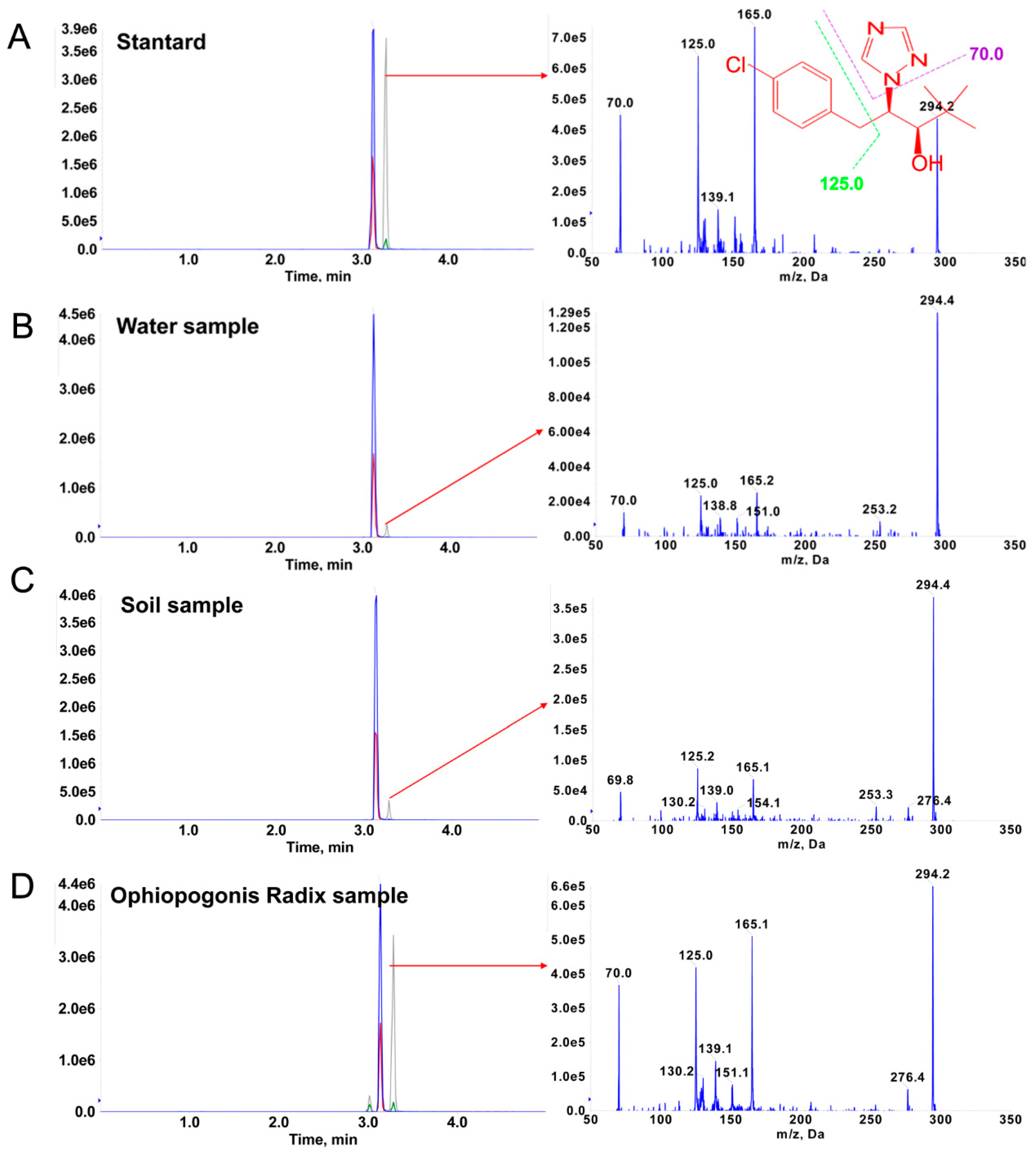
2.4.1. Residue Analysis of Paclobutrazol in Real Samples and EPI Spectra Confirmation
2.4.2. Quantitative Analysis of the Secondary Metabolites in Ophiopogonis Radix Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials and Paclobutrazol Treatment
3.3. Preparation of Standard Solutions
3.4. Preparation of Sample Solutions
3.5. Apparatus and Analytical Conditions
3.6. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FNA | methylophiopogonanone A |
| FA | methylophiopogonone A |
| FNB | methylophiopogonanone B |
| FC | ophiopogonanone C |
| FE | ophiopogonanone E |
| SD | ophiopogonin D |
| SD’ | ophiopogonin D’ |
| SRa | ophiopogon Ra |
| SC | ophiopojaponin C |
| MRM | multiple reaction monitoring |
| TCM | traditional Chinese medicine |
| PGR | plant growth regulator |
| MRM | multiple reaction monitoring |
| IDA | Information-dependent acquisition |
| EPI | enhanced product ion |
| ME | matrix effect |
| MRL | maximum residue limit |
References
- Chen, M.H.; Chen, X.J.; Wang, M.; Lin, L.G.; Wang, Y.T. Ophiopogon japonicus—A phytochemical, ethnomedicinal and pharmacological review. J. Ethnopharmacol. 2016, 181, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, X.; Lu, M.; He, X.; Yang, X. Recent advances in polysaccharides from ophiopogon japonicus and liriope spicata var. Prolifera. Int. J. Biol. Macromol. 2018, 114, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Chinese Herbalism Editorial Board. Zhonghua Bencao; Shanghai Science and Technology Press: Shanghai, China, 1999; pp. 7194–7195. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; Chemical Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Lin, W.L.; Su, W.W.; Cai, X.Y.; Luo, L.K.; Li, P.B.; Wang, Y.G. Fermentation effects of oligosaccharides of radix ophiopogonis on alloxan-induced diabetes in mice. Int. J. Biol. Macromol. 2011, 49, 194–200. [Google Scholar] [CrossRef] [PubMed]
- China Food and Drug Administration. Available online: http://www.sda.gov.cn/WS01/CL0001/2019 (accessed on 15 August 2019).
- Yin, W.U.; Dong, Z.; Huizhen, W.U.; Ding, W.; Zhao, M.; Shi, Q.; Qiao, W. Comparative studies on ophiopogonis and liriopes based on the determination of 11 bioactive components using LC-MS/MS and hierarchical clustering analysis. Food Res. Int. 2014, 57, 15–25. [Google Scholar]
- Ye, M.; Guo, D.; Ye, G.; Huang, C. Analysis of homoisoflavonoids in ophiopogon japonicus by hplc-dad-esi-msn. J. Am. Soc. Mass Spectrom. 2005, 16, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Wang, Y.; Wang, T.; Ying, X.; Wu, R.; Chen, H. De novoassembly and annotation of the zhe-maidong (ophiopogon japonicus(l.F.) ker-gawl) transcriptome in different growth stages. Sci. Rep. 2017, 7, 3616. [Google Scholar] [CrossRef]
- Jiang, X.W.Y.; Xie, H.; Li, R.; Wei, J.; Liu, Y. Environmental behavior of paclobutrazol in soil and its toxicity on potato and taro plants. Environ. Sci. Pollut. Res. Int. 2019. [Google Scholar] [CrossRef]
- Zhai, Y.Y.; Guo, B.L.; Cheng, M. Review on application of plant growth retardants in medicinal plants cultivation. Chin. Pharm. J. 2013, 38, 2739–2744. [Google Scholar]
- Luo, Z.; Zhang, L.; Mou, Y.; Cui, S.; Gu, Z.; Yu, J.; Ma, X. Multi-residue analysis of plant growth regulators and pesticides in traditional chinese medicines by high-performance liquid chromatography coupled with tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 2447–2460. [Google Scholar] [CrossRef]
- Zhao, X.; Mu, Y.; Yang, M. A simple multi-residue method for determination of plant growth retardants in ophiopogon japonicus and soil using ultra-performance liquid chromatography-tandem mass spectrometry. Chemosphere 2018, 8, 329–336. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, S.C.; Kim, K.M.; Lee, B.H.; Lee, I.J. Possible residual effects of gibberellic acid and gibberellin biosynthesis inhibitors on sprouting, early bulbil formation and tuber yield in chinese yam. J. Agron. Crop Sci. 2010, 189, 428–432. [Google Scholar] [CrossRef]
- Ministry of Health Labour and Welfare, Japan. Maximum Residue Limits (MRLs) List of Agricultural Chemicals in Foods. Available online: http://www.m5.ws001.squarestart.ne.jp/foundation/search.html (accessed on 20 August 2019).
- United States Environmental Protection Agency. Index to Pesticide Chemical Names, Part 180 Tolerance Information for Pesticide Chemicals in Food and Feed Commodities. Available online: https://www.ecfr.gov/cgi-bin/text-idx?c=ecfr&sid=1a0ecaf51aa3dba662c9cbf1b4336eaf&tpl=/ecfrbrowse/Title40/40cfr180_main_02.tpl (accessed on 20 August 2019).
- European Commission. Commission Regulation (EU) No 396/2005 of 23 February 2005 Amending Council Directive 91/414/EEC on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=download.MRL (accessed on 20 August 2019).
- Food and Agriculture Organization/World Health Organization. Food standards programme. In Proceedings of the Codex Alimentarius Commission, Twenty-seventh Session, Geneva, Switzerland, 28 June–3 July 2004; Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/ (accessed on 20 August 2019).
- Yang, Y.; Zhang, X.; Hao, A.; Li, T.; Luo, X. Determination of pacloburtrazol remained in Maidong and its growth environment by HPLC. West. J. Tradit. Chin. Med. 2016, 29, 37–39. [Google Scholar]
- Zhan, N.; Tao, S.S. Effect of MET on the effective components of Ophiopogon japonicus. J. Anhui Agri. Sci. 2014, 42, 52–53. [Google Scholar]
- Zhan, N.; Tao, S.S.; Xie, M.C. Effect of MET on growth, development and yield of Dwarf Liyturf Ophiopogon japonicas (L.f.) Ker-Gawl. Hubei Agr. Sci. 2014, 53, 2835–2837. [Google Scholar]
- Luo, Z.; Shi, H.; Zhang, K.; Qin, X.; Guo, Y.; Ma, X. Liquid chromatography with tandem mass spectrometry method for the simultaneous determination of multiple sweet mogrosides in the fruits of Siraitia grosvenorii and its marketed sweeteners. J. Sep. Sci. 2016, 39, 4124–4135. [Google Scholar] [CrossRef]
- Xu, D.; Huang, H.; Lu, M.; Shan, Z.; Yu, Z. Simultaneous Determination of 21 Plant Growth Regulators in Various Fruits Using Quechers Coupled with an Hplc-ms/ms Technique. Available online: https://www.agilent.com/cs/library/applications/5991-5506EN.pdf (accessed on 17 August 2019).
- Wei, H.; Jin, H.Y.; Wang, Y.; Ma, S.C. Simultaneous determination of 23 plant growth regulator residues in Chinese materia medica by ultra performance liquid chromatography-tandem mass spectrometry. Chin. Tradit. Herb. Drugs 2017, 48, 1653–1660. [Google Scholar]
- Danezis, G.P.; Anagnostopoulos, C.J.; Liapis, K.; Koupparis, M.A. Multi-residue analysis of pesticides, plant hormones, veterinary drugs and mycotoxins using HILIC chromatography—MS/MS in various food matrices. Anal. Chim. Acta 2016, 942, 121–138. [Google Scholar] [CrossRef] [PubMed]
- National Health and Family Planning Commission and Ministry of Agriculture of the People’s Republic of China. National Food Safety Standard-Maximum Residue Limits for Pesticides in Food (GB 2763-2016). Available online: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=ed7b47492d7a42359f839daf3f70eb4b (accessed on 20 August 2019).
- Jackson, M.; Line, M.; Hasan, O. Microbial degradation of a recalcitrant plant growth retardant—Paclobutrazol. Soil. Biol. Biochem. 1996, 28, 1265–1267. [Google Scholar] [CrossRef]
- Gonçalves, I.C.R.; Araújo, A.S.F.; Carvalho, E.M.S.; Carneiro, R.F.V. Effect of paclobutrazol on microbial biomass, respiration and cellulose decomposition in soil. Eur. J. Soil Biol. 2009, 45, 235–238. [Google Scholar] [CrossRef]
- Jamwal, K.; Bhattacharya, S.; Puri, S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants 2018, 9, 26–38. [Google Scholar] [CrossRef]
- Kanwal, N.; Siddiqui, A.J.; Haq, F.U.; El-Seedi, H.R.; Musharraf, S.G. Two-stage mass spectrometry approach for the analysis of triterpenoid glycos. RSC Adv. 2018, 8, 41023–41031. [Google Scholar] [CrossRef]
- European Commission. Document no. SANTE/11813/2017, Guidance Document on Analytica Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_mrl_guidelines_wrkdoc_2017-11813.pdf (accessed on 20 August 2019).
- International Conference on Harmonization (ICH). Q2 (R1): Text on Validation of Analytical Procedures; SANCO. Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed; ICH: Geneva, Switzerland, 2005. [Google Scholar]
Sample Availability: Samples of the compounds Ophiopogon Ra, Ophiopojaponin C, Ophiopogonanone E, Ophiopogonin D, Ophiopogonin D’, Methylophiopogonone A, Methylophiopogonanone A, Methylophiopogonanone B, and Ophiopogonanone C are available from the authors. |

| No. | Analytes | Precursor Ion (m/z) | Product Ions (m/z) | Ionization Mode | Retention Time (min) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 | DP (V) | PI q | CE (V) | PI i | CE (V) | ||||
| 1 | Forchlorfenuron | 248 | 22 | 129 | 30 | 92.9 | 35 | ESI+ | 3.13 |
| 2 | Paclobutrazol | 294.1 | 60 | 69.9 | 30 | 124.8 | 25 | ESI+ | 3.28 |
| 3 | Ophiopogon Ra (SRa) | 719.6 | 220 | 393.2 | 45 | 411.4 | 40 | ESI+ | 1.8 |
| 4 | Ophiopojaponin C (SC) | 869.5 | −220 | 737.4 | −50 | 205.6 | −50 | ESI− | 2.48 |
| 5 | Ophiopogonanone E (FE) | 359.2 | −50 | 153.9 | −35 | 208 | −35 | ESI− | 3.98 |
| 6 | Ophiopogonin D (SD) | 853.6 | −220 | 575.3 | −50 | 721.6 | −55 | ESI− | 4.14 |
| 7 | Ophiopogonin D’ (SD’) | 853.6 | −220 | 204.9 | −55 | 721.6 | −55 | ESI− | 4.41 |
| 8 | Methylophiopogonone A (FA) | 339.2 | −68 | 131 | −55 | 217.2 | −55 | ESI− | 5.51 |
| 9 | Methylophiopogonanone A (FNA) | 341.3 | −68 | 178 | −45 | 206 | −38 | ESI− | 5.7 |
| 10 | Methylophiopogonanone B (FNB) | 327 | −60 | 206 | −35 | 178 | −40 | ESI− | 5.89 |
| 11 | Ophiopogonanone C (FC) | 355.2 | −60 | 193.1 | −40 | 164.2 | −45 | ESI− | 6.61 |
| Analytes | Linearity | LOQ (ng/mL) | LOD (ng/mL) | Precision (RSD, %) | Recovery% (RSD, %) | Stability (RSD, %) | ME (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | Range (ng/mL) | Intra-Day | Inter-Day | Low Level | Medium Level | High Level | |||||
| Paclobutrazol | 0.9993 | 2–100 | 0.5 | 0.2 | 5.0 | 4.3 | Soil sample | 5.7 | 94.9 | ||
| 90.2 (8.0) | 85.2 (8.2) | 95.1 (3.6) | |||||||||
| Ophiopogonis Radix | 91.0 | ||||||||||
| 92.1 (4.6) | 84.6 (4.3) | 90.6 (7.4) | |||||||||
| SRa | 0.9963 | 50–1000 | 50.0 | 20.0 | 5.6 | 6.2 | 84.4 (10.5) | 95.5 (4.6) | 92.6 (6.9) | 9.5 | 95.2 |
| SC | 0.9998 | 10–1000 | 10.0 | 4.0 | 4.4 | 6.3 | 93.6 (1.8) | 83.0 (9.1) | 92.6 (6.9) | 4.5 | 99.1 |
| FE | 0.9995 | 5–500 | 2.0 | 0.8 | 2.5 | 3.5 | 93.7 (4.4) | 92.5 (10.4) | 96.7 (2.7) | 2.8 | 90.8 |
| SD | 0.9944 | 50–1000 | 30.0 | 10.0 | 5.9 | 7.3 | 86.3 (2.3) | 84.4 (5.4) | 88.6 (6.1) | 8.1 | 86.4 |
| SD’ | 0.9998 | 50–1000 | 30.0 | 10.0 | 4.2 | 4.8 | 85.0 (9.4) | 90.1 (8.9) | 97.6 (3.0) | 2.5 | 84.3 |
| FA | 0.9998 | 5–500 | 5.0 | 2.0 | 3.1 | 5.1 | 84.3 (11.1) | 93.3 (9.1) | 97.9 (1.8) | 5.7 | 96.7 |
| FNA | 0.9996 | 5–500 | 5.0 | 2.0 | 4.0 | 2.5 | 95.7 (2.3) | 95.3 (2.3) | 97.4 (2.1) | 3.8 | 93.9 |
| FNB | 0.9995 | 5–500 | 2.0 | 0.8 | 5.1 | 8.3 | 92.2 (2.5) | 89.2 (5.5) | 95.8 (2.7) | 6.0 | 84.8 |
| FC | 0.9998 | 5–500 | 5.0 | 2.0 | 4.6 | 3.5 | 82.6 (10.8) | 88.1 (12.1) | 92.0 (8.8) | 5.4 | 85.9 |
| Ophiopogonis Radix Sample | Residue Level (μg/kg) | Soil Sample | Residue Level (μg/kg) | Water Sample | Residue Level (μg/L) |
|---|---|---|---|---|---|
| CK1 | 31.67 | S50-1-1 | 26.96 | Groundwater 1 | ND |
| CK2 | 28.39 | S50-1-2 | 27.17 | Groundwater 2 | ND |
| CK3 | 37.66 | S50-1-3 | 27.72 | Groundwater 3 | <LOQ |
| P1-1 | 58.94 | S50-2-1 | 4.39 | Surface water 1 | 1.16 |
| P1-2 | 49.72 | S50-2-2 | 3.65 | Surface water 2 | 1.62 |
| P1-3 | 61.38 | S50-2-3 | 4.06 | Surface water 3 | 1.08 |
| P5-1 | 125.03 | S50-3-1 | 6.46 | ||
| P5-2 | 101.94 | S50-3-2 | 5.68 | ||
| P5-3 | 128.76 | S50-3-3 | 4.69 | ||
| P10-1 | 269.21 | S50-6-1 | 3.92 | ||
| P10-2 | 233.74 | S50-6-2 | 3.69 | ||
| P10-3 | 282.00 | S50-6-3 | 3.74 | ||
| P25-1 | 675.57 | ||||
| P25-2 | 851.59 | ||||
| P25-3 | 760.64 | ||||
| P50-1 | 1081.37 | ||||
| P50-2 | 1174.25 | ||||
| P50-3 | 1349.59 |
| Samples | SRa | FNB | FA | FNA | FC | FE | SD | SD’ | SC |
|---|---|---|---|---|---|---|---|---|---|
| CK1 | 3.37 | 33.08 | 1.35 | 78.64 | 2.41 | 21.30 | 781.35 | 56.72 | 297.40 |
| CK2 | 3.43 | 38.65 | 1.41 | 84.30 | 2.88 | 21.30 | 891.19 | 65.02 | 331.17 |
| CK3 | 4.42 | 32.88 | 1.43 | 72.42 | 2.68 | 21.06 | 802.07 | 63.68 | 322.08 |
| P1-1 | 2.78 | 39.23 | 1.59 | 79.77 | 2.70 | 23.90 | 341.97 | 38.39 | 155.84 |
| P1-2 | 2.43 | 35.19 | 1.57 | 84.30 | 2.75 | 24.51 | 315.03 | 35.59 | 151.95 |
| P1-3 | 3.31 | 36.35 | 1.37 | 83.73 | 2.48 | 23.69 | 431.09 | 47.09 | 163.64 |
| P5-1 | 2.32 | 40.19 | 1.65 | 89.39 | 1.85 | 18.80 | 292.23 | 33.31 | 149.35 |
| P5-2 | 2.15 | 33.65 | 1.22 | 78.64 | 1.63 | 17.98 | 230.05 | 41.47 | 154.55 |
| P5-3 | 2.66 | 34.81 | 1.33 | 79.21 | 1.55 | 17.74 | 275.65 | 40.94 | 211.69 |
| P10-1 | 2.33 | 30.96 | 1.49 | 67.89 | 1.40 | 23.56 | 397.93 | 46.02 | 180.52 |
| P10-2 | 1.75 | 37.12 | 1.22 | 74.12 | 1.44 | 19.49 | 395.85 | 38.13 | 225.97 |
| P10-3 | 2.57 | 33.08 | 1.02 | 63.37 | 1.15 | 18.74 | 420.73 | 38.53 | 185.71 |
| P25-1 | 2.92 | 32.88 | 1.26 | 74.12 | 1.26 | 22.60 | 191.71 | 40.40 | 166.23 |
| P25-2 | 2.55 | 32.88 | 1.11 | 74.12 | 1.54 | 18.87 | 250.78 | 37.86 | 146.75 |
| P25-3 | 3.35 | 33.65 | 1.22 | 76.38 | 1.52 | 24.14 | 271.50 | 41.74 | 181.82 |
| P50-1 | 1.55 | 35.19 | 1.22 | 78.08 | 1.66 | 23.38 | 167.67 | 20.74 | 95.19 |
| P50-2 | 1.95 | 31.35 | 1.00 | 67.89 | 1.39 | 16.79 | 157.10 | 20.60 | 90.65 |
| P50-3 | 2.11 | 29.04 | 1.20 | 66.20 | 1.57 | 17.26 | 192.75 | 22.61 | 102.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Luo, Z.; Cui, S.; Xie, L.; Yu, J.; Tang, D.; Ma, X.; Mou, Y. Residue of Paclobutrazol and Its Regulatory Effects on the Secondary Metabolites of Ophiopogon japonicas. Molecules 2019, 24, 3504. https://doi.org/10.3390/molecules24193504
Zhang L, Luo Z, Cui S, Xie L, Yu J, Tang D, Ma X, Mou Y. Residue of Paclobutrazol and Its Regulatory Effects on the Secondary Metabolites of Ophiopogon japonicas. Molecules. 2019; 24(19):3504. https://doi.org/10.3390/molecules24193504
Chicago/Turabian StyleZhang, Lixia, Zuliang Luo, Shengrong Cui, Lei Xie, Jing Yu, Deying Tang, Xiaojun Ma, and Yan Mou. 2019. "Residue of Paclobutrazol and Its Regulatory Effects on the Secondary Metabolites of Ophiopogon japonicas" Molecules 24, no. 19: 3504. https://doi.org/10.3390/molecules24193504
APA StyleZhang, L., Luo, Z., Cui, S., Xie, L., Yu, J., Tang, D., Ma, X., & Mou, Y. (2019). Residue of Paclobutrazol and Its Regulatory Effects on the Secondary Metabolites of Ophiopogon japonicas. Molecules, 24(19), 3504. https://doi.org/10.3390/molecules24193504