Unraveling the Molecular Mechanism of Traditional Chinese Medicine: Formulas Against Acute Airway Viral Infections as Examples
Abstract
:1. Significance of Herbal Medicine and Traditional Chinese Medicine (TCM)
2. Indications of Use of TCM Formulas for Diseases Management
2.1. Indications of R-Physicians’ Formulas
2.2. Indications of A-Physicians’ Formulas
3. Factors and Mechanisms Affecting Clinical Effects and Side Effects of the Formulas of A-Physicians
3.1. Individual Gene Affecting Pharmacodynamics and Pharmacokinetics
3.2. Complex Active Molecules vary in TCM Formulas
3.3. Complex Interplays between Herb-Drug, Herb-Food, Herb-Herb, Herb-Microbiome, and Herb-Disease
4. Unraveling the Molecular Mechanism of TCM Formulas, Using Airway Viral Infections as Examples
4.1. Ge-Gen-Tang (GGT; Kakkon-To in Japan; Galgeun-Tang in Korea; Gegen Decoction in China)
4.2. Ma-Huang-Tang (MHT; Maoto in Japan; Mahuang Decoction in China)
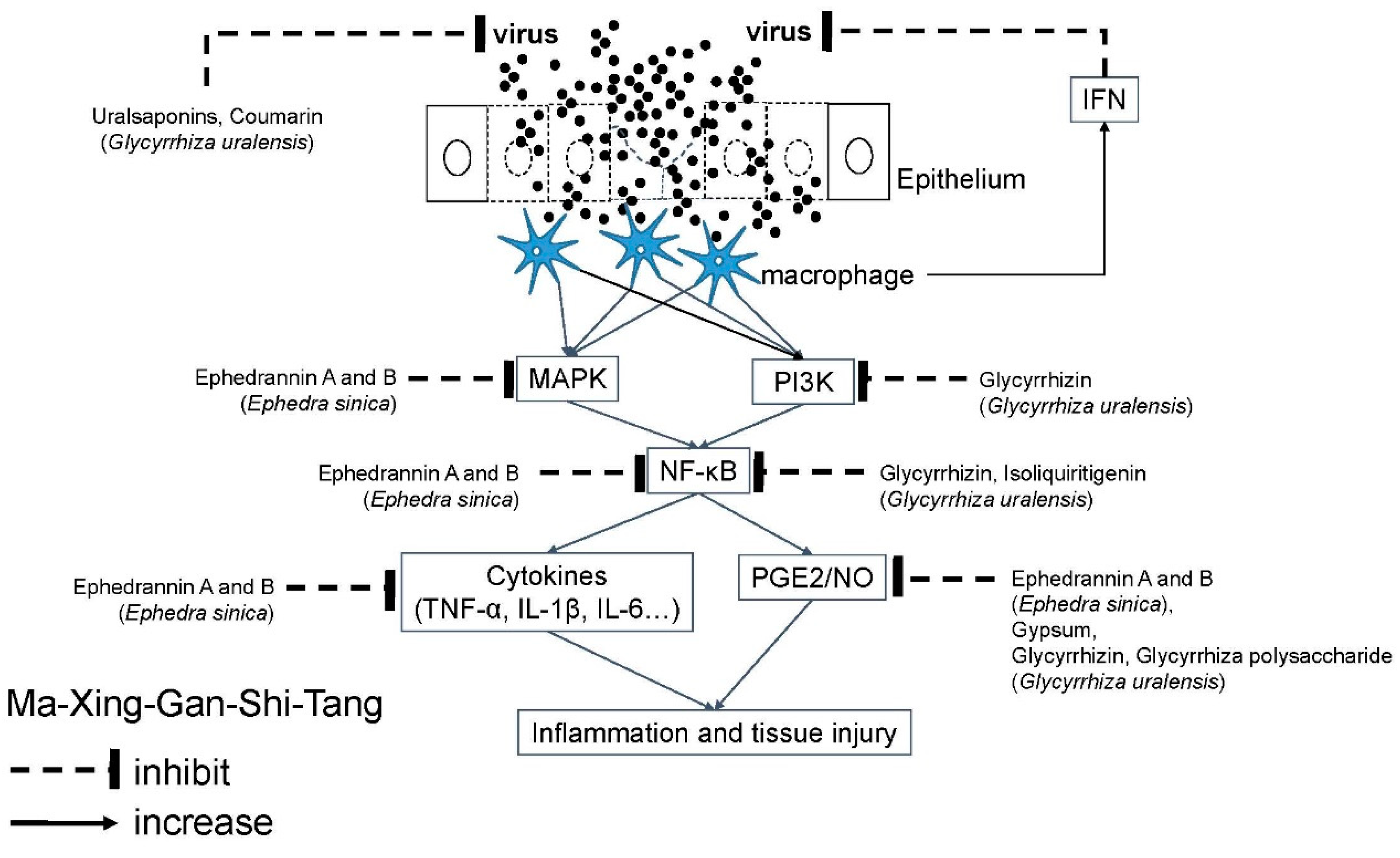
4.3. Ma-Xing-Gan-Shi-Tang (MXGST; Maxing Shigan decoction in China)
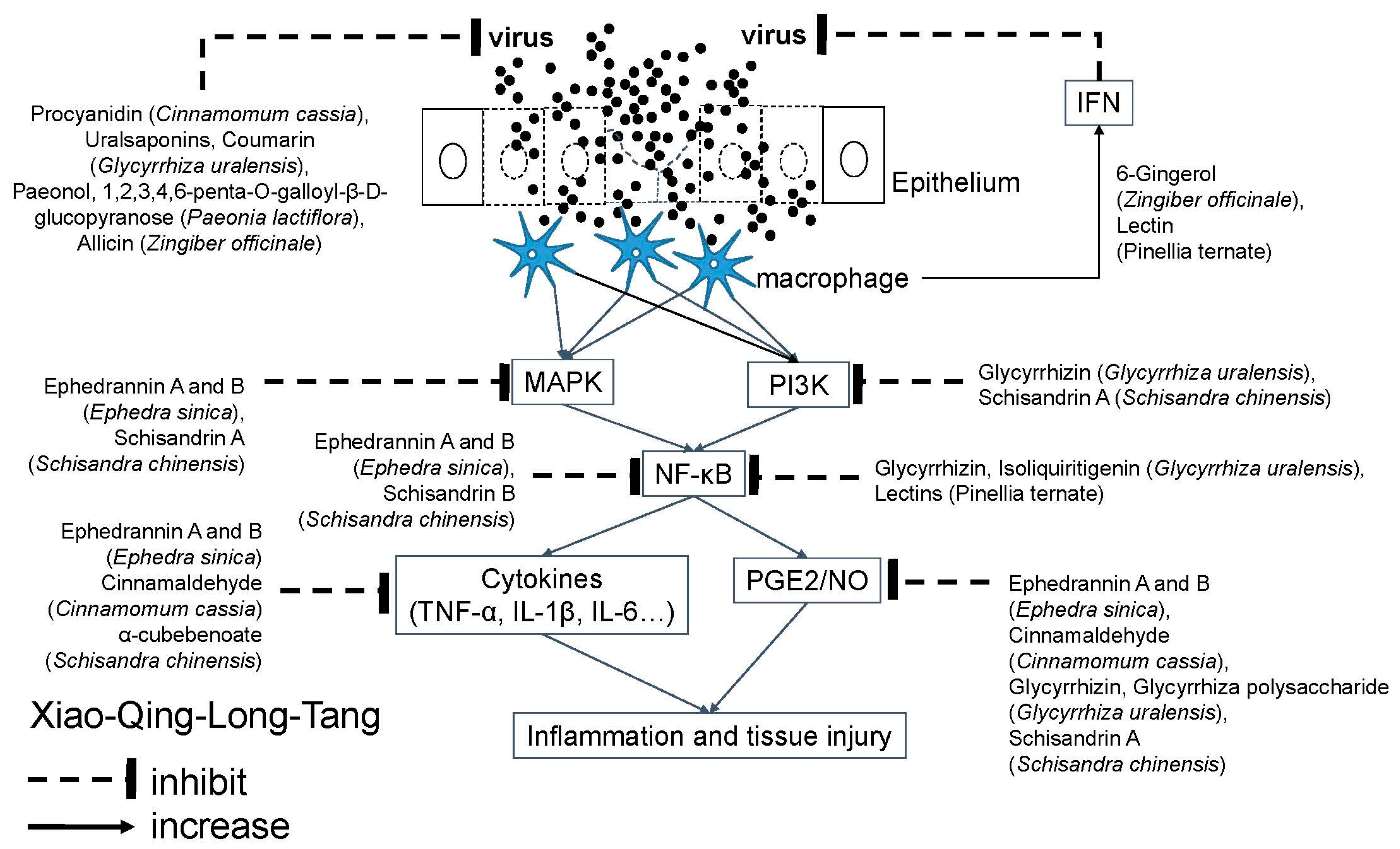
4.4. Xiao-Qing-Long-Tang (XQLT; Sho-Seiryu-To in Japan; So-Cheong-Ryong-Tang in Korea; Minor Blue-Green Dragon Decoction in China)
4.5. Ye-Gan-Ma-Huang-Tang (YGMHT; Yakammaoto in Japan; Shegan-Mahuang-Tang or Sheganmahuang Decoction in Chinese)
5. Limitations of Herbal Medicine and TCM
5.1. Away from Medical Education of Orthodox Medicine
5.2. Lack of Tough Evidence of Clinical Efficacy
5.3. Safety Issue is not Completely Resolved
5.4. Complex Interactions are not Fully Understood
5.5. Quality Uncertainty of Commercially Available Natural Products
5.6. Pitfall of Interpretation of Benefits
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brinckmann, J.A.; Wollschlaeger, B. The ABC Clinical Guide to Herbs; American Botanical Council: Austin, TX, USA, 2003. [Google Scholar]
- Eisenberg, D.M.; Davis, R.B.; Ettner, S.L.; Appel, S.; Wilkey, S.; Van Rompay, M.; Kessler, R.C. Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. JAMA 1998, 280, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl. Health Stat. Rep. 2015, 79, 1–16. [Google Scholar]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and alternative medicine use among adults and children: United States, 2007. Natl. Health Stat. Rep. 2008, 12, 1–23. [Google Scholar]
- Schilcher, H. Current state of phytotherapy in Germany. Deutsche Apotheker-Zeitung 1998, 138, 144. [Google Scholar]
- WHO. Traditional Medicine Strategy 2002–2005; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- 大塚敬節; 矢數道明; 清水藤太郎. 漢方診療醫典; 南山堂: Tokyo, Japan, 1969. [Google Scholar]
- Pompei, R.; Flore, O.; Marccialis, M.A.; Pani, A.; Loddo, B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 1979, 281, 689–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Chang, J.S.; Lin, L.T.; Chiang, L.C.; Lin, C.C. Antiviral effect of cimicifugin from Cimicifuga foetida against human respiratory syncytial virus. Am. J. Chin. Med. 2012, 40, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Li, J.; Chen, Q.; Jiang, Z.; Zhang, R.; Wang, X.; Yang, Z.; Pan, X. Pterodontic Acid Isolated from Laggera pterodonta Inhibits Viral Replication and Inflammation Induced by Influenza A Virus. Molecules 2017, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Okajima, K.; Sakata, K.; Takatsuki, K. A possible mechanism of interstitial pneumonia during interferon therapy with sho-saiko-to. Nihon Kyobu Shikkan Gakkai Zasshi 1995, 33, 389–394. [Google Scholar]
- Kobashi, Y.; Nakajima, M.; Niki, Y.; Matsushima, T. A case of acute eosinophilic pneumonia due to Sho-saiko-to. Nihon Kyobu Shikkan Gakkai Zasshi 1997, 35, 1372–1377. [Google Scholar]
- Katou, K.; Mori, K. Autoimmune hepatitis with drug-induced pneumonia due to Sho-saiko-to. Nihon Kokyuki Gakkai Zasshi 1999, 37, 641–646. [Google Scholar]
- Tojima, H.; Yamazaki, T.; Tokudome, T. Two cases of pneumonia caused by Sho-saiko-to. Nihon Kyobu Shikkan Gakkai Zasshi 1996, 34, 904–910. [Google Scholar]
- Wada, Y.; Kubo, M. Acute lymphoblastic leukemia complicated by type C hepatitis during treatment and further by acute interstitial pneumonia due to sho-saiko-to in 7-year-old. Arerugi 1997, 46, 1148–1155. [Google Scholar] [PubMed]
- Tomioka, H.; Hashimoto, K.; Ohnishi, H.; Fujiyama, R.; Sakurai, T.; Tada, K.; Sakamoto, H.; Iwasaki, H. An autopsy case of interstitial pneumonia probably induced by Sho-saiko-to. Nihon Kokyuki Gakkai Zasshi 1999, 37, 1013–1018. [Google Scholar] [PubMed]
- Sugiyama, H.; Nagai, M.; Kotajima, F.; Yoshizawa, A.; Kamimura, M.; Horiuchi, T.; Kudo, K.; Kabe, J.; Hayashi, S.; Umeda, N. A case of interstitial pneumonia with chronic hepatitis C following interferon-alfa and sho-saiko-to therapy. Arerugi 1995, 44, 711–714. [Google Scholar] [PubMed]
- Li, C.T.; Wang, H.B.; Xu, B.J. A comparative study on anticoagulant activities of three Chinese herbal medicines from the genus Panax and anticoagulant activities of ginsenosides Rg1 and Rg2. Pharm. Biol. 2013, 51, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.S.; Wei, G.; Dey, L.; Karrison, T.; Nahlik, L.; Maleckar, S.; Kasza, K.; Ang-Lee, M.; Moss, J. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: A randomized, controlled Trial. Ann. Intern. Med. 2004, 141, 23–27. [Google Scholar] [CrossRef]
- Rosado, M.F. Thrombosis of a prosthetic aortic valve disclosing a hazardous interaction between warfarin and a commercial ginseng product. Cardiology 2003, 99, 111. [Google Scholar] [CrossRef]
- Greenspan, E.M. Ginseng and vaginal bleeding. JAMA 1983, 249, 2018. [Google Scholar] [CrossRef]
- Hopkins, M.P.; Androff, L.; Benninghoff, A.S. Ginseng face cream and unexplained vaginal bleeding. Am. J. Obstet. Gynecol. 1988, 159, 1121–1122. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, Y.M.; Ahn, S.Y.; Doo, H.K.; Lee, B.C. Interaction between warfarin and Panax ginseng in ischemic stroke patients. J. Altern. Complement. Med. 2008, 14, 715–721. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Zhang, A.; Sun, W.; Wang, P.; Wang, Z. Potential role of metabolomics apporoaches in the area of traditional Chinese medicine: As pillars of the bridge between Chinese and Western medicine. J. Pharm. Biomed. Anal. 2011, 55, 859–868. [Google Scholar] [CrossRef]
- Lu, C.; Deng, J.; Li, L.; Wang, D.; Li, G. Application of metabolomics on diagnosis and treatment of patients with psoriasis in traditional Chinese medicine. Biochim. Biophys. Acta 2014, 1844, 280–288. [Google Scholar] [CrossRef]
- Liu, P.; Duan, J.; Wang, P.; Qian, D.; Guo, J.; Shang, E.; Su, S.; Tang, Y.; Tang, Z. Biomarkers of primary dysmenorrhea and herbal formula intervention: An exploratory metabonomics study of blood plasma and urine. Mol. Biosyst. 2013, 9, 77–87. [Google Scholar] [CrossRef]
- Liu, P.; Duan, J.A.; Guo, J.M.; Qian, D.W.; Shang, E.X.; Tang, Y.P.; Su, S.L. Plasma metabolic profiling of normal and dysmenorrhea syndrome rats and the effects of Xiang-Fu-Si-Wu Decoction intervention. Pharm. Biol. 2014, 52, 603–613. [Google Scholar] [CrossRef]
- Ko, R.J. Adulterants in Asian patent medicines. N. Engl. J. Med. 1998, 339, 847. [Google Scholar] [CrossRef]
- Saper, R.B.; Kales, S.N.; Paquin, J.; Burns, M.J.; Eisenberg, D.M.; Davis, R.B.; Phillips, R.S. Heavy metal content of ayurvedic herbal medicine products. JAMA 2004, 292, 2868–2873. [Google Scholar] [CrossRef]
- Kew, J.; Morris, C.; Aihie, A.; Fysh, R.; Jones, S.; Brooks, D. Arsenic and mercury intoxication due to Indian ethnic remedies. BMJ Clinic. Res. 1993, 306, 506–507. [Google Scholar] [CrossRef]
- Saper, R.B.; Phillips, R.S.; Sehgal, A.; Khouri, N.; Davis, R.B.; Paquin, J.; Thuppil, V.; Kales, S.N. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA 2008, 300, 915–923. [Google Scholar] [CrossRef]
- Field, T.S.; Gurwitz, J.H.; Avorn, J.; McCormick, D.; Jain, S.; Eckler, M.; Benser, M.; Bates, D.W. Risk factors for adverse drug events among nursing home residents. Arch. Intern. Med. 2001, 161, 1629–1634. [Google Scholar] [CrossRef]
- Noh, K.; Kang, Y.; Nepal, M.R.; Jeong, K.S.; Oh, D.G.; Kang, M.J.; Lee, S.; Kang, W.; Jeong, H.G.; Jeong, T.C. Role of Intestinal Microbiota in Baicalin-Induced Drug Interaction and Its Pharmacokinetics. Molecules 2016, 21, 337. [Google Scholar] [CrossRef]
- Tian, X.; Cheng, Z.Y.; Jin, H.; Gao, J.; Qiao, H.L. Inhibitory Effects of Baicalin on the Expression and Activity of CYP3A Induce the Pharmacokinetic Changes of Midazolam in Rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 179643. [Google Scholar] [CrossRef]
- Fong, S.Y.; Wong, Y.C.; Xie, C.; Zuo, Z. Herb-drug interactions between Scutellariae Radix and mefenamic acid: Simultaneous investigation of pharmacokinetics, anti-inflammatory effect and gastric damage in rats. J. Ethnopharmacol. 2015, 170, 106–116. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, W.; Guo, D.; Tan, Z.R.; Xu, P.; Li, Q.; Liu, Y.Z.; Zhang, L.; He, T.Y.; Hu, D.L.; et al. The effect of herbal medicine baicalin on pharmacokinetics of rosuvastatin, substrate of organic anion-transporting polypeptide 1B1. Clin. Pharmacol. Ther. 2008, 83, 471–476. [Google Scholar] [CrossRef]
- Liang, R.; Han, R.M.; Fu, L.M.; Ai, X.C.; Zhang, J.P.; Skibsted, L.H. Baicalin in radical scavenging and its synergistic effect with beta-carotene in antilipoxidation. J. Agric. Food Chem. 2009, 57, 7118–7124. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.Y.; Xu, L.; Liu, B.; Zhou, L.L.; Wang, X.F.; Wang, D.; Sun, T. Studies on the competitive binding of cleviprex and flavonoids to plasma protein by multi-spectroscopic methods: A prediction of food-drug interaction. J. Photochem. Photobiol. B 2017, 175, 192–199. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; He, L.L.; Liu, B.; Zhang, S.Y.; Ye, X.; Jing, J.J.; Zhang, J.F.; Gao, M.; Wang, X. Spectroscopic investigation on the food components-drug interaction: The influence of flavonoids on the affinity of nifedipine to human serum albumin. Food Chem. Toxicol. 2015, 78, 42–51. [Google Scholar] [CrossRef]
- Liu, B.M.; Zhang, J.; Hao, A.J.; Xu, L.; Wang, D.; Ji, H.; Sun, S.J.; Chen, B.Q.; Liu, B. The increased binding affinity of curcumin with human serum albumin in the presence of rutin and baicalin: A potential for drug delivery system. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 155, 88–94. [Google Scholar] [CrossRef]
- Zhang, C.H.; Yu, R.Y.; Liu, Y.H.; Tu, X.Y.; Tu, J.; Wang, Y.S.; Xu, G.L. Interaction of baicalin with berberine for glucose uptake in 3T3-L1 adipocytes and HepG2 hepatocytes. J. Ethnopharmacol. 2014, 151, 864–872. [Google Scholar] [CrossRef]
- Murtaza, N.; Ó Cuív, P.; Morrison, M. Diet and the Microbiome. Gastroenterol. Clin. North. Am. 2017, 46, 49–60. [Google Scholar] [CrossRef]
- Chen, F.; Wen, Q.; Jiang, J.; Li, H.L.; Tan, Y.F.; Li, Y.H.; Zeng, N.K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 2016, 179, 253–264. [Google Scholar] [CrossRef]
- Kang, M.J.; Ko, G.S.; Oh, D.G.; Kim, J.S.; Noh, K.; Kang, W.; Yoon, W.K.; Kim, H.C.; Jeong, H.G.; Jeong, T.C. Role of metabolism by intestinal microbiota in pharmacokinetics of oral baicalin. Arch. Pharm. Res. 2014, 37, 371–378. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, M.; Qu, F.; Li, H.L.; Yu, L.B.; Rao, Y.; Wang, Y.S.; Xu, G.L. Pharmacokinetics of Maxing Shigan decoction in normal rats and RSV pneumonia model rats by HPLC-MS/MS. Zhongguo Zhong Yao Za Zhi 2015, 40, 2649–2655. [Google Scholar]
- Grief, S.N. Upper respiratory infections. Prim. Care 2013, 40, 757–770. [Google Scholar] [CrossRef]
- Jameson, J.L.; Dennis, A.S.F.; Kasper, L.; Hauser, S.L.; Longo, D.L.; Loscalzo, J. Common viral respiratory infections. In Harrison’s Principles of Internal Medicine 20e; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Ministry of Health and Welfare. Taiwan Herbal Pharmacopeia, 3rd ed.; National Central Library: Taipei City, Taiwan, 2018.
- Muraoka, K.; Yoshida, S.; Hasegawa, K.; Nakanishi, N.; Fukuzawa, I.; Tomita, A.; Cyong, J.C. A pharmacologic study on the mechanism of action of Kakkon-to: Body temperature elevation and phagocytic activation of macrophages in dogs. J. Altern. Complement. Med. 2004, 10, 841–849. [Google Scholar]
- Chang, J.S.; Wang, K.C.; Shieh, D.E.; Hsu, F.F.; Chiang, L.C. Ge-Gen-Tang has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2012, 139, 305–310. [Google Scholar] [CrossRef]
- Wu, M.S.; Yen, H.R.; Chang, C.W.; Peng, T.Y.; Hsieh, C.F.; Chen, C.J.; Lin, T.Y.; Horng, J.T. Mechanism of action of the suppression of influenza virus replication by Ko-Ken Tang through inhibition of the phosphatidylinositol 3-kinase/Akt signaling pathway and viral RNP nuclear export. J. Ethnopharmacol. 2011, 134, 614–623. [Google Scholar] [CrossRef]
- Kurokawa, M.; Tsurita, M.; Brown, J.; Fukuda, Y.; Shiraki, K. Effect of interleukin-12 level augmented by Kakkon-to, a herbal medicine, on the early stage of influenza infection in mice. Antiviral Res. 2002, 56, 183–188. [Google Scholar] [CrossRef]
- Song, W.; Si, L.; Ji, S.; Wang, H.; Fang, X.M.; Yu, L.Y.; Li, R.Y.; Liang, L.N.; Zhou, D.; Ye, M. Uralsaponins M-Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef]
- Dai, J.; Wang, G.; Li, W.; Zhang, L.; Yang, J.; Zhao, X.; Chen, X.; Xu, Y.; Li, K. High-throughput screening for anti-influenza A virus drugs and study of the mechanism of procyanidin on influenza A virus-induced autophagy. J. Biomol. Screen. 2012, 17, 605–617. [Google Scholar] [CrossRef]
- Sahoo, M.; Jena, L.; Rath, S.N.; Kumar, S. Identification of Suitable Natural Inhibitor against Influenza A (H1N1) Neuraminidase Protein by Molecular Docking. Genom. Inform. 2016, 14, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Ngan, L.T.; Jang, M.J.; Kwon, M.J.; Ahn, Y.J. Antiviral activity and possible mechanism of action of constituents identified in Paeonia lactiflora root toward human rhinoviruses. PLoS ONE 2015, 10, e0121629. [Google Scholar] [CrossRef]
- Kurokawa, M.; Imakita, M.; Kumeda, C.A.; Yukawa, T.A.; Shiraki, K. Kakkon-to suppressed interleukin—La production responsive. J. Tradit. Med. 1996, 13, 201–209. [Google Scholar]
- Shin, N.R.; Kim, C.; Seo, C.S.; Ko, J.W.; Cho, Y.K.; Shin, I.S.; Kim, J.S. Galgeun-tang Attenuates Cigarette Smoke and Lipopolysaccharide Induced Pulmonary Inflammation via IkappaBalpha/NF-kappaB Signaling. Molecules 2018, 23, 10. [Google Scholar] [CrossRef]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of paeonia lactiflora pall. a traditional chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.Q.; Yuan, B.C.; Liu, Y. The Pharmacological Activities of Licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Wan, F.; Jin, Z.; Wang, J.; Xu, X. Nitrite oxide and inducible nitric oxide synthase were regulated by polysaccharides isolated from Glycyrrhiza uralensis Fisch. J. Ethnopharmacol. 2008, 118, 59–64. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, Y.; Honda, H.; Okamoto, N.; Yamamoto, S.; Hamashima, T.; Ishii, Y.; Tanaka, M.; Suganami, T.; Sasahara, M.; et al. Isoliquiritigenin Attenuates Adipose Tissue Inflammation in vitro and Adipose Tissue Fibrosis through Inhibition of Innate Immune Responses in Mice. Sci. Rep. 2016, 6, 23097. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.Y.; Huo, H.R.; Zhao, B.S.; Liu, H.B.; Li, L.F.; Ma, Y.Y.; Guo, S.Y.; Jiang, T.L. Cinnamaldehyde reduces IL-1beta-induced cyclooxygenase-2 activity in rat cerebral microvascular endothelial cells. Eur. J. Pharmacol. 2006, 537, 174–180. [Google Scholar] [CrossRef]
- Chao, L.K.; Hua, K.F.; Hsu, H.Y.; Cheng, S.S.; Lin, I.F.; Chen, C.J.; Chen, S.T.; Chang, S.T. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem. Toxicol. 2008, 46, 220–231. [Google Scholar] [CrossRef]
- Reddy, A.M.; Seo, J.H.; Ryu, S.Y.; Kim, Y.S.; Kim, Y.S.; Min, K.R.; Kim, Y. Cinnamaldehyde and 2-methoxycinnamaldehyde as NF-kappaB inhibitors from Cinnamomum cassia. Planta Med. 2004, 70, 823–827. [Google Scholar] [CrossRef]
- Gunawardena, D.; Karunaweera, N.; Lee, S.; van Der Kooy, F.; Harman, D.G.; Raju, R.; Bennett, L.; Gyengesi, E.; Sucher, N.J.; Munch, G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts—Identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015, 6, 910–919. [Google Scholar] [CrossRef]
- Bruder, D.; Srikiatkhachorn, A.; Enelow, R.I. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol. 2006, 19, 147–155. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Ma, C.; Wang, S. Puerarin attenuates airway inflammation by regulation of eotaxin-3. Immunol. Lett. 2015, 163, 173–178. [Google Scholar] [CrossRef]
- Yao, J.; Du, Z.; Li, Z.; Zhang, S.; Lin, Y.; Li, H.; Zhou, L.; Wang, Y.; Yan, G.; Wu, X.; et al. 6-Gingerol as an arginase inhibitor prevents urethane-induced lung carcinogenesis by reprogramming tumor supporting M2 macrophages to M1 phenotype. Food Funct. 2018, 9, 4611–4620. [Google Scholar] [CrossRef]
- Bernard, M.; Furlong, S.J.; Power Coombs, M.R.; Hoskin, D.W. Differential Inhibition of T Lymphocyte Proliferation and Cytokine Synthesis by [6]-Gingerol, [8]-Gingerol, and [10]-Gingerol. Phytother. Res. 2015, 29, 1707–1713. [Google Scholar] [CrossRef]
- Kubo, T.; Nishimura, H. Antipyretic effect of Mao-to, a Japanese herbal medicine, for treatment of type A influenza infection in children. Phytomedicine 2007, 14, 96–101. [Google Scholar] [CrossRef]
- Wang, H.M.; Lin, S.K.; Yeh, C.H.; Lai, J.N. Prescription pattern of Chinese herbal products for adult-onset asthma in Taiwan: A population-based study. Ann. Allergy Asthma Immunol. 2014, 112, 465–470. [Google Scholar] [CrossRef]
- Saita, M.; Naito, T.; Boku, S.; Watanabe, Y.; Suzuki, M.; Oka, F.; Takahashi, M.; Sakurai, T.; Sugihara, E.; Tomomi, H.; et al. The efficacy of ma-huang-tang (maoto) against influenza. Health 2011, 3, 300–303. [Google Scholar] [CrossRef] [Green Version]
- Miyagoshi, M.; Amagaya, S.; Ogihara, Y. Antitussive effects of L-ephedrine, amygdalin, and makyokansekito (Chinese traditional medicine) using a cough model induced by sulfur dioxide gas in mice. Planta Med. 1986, 4, 275–278. [Google Scholar] [CrossRef]
- Xiaomei, T.; Yang, G.; Linzhong, Y.; Huifang, Z.; Ying, X.; Jiabo, L. Effect of Ephedra with Semen Armeniacae Amarum by compatibility of different ratio in acute toxicity test and antiasthmatic. Pharmacol. Clinric. Chin. Mater. Med. 2013, 29, 82–84. [Google Scholar]
- Seo, C.; Yoo, S.R.; Jeong, S.J.; Ha, H. Simultaneous determination and anti-inflammatory effects of traditional herbal medicine, Mahwang-tang. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 166–180. [Google Scholar] [CrossRef]
- Xiao, M.M.; Pan, C.S.; Liu, Y.Y.; Ma, L.Q.; Yan, L.; Fan, J.Y.; Wang, C.S.; Huang, R.; Han, J.Y. Post-treatment with Ma-Huang-Tang ameliorates cold-warm-cycles induced rat lung injury. Sci. Rep. 2017, 7, 312. [Google Scholar] [CrossRef]
- Jiao, J.; Wu, J.; Wang, J.; Guo, Y.; Gao, L.; Liang, H.; Huang, J.; Wang, J. Ma Huang Tang ameliorates bronchial asthma symptoms through the TLR9 pathway. Pharm. Biol. 2018, 56, 580–593. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.H.; Ma, Z.Q.; Fu, Q.; Ma, S.P. Ma Huang Tang ameliorates asthma though modulation of Th1/Th2 cytokines and inhibition of Th17 cells in ovalbumin-sensitized mice. Chin. J. Nat. Med. 2014, 12, 361–366. [Google Scholar] [CrossRef]
- Wei, W.; Wan, H.; Peng, X.; Zhou, H.; Lu, Y.; He, Y. Antiviral effects of Ma Huang Tang against H1N1 influenza virus infection in vitro and in an ICR pneumonia mouse model. Biomed. Pharmacother. 2018, 102, 1161–1175. [Google Scholar] [CrossRef]
- Zhang, B.M.; Wang, Z.B.; Xin, P.; Wang, Q.H.; Bu, H.; Kuang, H.X. Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 2018, 16, 811–828. [Google Scholar] [CrossRef]
- Ling, M.; Piddlesden, S.J.; Morgan, B.P. A component of the medicinal herb ephedra blocks activation in the classical and alternative pathways of complement. Clin. Exp. Immunol. 1995, 102, 582–588. [Google Scholar] [CrossRef]
- Abourashed, E.A.; El-Alfy, A.T.; Khan, I.A.; Walker, L. Ephedra in perspective—A current review. Phytother. Res. 2003, 17, 703–712. [Google Scholar] [CrossRef]
- Kim, I.S.; Park, Y.J.; Yoon, S.J.; Lee, H.B. Ephedrannin A and B from roots of Ephedra sinica inhibit lipopolysaccharide-induced inflammatory mediators by suppressing nuclear factor-kappaB activation in RAW 264.7 macrophages. Int. Immunopharmacol. 2010, 10, 1616–1625. [Google Scholar] [CrossRef]
- Hyuga, S. The Pharmacological Actions of Ephedrine Alkaloids-free Ephreda Herb Extract and Preparation for Clinical Application. Yakugaku Zasshi 2017, 137, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Jia, W.; Zhao, A.; Wang, X. Anti-influenza agents from plants and traditional Chinese medicine. Phytother. Res. 2006, 20, 335–341. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Li, M.; Tang, T.S.; Wang, B.; Zhang, B. An study on Gypsum Compounds and Their Antipyretic Function and Anti-inflammatory Mechanisms. J. Shaanxi College Tradit. Chin. Med. 2012, 35, 033. [Google Scholar]
- Mei, F.; Xing, X.F.; Tang, Q.F.; Chen, F.L.; Guo, Y.; Song, S.; Tan, X.M.; Luo, J.B. Antipyretic and anti-asthmatic activities of traditional Chinese herb-pairs, Ephedra and Gypsum. Chin. J. Integr. Med. 2016, 22, 445–450. [Google Scholar] [CrossRef]
- Wang, X. Maxingshigan decoction for treating AECOPD in 39 cases and nursing Measures. China Pharm. 2015, 24, 93–95. [Google Scholar]
- Lin, S.K.; Tsai, Y.T.; Lo, P.C.; Lai, J.N. Traditional Chinese medicine therapy decreases the pneumonia risk in patients with dementia. Medicine 2016, 95, e4917. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chang, C.W.; Wu, C.R. Antitussive, anti-pyretic and toxicological evaluation of Ma-Xing-Gan-Shi-Tang in rodents. BMC Complement. Altern. Med. 2016, 16, 456. [Google Scholar] [CrossRef]
- Kao, S.T.; Yeh, T.J.; Hsieh, C.C.; Shiau, H.B.; Yeh, F.T.; Lin, J.G. The effects of Ma-Xing-Gan-Shi-Tang on respiratory resistance and airway leukocyte infiltration in asthmatic guinea pigs. Immunopharmacol. Immunotoxicol. 2001, 23, 445–458. [Google Scholar] [CrossRef]
- Zhang, W.; Xinyue, Z.; Shao, Y. Changes in the level of cytokine in rats with chronic obstructive pulmonary disease of phlegm heat cumber lung type after treatment of Maxing Shigan decoction. Chin. J. Tissue Eng. Res. 2006, 10, 167–170. [Google Scholar]
- Kung, Y.Y.; Chen, Y.C.; Hwang, S.J.; Chen, T.J.; Chen, F.P. The prescriptions frequencies and patterns of Chinese herbal medicine for allergic rhinitis in Taiwan. Allergy 2006, 61, 1316–1318. [Google Scholar] [CrossRef]
- Liao, Y.N.; Hu, W.L.; Chen, H.J.; Hung, Y.C. The Use of Chinese Herbal Medicine in the Treatment of Chronic Obstructive Pulmonary Disease (COPD). Am. J. Chin. Med. 2017, 45, 225–238. [Google Scholar] [CrossRef]
- Chang, J.S.; Yeh, C.F.; Wang, K.C.; Shieh, D.E.; Yen, M.H.; Chiang, L.C. Xiao-Qing-Long-Tang (Sho-seiryu-to) inhibited cytopathic effect of human respiratory syncytial virus in cell lines of human respiratory tract. J. Ethnopharmacol. 2013, 147, 481–487. [Google Scholar] [CrossRef]
- Nagai, T.; Yamada, H. In vivo anti-influenza virus activity of kampo (Japanese herbal) medicine “sho-seiryu-to” to indicate a break in thought or interpretation and its mode of action. Int. J. Immunopharmacol. 1994, 16, 605–613. [Google Scholar] [CrossRef]
- Nagai, T.; Urata, M.; Yamada, H. In vivo anti-influenza virus activity of Kampo (Japanese herbal) medicine “Sho-seiryu-to”—Effects on aged mice, against subtypes of a viruses and B virus, and therapeutic effect. Immunopharmacol. Immunotoxicol. 1996, 18, 193–208. [Google Scholar] [CrossRef]
- Nagai, T.; Nakao, M.; Shimizu, Y.; Kodera, Y.; Oh-Ishi, M.; Maeda, T.; Yamada, H. Proteomic Analysis of Anti-inflammatory Effects of a Kampo (Japanese Herbal) Medicine “Shoseiryuto (Xiao-Qing-Long-Tang)” on Airway Inflammation in a Mouse Model. Evid. Based Complement. Alternat. Med. 2011, 2011, 604196. [Google Scholar] [CrossRef]
- Wang, S.D.; Lin, L.J.; Chen, C.L.; Lee, S.C.; Lin, C.C.; Wang, J.Y.; Kao, S.T. Xiao-Qing-Long-Tang attenuates allergic airway inflammation and remodeling in repetitive Dermatogoides pteronyssinus challenged chronic asthmatic mice model. J. Ethnopharmacol. 2012, 142, 531–538. [Google Scholar] [CrossRef]
- Cao, D.R. Effects of Xiaoqiongtang decoction on airway inflammation and airway remodeling in patients with COPD. Med. J. West. China 2009, 21, 3. [Google Scholar]
- Nagai, T.; Arai, Y.; Emori, M.; Nunome, S.Y.; Yabe, T.; Takeda, T.; Yamada, H. Anti-allergic activity of a Kampo (Japanese herbal) medicine “Sho-seiryu-to (Xiao-Qing-Long-Tang)” on airway inflammation in a mouse model. Int. Immunopharmacol. 2004, 4, 1353–1365. [Google Scholar] [CrossRef]
- Tu, C.; Huang, X.; Xiao, Y.; Song, M.; Ma, Y.; Yan, J.; You, H.; Wu, H. Schisandrin A Inhibits the IL-1beta-Induced Inflammation and Cartilage Degradation via Suppression of MAPK and NF-kappaB Signal Pathways in Rat Chondrocytes. Front. Pharmacol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Choi, E.O.; Leem, S.H.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Yun, S.J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-kappaB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar]
- Lin, Q.; Qin, X.; Shi, M.; Qin, Z.; Meng, Y.; Qin, Z.; Guo, S. Schisandrin B inhibits LPS-induced inflammatory response in human umbilical vein endothelial cells by activating Nrf2. Int. Immunopharmacol. 2017, 49, 142–147. [Google Scholar] [CrossRef]
- Kook, M.; Lee, S.K.; Kim, S.D.; Lee, H.Y.; Hwang, J.S.; Choi, Y.W.; Bae, Y.S. Anti-septic activity of alpha-cubebenoate isolated from Schisandra chinensis. BMB Rep. 2015, 48, 336–341. [Google Scholar] [CrossRef]
- Kang, S.; Lee, K.P.; Park, S.J.; Noh, D.Y.; Kim, J.M.; Moon, H.R.; Lee, Y.G.; Choi, Y.W.; Im, D.S. Identification of a novel anti-inflammatory compound, alpha-cubebenoate from Schisandra chinensis. J. Ethnopharmacol. 2014, 153, 242–249. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, X.D.; Nie, Y.C.; Gan, Z.Y.; Yang, L.Q.; Huang, C.Q.; Lai, K.F.; Zhong, N.S. Antitussive activity of the Schisandra chinensis fruit polysaccharide (SCFP-1) in guinea pigs models. J. Ethnopharmacol. 2016, 194, 378–385. [Google Scholar] [CrossRef]
- Yu, H.L.; Zhao, T.F.; Wu, H.; Pan, Y.Z.; Zhang, Q.; Wang, K.L.; Zhang, C.C.; Jin, Y.P. Pinellia ternata lectin exerts a pro-inflammatory effect on macrophages by inducing the release of pro-inflammatory cytokines, the activation of the nuclear factor-kappaB signaling pathway and the overproduction of reactive oxygen species. Int. J. Mol. Med. 2015, 36, 1127–1135. [Google Scholar] [CrossRef]
- Cai, S.; Zhongde, Z. Meta-Analysis on Shegan Mahuang Tang for Refractory Asthma. J. New Chin. Med. 2017, 12, 52. [Google Scholar]
- Yunfeng, J. Clinical observation on therapeutic effect of traditional Chinese medicine granules made by formula of Shegan Mahuang decoction for patients with asthma. Life Sci. J. 2015, 12, 4. [Google Scholar]
- Yen, M.H.; Lee, J.J.; Yeh, C.F.; Wang, K.C.; Chiang, Y.W.; Chiang, L.C.; Chang, J.S. Yakammaoto inhibited human coxsackievirus B4 (CVB4)-induced airway and renal tubular injuries by preventing viral attachment, internalization, and replication. J. Ethnopharmacol. 2014, 151, 1056–1063. [Google Scholar] [CrossRef]
- Yeh, C.F.; Wang, K.C.; Lu, C.Y.; Chiang, L.C.; Shieh, D.E.; Yen, M.H.; Chang, J.S. Yakammaoto inhibits enterovirus 71 infection by reducing viral attachment, internalization, replication, and translation. Kaohsiung J. Med. Sci. 2015, 31, 293–302. [Google Scholar] [CrossRef]
- Chen, Z.X.; Hu, G.H. Effect of modified shegan mahuang decoction on cytokines in children patients with cough and variant asthma. Chin. J. Integrat. Tradit. West. Med. 2010, 30, 208–210. [Google Scholar]
- Zhu, B.; Dong, J.; Gao, X.; He, Y.; Sun, H. Antiasthmatic Effects of Sanglong Pingchuan Decoction through Inducing a Balanced Th1/Th2 Immune Response. Evid. Based Complement. Alternat. Med. 2018, 2018, 2629565. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, J.; Liu, J.; Xu, W.; Wei, Z.; Li, Y.; Wu, H.; Xiao, H. Network Pharmacology-Based Investigation of Protective Mechanism of Aster tataricus on Lipopolysaccharide-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 543. [Google Scholar] [CrossRef]
- Ahn, K.S.; Noh, E.J.; Cha, K.H.; Kim, Y.S.; Lim, S.S.; Shin, K.H.; Jung, S.H. Inhibitory effects of Irigenin from the rhizomes of Belamcanda chinensis on nitric oxide and prostaglandin E(2) production in murine macrophage RAW 264.7 cells. Life Sci. 2006, 78, 2336–2342. [Google Scholar] [CrossRef]
- Karsch-Volk, M.; Barrett, B.; Kiefer, D.; Bauer, R.; Ardjomand-Woelkart, K.; Linde, K. Echinacea for preventing and treating the common cold. Cochrane Database Syst. Rev. 2014, 2, CD000530. [Google Scholar] [CrossRef]
- Snitz, B.E.; O’Meara, E.S.; Carlson, M.C.; Arnold, A.M.; Ives, D.G.; Rapp, S.R.; Saxton, J.; Lopez, O.L.; Dunn, L.O.; Sink, K.M.; et al. Ginkgo biloba for preventing cognitive decline in older adults: A randomized trial. JAMA 2009, 302, 2663–2670. [Google Scholar] [CrossRef]
- Nabeshima, S.; Kashiwagi, K.; Ajisaka, K.; Masui, S.; Takeoka, H.; Ikematsu, H.; Kashiwagi, S. A randomized, controlled trial comparing traditional herbal medicine and neuraminidase inhibitors in the treatment of seasonal influenza. J. Infect. Chemother. 2012, 18, 534–543. [Google Scholar] [CrossRef]
- Yu, J.S.; Ho, C.H.; Hsu, Y.C.; Wang, J.J.; Hsieh, C.L. Traditional Chinese medicine treatments for upper respiratory tract infections/common colds in Taiwan. Eur. J. Integr. Med. 2014, 6, 538–544. [Google Scholar] [CrossRef]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–966. [Google Scholar] [CrossRef]
- Angerhofer, C.K. Herbal Medicines: A Guide for Health-Care Professionals. J. Nat. Prod. 2002, 65, 1964. [Google Scholar]
- Bashir, R.M.; Lewis, J.H. Hepatotoxicity of drug used in the treatment of gastrointestinal disorders. Gastroenterol Clin. North Am. 1995, 24, 957. [Google Scholar]
- Haller, C.A.; Benowitz, N.L. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N. Engl. J. Med. 2000, 343, 1833–1838. [Google Scholar] [CrossRef]
- Peters, C.M.; O’Neill, J.O.; Young, J.B.; Bott-Silverman, C. Is there an association between ephedra and heart failure? A case series. J. Card. Fail. 2005, 11, 9–11. [Google Scholar] [CrossRef]
- Shekelle, P.G.; Hardy, M.L.; Morton, S.C.; Maglione, M.; Mojica, W.A.; Suttorp, M.J.; Rhodes, S.L.; Jungvig, L.; Gagne, J. Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: A meta-analysis. JAMA 2003, 289, 1537–1545. [Google Scholar]
- Larrey, D. Hepatotoxicity of herbal remedies. J. Hepatol. 1997, 26, 47–51. [Google Scholar] [CrossRef]
- Vanherweghem, J.L.; Depierreux, M.; Tielemans, C.; Abramowicz, D.; Dratwa, M.; Jadoul, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; Vanhaelen-Fastre, R.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Cosyns, J.P.; Jadoul, M.; Squifflet, J.P.; De Plaen, J.F.; Ferluga, D.; van Ypersele de Strihou, C. Chinese herbs nephropathy: A clue to Balkan endemic nephropathy? Kidney Int. 1994, 45, 1680–1688. [Google Scholar] [CrossRef] [Green Version]
- Arlt, V.M.; Stiborova, M.; Schmeiser, H.H. Aristolochic acid as a probable human cancer hazard in herbal remedies: A review. Mutagenesis 2002, 17, 265–277. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Liao, N.S.; Chen, W. Progress in cytochrome P450 enzyme in toxicity of traditional Chinese medicines. Chin. J. Pharmacol. Toxicol. 2012, 26, 402–405. [Google Scholar]
- The State Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China, 6th ed.; People’s Medical Publishing House: Beijing, China, 2005. [Google Scholar]
- WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants. Available online: http://apps.who.int/medicinedocs/en/d/Js4928e/ (accessed on 16 June 2019).
- FDA. Botanical Drug Development Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/botanical-drug-development-guidance-industry (DECEMBER 2016) (accessed on 28 June 2019).
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or Negative Actors? Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]




| Chinese Medicine Plant | Family | Weight (gm) | Used Part | Identified Molecules |
|---|---|---|---|---|
| Cinnamomum cassia (L.) J. Presl | Lauraceae | 3.0 | Twig | Coumarin, cinnamic alcohol, cinnamic acid, 2-methoxy cinnamic acid, cinnamaldehyde, E-cinnamaldehyde, 2-methoxy cinnamaldehyde, 6-methoxy cinnamaldehyde |
| Ephedra sinica Stapf. | Ephedraceae | 4.5 | Aerial part | Ephedrine, L-ephedrannin, ephedrannin A and B, pseudoephedrine alkaloid, flavonoids, and organic acids |
| Glycyrrhiza uralensis Fisch. | Leguminosae | 3.0 | Root and Stolon | Glycyrrhizin, glycyrrhinic acid, glycyrrhetic acic or glycyrrhetinic acid, liquiritin, liquiritigenin, glycyamarin, iso-liquiritin, grabric acid, licoricidin, glycyrol, 5-0-methyl glycerol, iso-glycyrol |
| Paeonia lactiflora Pall. | Ranunculaceae | 3.0 | Radix | Paeoniflorin, oxypaeoniflorin, albiflorin, benzoylpaeoniflorin, paeoniflorigenone, paeonolide, paeonol |
| Pueraria lobata Ohwi | Leguminosae | 6.0 | Radix | Puerarin, daidzin, genistin, daidzein, genistein |
| Zingiber officinale Roscoe | Zingiberaceae | 4.5 | Root-like stem | 6-Gingerol, 6-Shogaol, zingerone, allicin |
| Ziziphus jujuba Mill. | Rhamnaceae | 4.0 | Fruit | 3-O-(trans-p-coumaroyl)-alphitolic acid, 3-O-(cis-p-coumaroyl)-alphitolic acid, 3β-O-(trans-p-coumaroyl)-maslinic acid, pomonic acid, 2-oxo-pomolic acid, benthamic acid, terminic acid, oleanic acid, betulinic acid, quercetin 3-O-rutinoside, quercetin 3-O-robinobioside, apigenin, traumatic acid, (Z)-4-oxotetradec-5-enoic acid, 7(E)-9-keto-hexadec-7-enoic acid, 9(E)-11-oxo-octadecenoic acid (9CI), and magnoflorine |
| Chinese Medicine Plant | Family | Weight (gm) | Used Part | Identified Molecules |
|---|---|---|---|---|
| Ephedra sinica Stapf. | Ephedraceae | 9.0 | Stalk | Ephedrine, L-ephedrannin, ephedrannin A and B, pseudoephedrine alkaloids, flavonoids, and organic acids |
| Cinnamomum cassia (L.) J. Presl | Lauraceae | 6.0 | Twig | Coumarin, cinnamic alcohol, cinnamic acid, 2-methoxy cinnamic acid, cinnamaldehyde, E-cinnamaldehyde, 2-methoxy cinnamaldehyde, 6-methoxy cinnamaldehyde |
| Glycyrrhiza uralensis Fisch. | Fabaceae | 3.0 | Root & Rhizome | Glycyrrhizin, glycyrrhinic acid, glycyrrhetic acic or glycyrrhetinic acid, liquiritin, liquiritigenin, glycyamarin, iso-liquiritin, grabric acid, licoricidin, glycyrol, 5-0-methyl glycerol, iso-glycyrol |
| Prunus armeniaca L. var. ansu Maxium | Rosaceae | 5.0 | Ripe seed | Amygdalin |
| Chinese Medicine Plant | Family | Weight (gm) | Used Part | Identified Molecules |
|---|---|---|---|---|
| Ephedra sinica Stapf. | Ephedraceae | 8.0 | Stalk | Ephedrine, L-ephedrannin, ephedrannin A and B, pseudoephedrine alkaloids, flavonoids, and organic acids |
| Prunus armeniaca L. var. ansu Maxium | Rosaceae | 6.0 | Ripe seed | Amygdalin |
| Glycyrrhiza uralensis Fisch. | Leguminosae | 4.0 | Root and Rhizome | Glycyrrhizin, glycyrrhinic acid, glycyrrhetic acic or glycyrrhetinic acid, liquiritin, liquiritigenin, glycyamarin, iso-liquiritin, grabric acid, licoricidin, glycyrol, 5-0-methyl glycerol, iso-glycyrol |
| Gypsum Fibrosum | CaSO4·2H2O | 16.0 |
| Chinese Medicine Plant | Family | Weight (gm) | Used Part | Identified Molecules |
|---|---|---|---|---|
| Ephedra sinica Stapf | Ephedraceae | 4.0 | Stem | Ephedrine, L-ephedrannin, ephedrannin A and B, pseudoephedrine alkaloids, flavonoids, and organic acids |
| Cinnamomum cassia (L.) J. Presl | Lauraceae | 4.0 | Twig | Coumarin, cinnamic alcohol, cinnamic acid, 2-methoxy cinnamic acid, cinnamaldehyde, E-cinnamaldehyde, 2-methoxy cinnamaldehyde, 6-methoxy cinnamaldehyde |
| Paeonia lactiflora Pall. | Ranuculaceae | 4.0 | Root | Paeoniflorin, oxypaeoniflorin, albiflorin, benzoylpaeoniflorin, paeoniflorigenone, paeonolide, paeonol |
| Glycyrrhiza uralensis Fisch. | Leguminosae | 4.0 | Root | Glycyrrhizin, glycyrrhinic acid, glycyrrhetic acic or glycyrrhetinic acid, liquiritin, liquiritigenin, glycyamarin, iso-liquiritin, grabric acid, licoricidin, glycyrol, 5-0-methyl glycerol, iso-glycyrol |
| Zingiber officinale Roscoe | Zingiberaceae | 4.0 | Rhizome | 6-Gingerol, 6-Shogaol, Zingerone, Allicin |
| Pinellia ternata (Thunb.) Breitenb. | Araceae | 4.0 | Tuber | 3-Acetoamino-5-methylisooxazole, butyl-ethylene ether, 3-methyleicosane, hexadecylendioic acid, methyl-2-chloropropenoate, anethole, benzaldehyde, 1,5-pentadiol, 2-methylpyrazine, 9-heptadecanol, ethylpalmitate, pentaldehyde oxime, ephedrine, choline, β-ssitosterol, daucosterol, homogentisic acid, protocatechualdehyde, shogaol, baicaline, baicalein, gingerol, 1,2,3,4,6-penta-Ogalloylglucose, 12,13-epoxy-9-hydroxynonadeca-7,10-dienoic acid, aminobutyric acid, aspartic acid |
| Asarum heterotropides F.Schmidt. f.mandshuricum (Maxim.) Kitag. | Aristolochiaceae | 1.5 | Root | Methylleugenol, safraole, asatone, α- and β-pinene, asaricin, eucarvone, estragole |
| Schisandra chinensis (Turcz.) Baill | Magnoliaceae | 1.5 | Fruit | Deoxyschizandrin, γ-schizandrin, schizandrin, aomisin, pseudo-r-schizandrin, schisantherin A |
| Chinese Medicine Plant | Family | Weight (gm) | Used Part | Main Identified Molecules |
|---|---|---|---|---|
| Ephedra sinica Stapf. | Ephedraceae | 4.0 | Stem | Ephedrine, L-ephedrannin, ephedrannin A and B, pseudoephedrine alkaloids, flavonoids, and organic acids |
| Pinellia ternata (Thunb.) Breitenb. | Araceae | 4.0 | Tuber | 3-acetoamino-5-methylisooxazole, butyl-ethylene ether, 3-methyleicosane, hexadecylendioic acid, methyl-2-chloropropenoate, anethole, benzaldehyde, 1,5-pentadiol, 2-methylpyrazine, 9-heptadecanol, ethylpalmitate, pentaldehyde oxime, ephedrine, choline, β-ssitosterol, daucosterol, homogentisic acid, protocatechualdehyde, shogaol, baicaline, baicalein, gingerol, 1,2,3,4,6-penta-Ogalloylglucose, 12,13-epoxy-9-hydroxynonadeca-7,10-dienoic acid, aminobutyric acid, aspartic acid |
| Zingiber officinale Roscoe | Zingiberaceae | 4.0 | Rhizome | 6-Gingerol, 6-Shogaol, zingerone, allicin |
| Tussilago farfara L. | Compositae | 3.0 | Flower | Faradiol, armiliot, rutin, hyperin, tussilagone, tannin, essential oil, wax |
| Aster tataricus L.f. | Compositae | 3.0 | Root & Rhizome | Shionone, epifriedelanol |
| Ziziphus jujube Mill. | Rhamnaceae | 2.0 | Fruit | 3-O-(trans-p-coumaroyl)-alphitolic acid, 3-O-(cis-p-coumaroyl)-alphitolic acid, 3β-O-(trans-p-coumaroyl)-maslinic acid, pomonic acid, 2-oxo-pomolic acid, benthamic acid, terminic acid, oleanic acid,betulinic acid, quercetin 3-O-rutinoside,quercetin 3-O-robinobioside, apigenin,traumatic acid, (Z)-4-oxotetradec-5-enoic acid, 7(E)-9-keto-hexadec-7-enoic acid, 9(E)-11-oxo-octadecenoic acid (9CI), magnoflorine |
| Belamcanda chinensis (L.) DC. | Iridaceae | 1.5 | Rhizome | Irisflorentin, isorhapontigenin, tectorigenin |
| Asarum heterotropides F.Schmidt. f.mandshuricum (Maxim.) Kitag. | Aristolochiaceae | 1.5 | Root | Methylleugenol, safraole, asatone, α- and β-pinene, asaricin, eucarvone, estragole |
| Schisandra chinensis (Turcz.) Baill | Magnoliaceae | 1..0 | Fruit | Deoxyschizandrin, γ-schizandrin, schizandrin, aomisin, pseudo-γ-schizandrin, schisantherin A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eng, Y.S.; Lee, C.H.; Lee, W.C.; Huang, C.C.; Chang, J.S. Unraveling the Molecular Mechanism of Traditional Chinese Medicine: Formulas Against Acute Airway Viral Infections as Examples. Molecules 2019, 24, 3505. https://doi.org/10.3390/molecules24193505
Eng YS, Lee CH, Lee WC, Huang CC, Chang JS. Unraveling the Molecular Mechanism of Traditional Chinese Medicine: Formulas Against Acute Airway Viral Infections as Examples. Molecules. 2019; 24(19):3505. https://doi.org/10.3390/molecules24193505
Chicago/Turabian StyleEng, Yi Shin, Chien Hsing Lee, Wei Chang Lee, Ching Chun Huang, and Jung San Chang. 2019. "Unraveling the Molecular Mechanism of Traditional Chinese Medicine: Formulas Against Acute Airway Viral Infections as Examples" Molecules 24, no. 19: 3505. https://doi.org/10.3390/molecules24193505






