Identification and Semi-Synthesis of 3-O-Protocatechuoylceanothic Acid, a Novel and Natural GPR120 Agonist †
Abstract
:1. Introduction
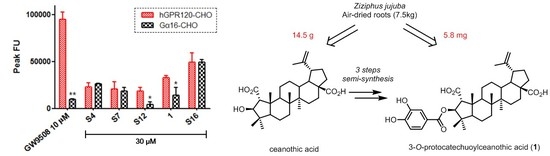
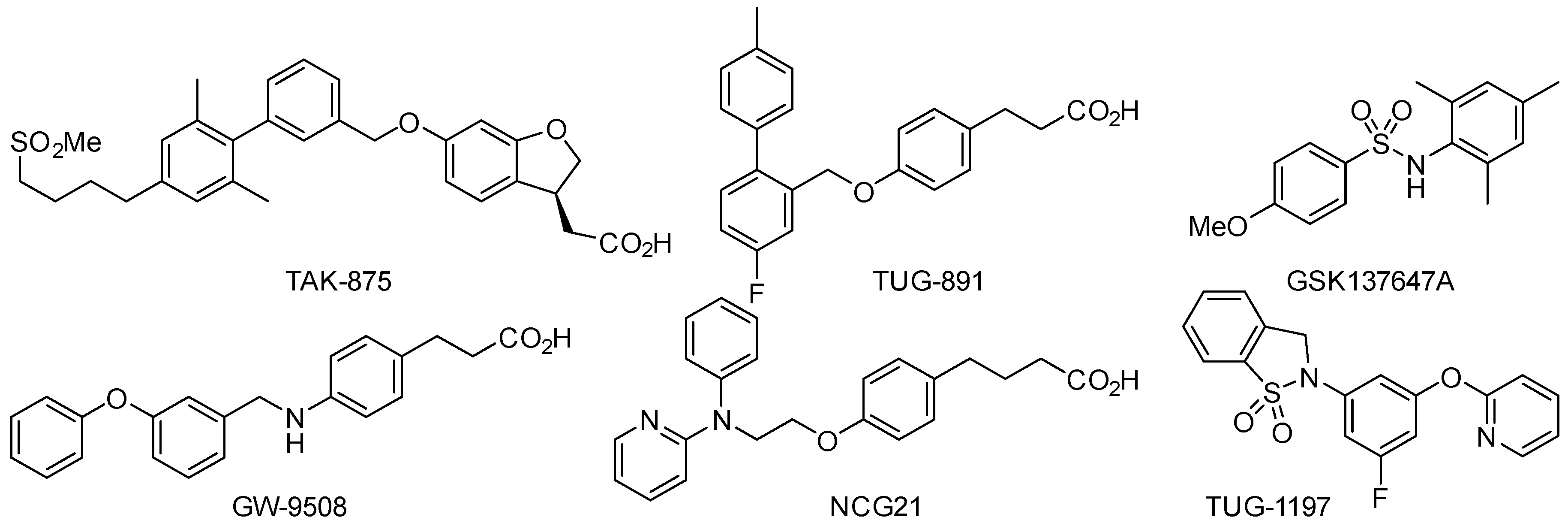
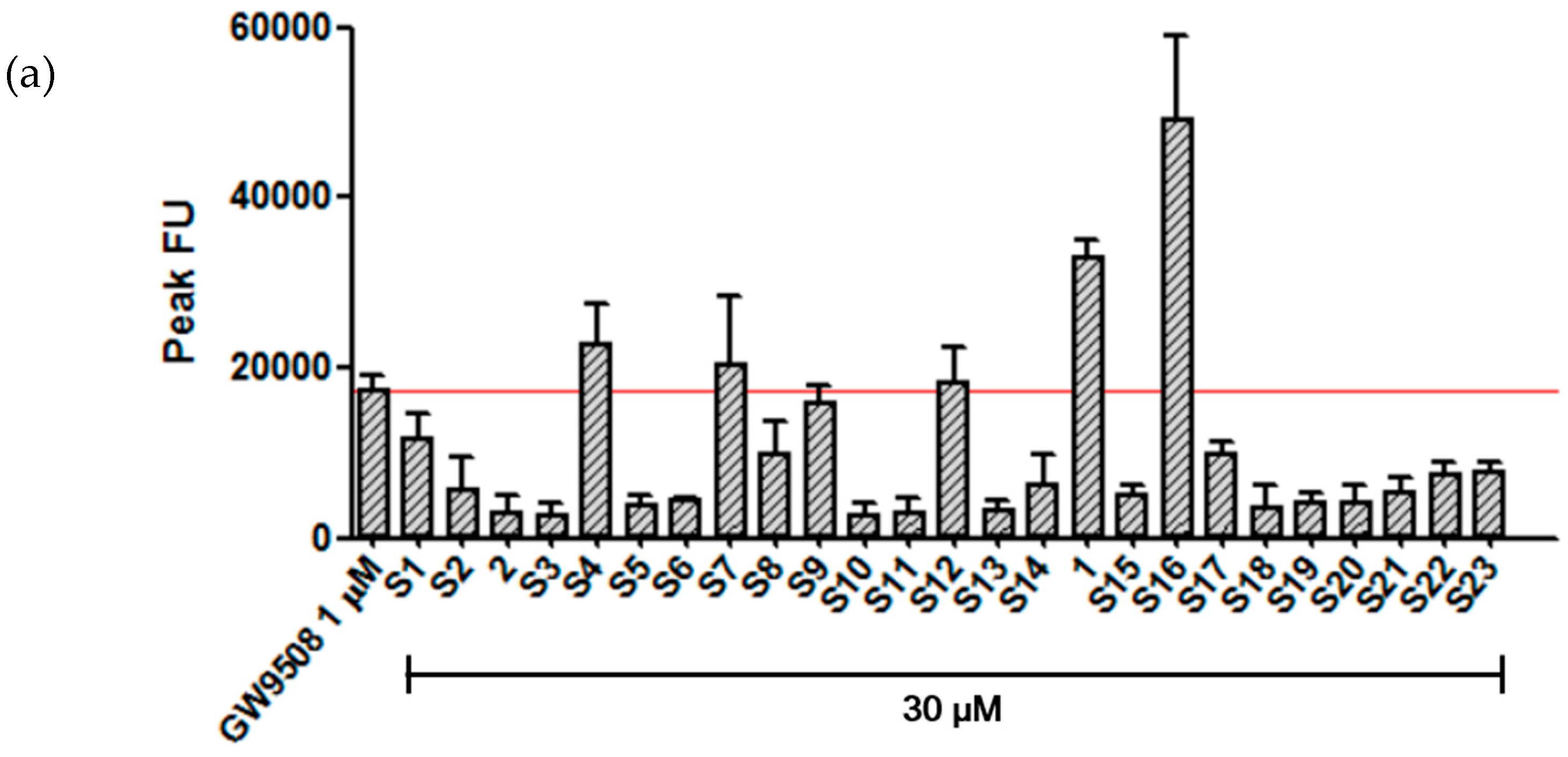
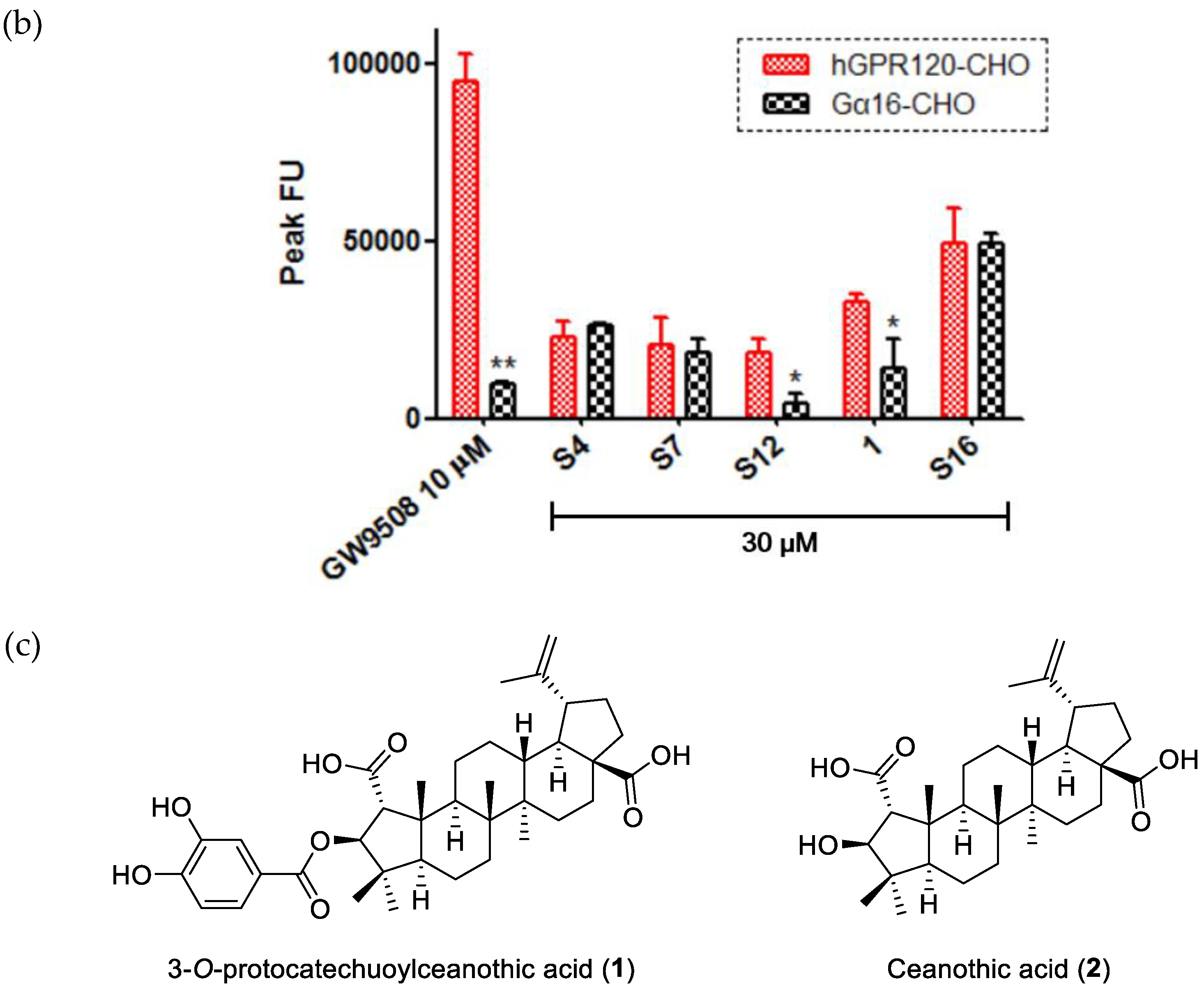
2. Results and Discussion
3. Materials and Methods
3.1. Biology
Analysis of GPR120 Ligand Activity
3.2. Chemistry
3.2.1. General Information
3.2.2. Experimental Part
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rayasam, G.V.; Tulasi, V.K.; Davis, J.A.; Bansal, V.S. Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin. Ther. Tar. 2007, 11, 661–671. [Google Scholar] [CrossRef]
- Cintra, D.E.; Ropelle, E.R.; Moraes, J.C.; Pauli, J.R.; Morari, J.; de Souza, C.T.; Grimaldi, R.; Stahl, M.; Carvalheira, J.B.; Saad, M.J.; et al. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS ONE 2012, 7, e30571. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A GPR120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Hirasawa, A.; Poulain-Godefroy, O.; Bonnefond, A.; Hara, T.; Yengo, L.; Kimura, I.; Leloire, A.; Liu, N.; Iida, K.; et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 2012, 483, 350–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, G.; Alvarez-Curto, E.; Hudson, B.D.; Prihandoko, R.; Tobin, A.B. FFA4/GPR120: Pharmacology and therapeutic opportunities. Trends Pharmacol. Sci. 2017, 38, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, C.P.; Peat, A.J.; McKeown, S.C.; Corbett, D.F.; Goetz, A.S.; Littleton, T.R.; McCoy, D.C.; Kenakin, T.P.; Andrews, J.L.; Ammala, C.; et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: Identification of agonist and antagonist small molecules. Br. J. Pharmacol. 2006, 148, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Igari, S.-I.; Hirasawa, A.; Hata, M.; Ishiguro, M.; Fujieda, H.; Itoh, Y.; Hirano, T.; Nakagawa, H.; Ogura, M.; et al. Identification of G protein-coupled receptor 120-selective agonists derived from PPARγ agonists. J. Med. Chem. 2008, 51, 7640–7644. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hirasawa, A.; Hara, T.; Kimura, I.; Adachi, T.; Awaji, T.; Ishiguro, M.; Suzuki, T.; Miyata, N.; Tsujimoto, G. Structure-activity relationships of GPR120 agonists based on a docking simulation. Mol. Pharmacol. 2010, 78, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Shimpukade, B.; Hudson, B.D.; Hovgaard, C.K.; Milligan, G.; Ulven, T. Discovery of a potent and selective GPR120 agonist. J. Med. Chem. 2012, 55, 4511–4515. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Passilly-Degrace, P.; Chevrot, M.; Ancel, D.; Sparks, S.M.; Drucker, D.J.; Besnard, P. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: Putative involvement of GPR120 and impact on taste sensitivity. J. Lipid Res. 2012, 53, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.D.; Shimpukade, B.; Mackenzie, A.E.; Butcher, A.J.; Pediani, J.D.; Christiansen, E.; Heathcote, H.; Tobin, A.B.; Ulven, T.; Milligan, G. The pharmacology of TUG-891, a potent and selective agonist of the free fatty acid receptor 4 (FFA4/GPR120), demonstrates both potential opportunity and possible challenges to therapeutic agonism. Mol. Pharmacol. 2013, 84, 710–725. [Google Scholar] [CrossRef] [PubMed]
- Sparks, S.M.; Chen, G.; Collins, J.L.; Danger, D.; Dock, S.T.; Jayawickreme, C.; Jenkinson, S.; Laudeman, C.; Leesnitzer, M.A.; Liang, X.; et al. Identification of diarylsulfonamides as agonists of the free fatty acid receptor 4 (FFA4/GPR120). Bioorg. Med. Chem. Lett. 2014, 24, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.M.; Watterson, K.R.; Wargent, E.T.; Hansen, S.V.; Hudson, B.D.; Kępczyńska, M.A.; Dunlop, J.; Shimpukade, B.; Christiansen, E.; Milligan, G.; et al. Non-acidic free fatty acid receptor 4 agonists with antidiabetic activity. J. Med. Chem. 2016, 59, 8868–8878. [Google Scholar] [CrossRef] [PubMed]
- Kaku, K.; Enya, K.; Nakaya, R.; Ohira, T.; Matsuno, R. Long-term safety and efficacy of fasiglifam (TAK-875), a G-protein-coupled receptor 40 agonist, as monotherapy and combination therapy in japanese patients with type 2 diabetes: A 52-week open-label phase III study. Diabetes Obes. Metab. 2016, 18, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.B.; Kim, J.W.; Oh, W.K.; Kim, J.; Sung, S.H. Cytotoxic ceanothane-and lupane-type triterpenoids from the roots of Ziziphus jujuba. J. Nat. Prod. 2016, 79, 2364–2375. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, J.; Phuong, N.T.; Park, J.G.; Park, J.-H.; Kim, Y.-C.; Baek, M.C.; Lim, S.C.; Kang, K.W. Involvement of the P2X7 receptor in the migration and metastasis of tamoxifen-resistant breast cancer: Effects on small extracellular vesicles production. Sci. Rep. 2019, 9, 11587. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.-C.; Fang, J.-M.; Jan, J.-T.; Cheng, T.-J.R.; Wang, S.-Y.; Yang, S.-T.; Cheng, Y.-S.E.; Wong, C.-H. Enhanced anti-influenza agents conjugated with anti-inflammatory activity. J. Med. Chem. 2012, 55, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4, 6, and 1 are available from the authors. |

| Coupling reagents | Solvents | Results |
| DCC, EDCI, HATU, DEPBT, PyBOP | CH2Cl2 | No reaction (>90% of 2) |
| DMF | 40–80% of 2 and complex mixture | |
| CH2Cl2:DMF = 2:1 | Complex mixture (none of 2) |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.; Park, J.G.; Kang, K.B.; Suh, Y.-G.
Identification and Semi-Synthesis of 3-O-Protocatechuoylceanothic Acid, a Novel and Natural GPR120 Agonist
Lim C, Park JG, Kang KB, Suh Y-G.
Identification and Semi-Synthesis of 3-O-Protocatechuoylceanothic Acid, a Novel and Natural GPR120 Agonist
Lim, Changjin, Jung Gyu Park, Kyo Bin Kang, and Young-Ger Suh.
2019. "Identification and Semi-Synthesis of 3-O-Protocatechuoylceanothic Acid, a Novel and Natural GPR120 Agonist