Non-Enzymatic Electrochemical Sensor Based on Sliver Nanoparticle-Decorated Carbon Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
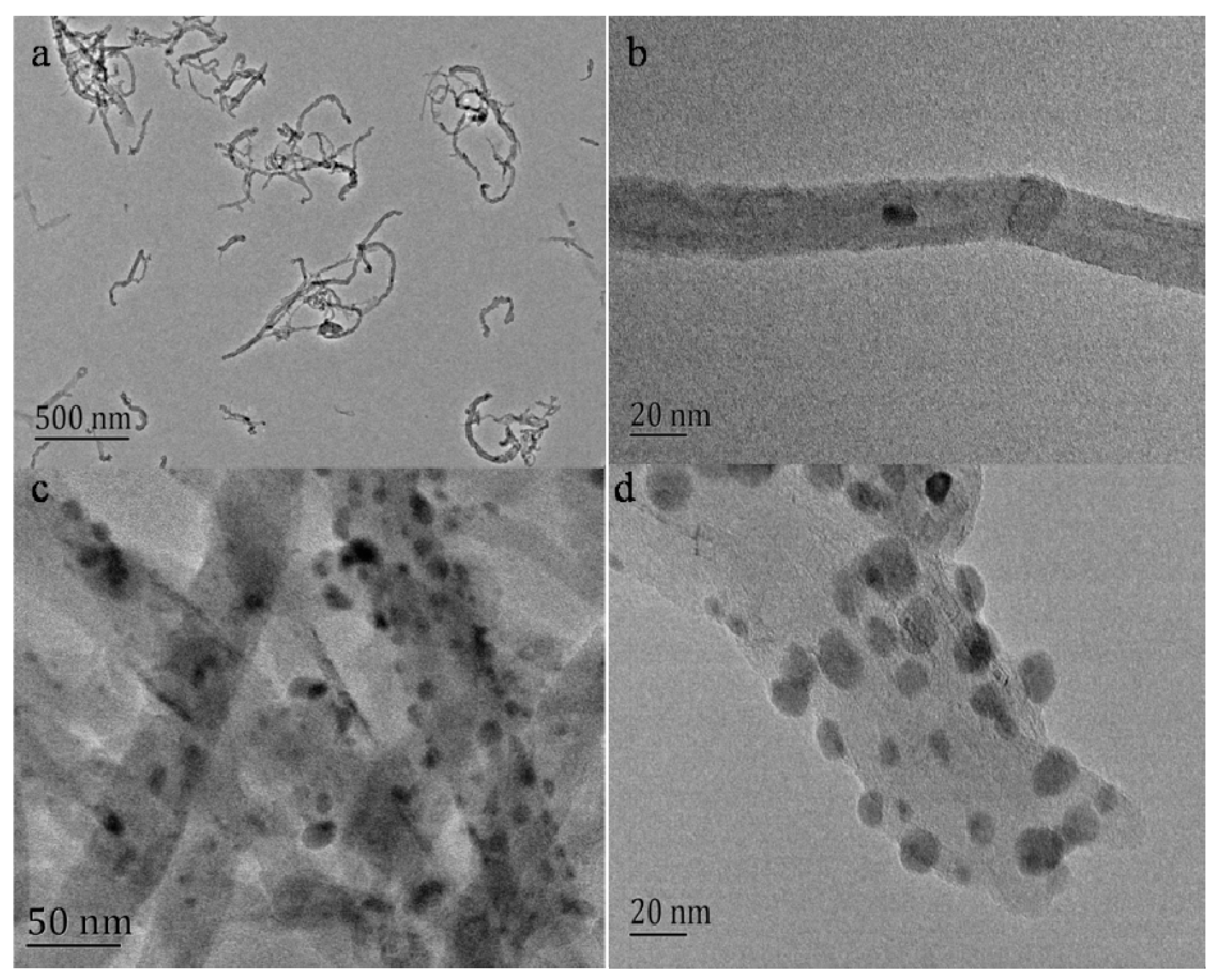
2.1. Characterization of AgNPs-MWCNT Nanocomposite
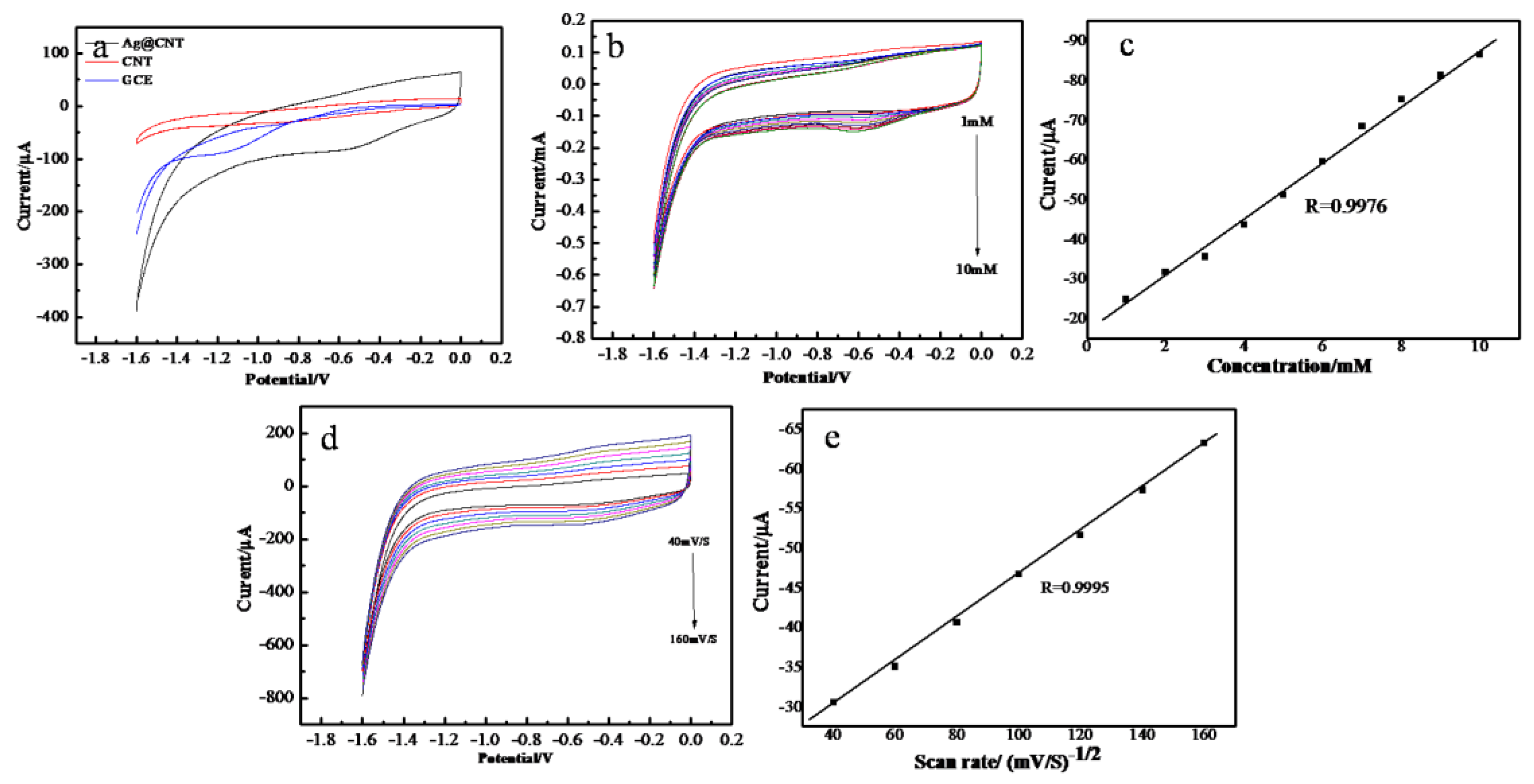
2.2. Electrocatalytic Activity of AgNPs-MWCNT Nanocomposite to H2O2
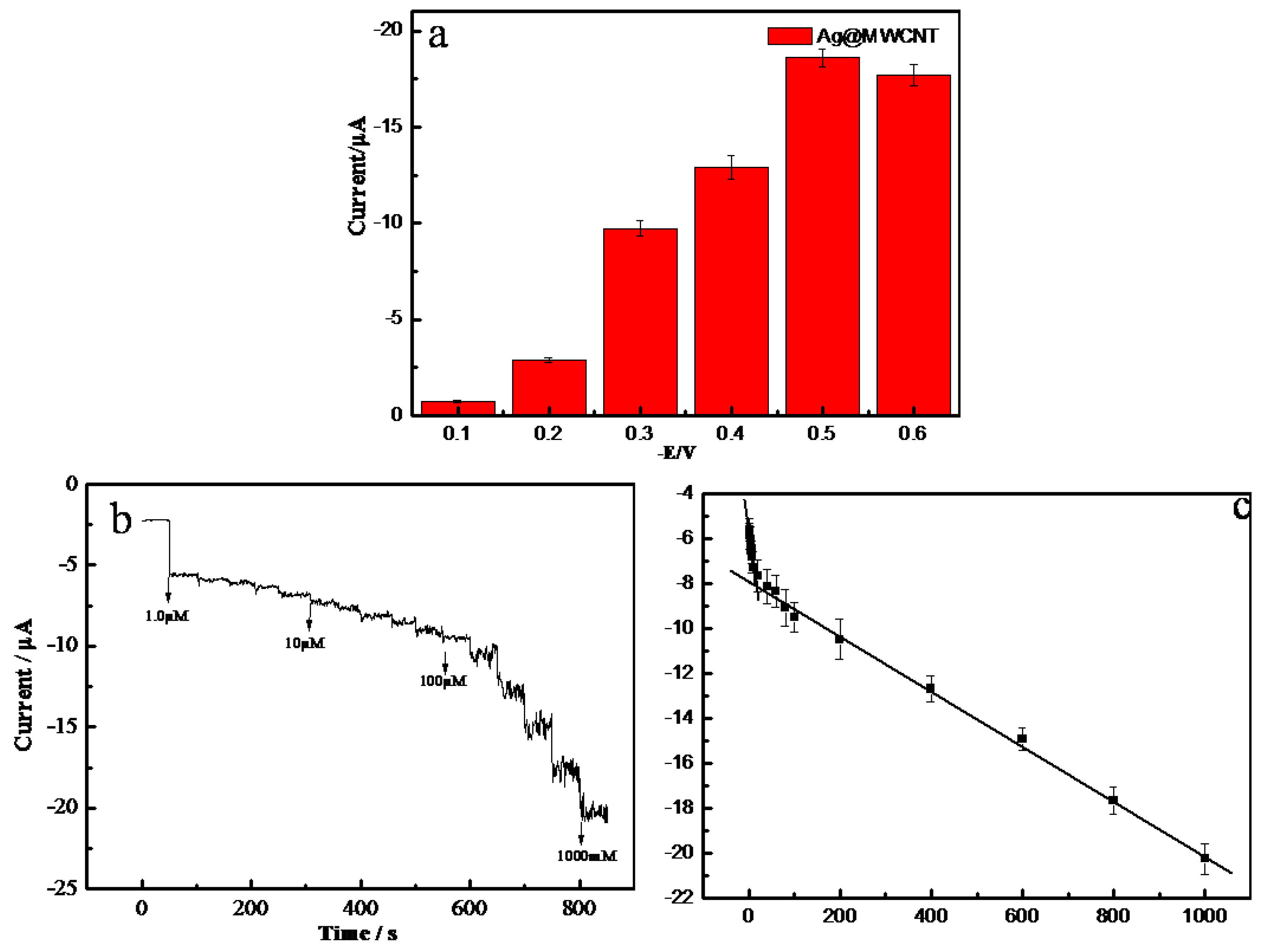
2.3. Amperommetric Response of H2O2 on AgNPs-MWCNT/GCE
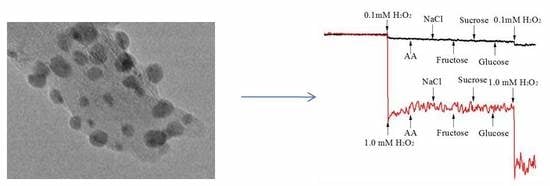
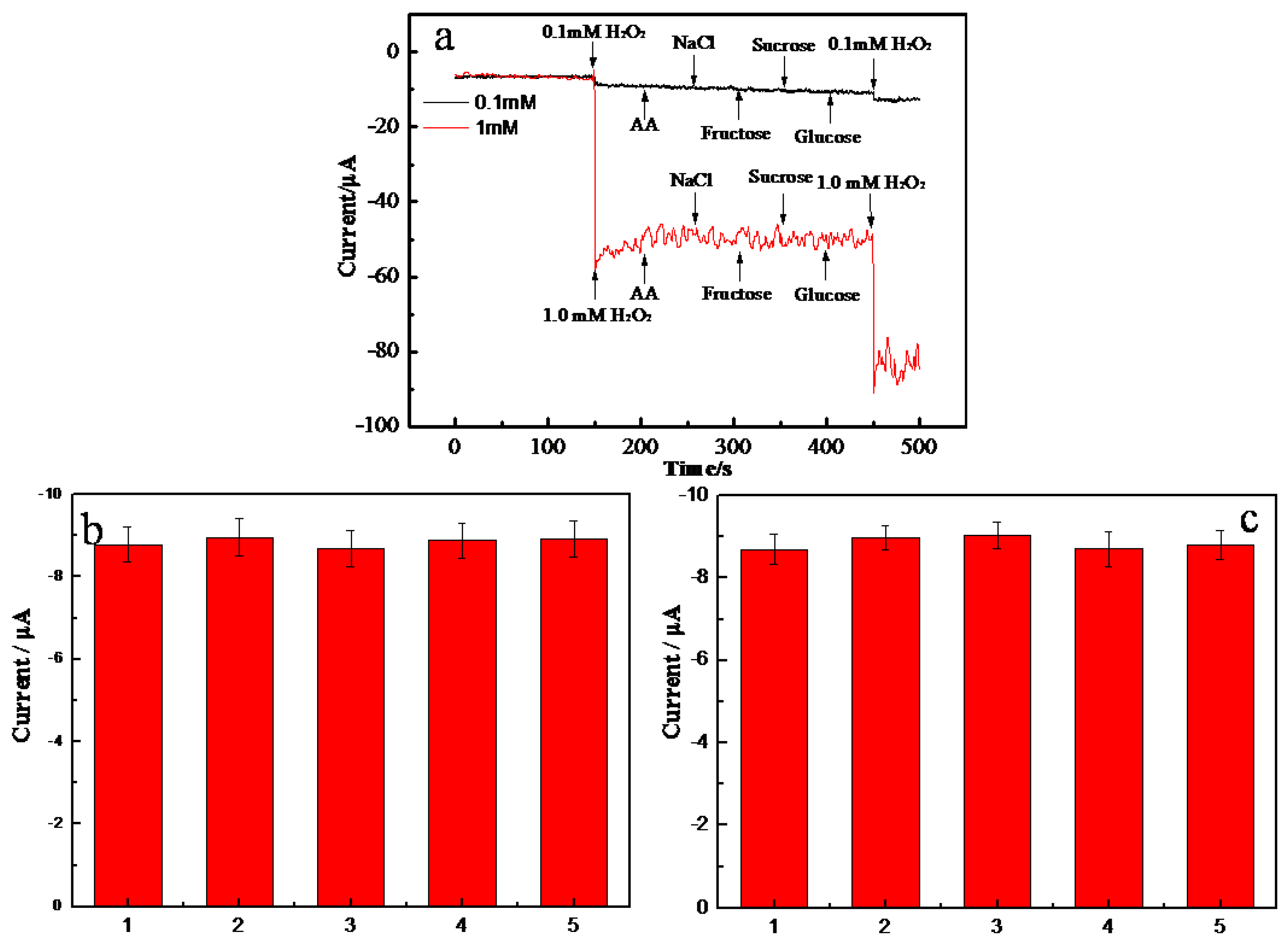
2.4. Selectivity, Reproducibility, and Stability of the AgNPs-MWCNT/GCE Sensor
2.5. Detection of H2O2 in Human Blood Serum
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of AgNPs-MWCNT Nanocomposite
3.3. Preparation of the AgNPs-MWCNT/GCE
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Varghese, S.H.; Nair, R.; Nair, B.G.; Hanajiri, T.; Maekawa, T.; Yoshida, Y.; Kumar, S. Sensors based on carbon nanotubes and their applications: A review. Curr. Nanosci. 2010, 6, 331–346. [Google Scholar] [CrossRef]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.J. Nanostructuring electrodes with carbon nanotubes: A review on electrochemistry and applications for sensing. Electrochim. Acta 2005, 50, 3049–3060. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, B.; Yan, J.; Song, S.; Min, R.; Fan, C. Nanotube-based colorimetric probe for ultrasensitive detection of ataxia telangiectasia mutated protein. Anal. Chem. 2011, 83, 9191–9196. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Xu, H.; Takalkar, S.; Gurung, A.S.; Liu, B.; Zheng, Y.; Guo, Z.; Baloda, M.; Baryeh, K.; Liu, G. Carbon nanotube-based lateral flow biosensor for sensitive and rapid detection of DNA sequence. Biosens. Bioelectron. 2015, 64, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Luksirikul, P.; Tedsree, K.; Moloney, M.G.; Green, M.L.H.; Tsang, S.C.E. Electron promotion by surface functional groups of single wall carbon nanotubes to overlying metal particles in a fuel-cell catalyst. Angew. Chem. 2012, 51, 6998–7001. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Shao, C.; Zhang, Z.; Zhang, M.; Mu, J.; Guo, Z.; Liu, Y.C. In situ assembly of well-dispersed Ag nanoparticles (AgNPs) on electrospun carbon nanofibers (CNFs) for catalytic reduction of 4-nitrophenol. Nanoscale 2011, 3, 3357–3363. [Google Scholar]
- Guo, D.J.; Li, H.L. High dispersion and electrocatalytic properties of palladium nanoparticles on single-walled carbon nanotubes. J. Colloid Interf. Sci. 2005, 286, 274–279. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef]
- Gilroy, K.D.; Yang, X.; Xie, S.; Zhao, M.; Qin, D.; Xia, Y. Shape-controlled synthesis of colloidal metal nanocrystals by replicating the surface atomic structure on the seed. Adv. Mater. 2018, 30, 1706312–1706337. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.; Mandal, T.K. Synthesis and catalytic application of nanostructured silver dendrites. J. Phys. Chem. C 2007, 111, 16750–16760. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.J.; Bai, J.; Jiang, X.; Fan, G. High sensitivity hydrogen peroxide and hydrazine sensor based on silver nanocubes with rich {100} facets as an enhanced electrochemical sensing platform. Biosens. Bioelectron. 2013, 43, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.J.; Gan, S.Y.; Fu, X.G.; Li, F.; Han, D.; Guo, L.; Niu, L. Electrochemically controlled growth of silver nanocrystals on graphene thin film and applications for efficient nonenzymatic H2O2 biosensor. Electrochim. Acta 2013, 89, 222–228. [Google Scholar] [CrossRef]
- Qu, F.L.; Lu, H.M.; Yang, M.H.; Deng, C. Electrochemical immunosensor based on electron transfer mediated by graphene oxide initiated silver enhancement. Biosens. Bioelectron. 2011, 26, 4810–4814. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Schulz, C.; Favero, G.; Mazzei, F.; Ludwig, R.; Gorton, L.; Antiochia, R. Green synthesis and characterization of gold and silver nanoparticles and their application for development of a third generation lactose biosensor. Electroanalysis 2016, 29, 77–86. [Google Scholar] [CrossRef]
- Lu, W.B.; Luo, Y.L.; Chang, G.H.; Sun, X. Synthesis of functional SiO2-coated graphene oxide nanosheets decorated with Ag nanoparticles for H2O2 and glucose detection. Biosens. Bioelectron. 2011, 26, 4791–4797. [Google Scholar] [CrossRef] [PubMed]
- Goulart, L.A.; Gonçalves, R.; Correa, A.A.; Pereira, E.C.; Mascaro, L.H. Synergic effect of silver nanoparticles and carbon nanotubes on the simultaneous voltammetric determination of hydroquinone, catechol, bisphenol A and phenol. Microchim. Acta 2018, 185, 12–20. [Google Scholar] [CrossRef]
- Castle, A.B.; Gracia-Espino, E.; Nieto-Delgado, C.; Terrones, H.; Terrones, M.; Hussain, S. Hydroxyl-functionalized and N-doped multiwalled carbon nanotubes decorated with silver nanoparticles preserve cellular function. ACS Nano 2011, 5, 2458–2466. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Iordache, M.S.; Iordache, A.M.; Bunghez, I.; Ghiurea, M.; Badea, N.; Fierascu, R.; Stamatin, I. Eco-designed biohybrids based on liposomes, mint–nanosilver and carbon nanotubes for antioxidant and antimicrobial coating. Mater. Sci. Eng. C 2014, 39, 177–185. [Google Scholar] [CrossRef]
- Azadbakht, A.; Abbasi, A.R.; Derikvand, Z.; Karimi, Z.; Roushani, M. Surface-Renewable AgNPs/CNT/rGO Nanocomposites as Bifunctional Impedimetric Sensors. Nano-MicroLett. 2017, 9, 1–11. [Google Scholar] [CrossRef]
- Tammeveski, L.; Erikson, H.; Sarapuu, A.; Kozlova, J.; Ritslaid, P.; Sammelselg, V.; Tammeveski, K. Electrocatalytic oxygen reduction on silver nanoparticle/multi-walled carbon nanotube modified glassy carbon electrodes in alkaline solution. Electrochem. Commun. 2012, 20, 15–18. [Google Scholar] [CrossRef]
- Larrude, D.G.; Maia da Costa, M.E.H.; Freire, F.L. Synthesis and Characterization of Silver Nanoparticle-Multiwalled Carbon Nanotube Composites. J. Nanomater. 2014, 654068, 1–7. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, H.X.; Fu, Y.; Guo, S.; Hu, Y.; Zhang, L.; Liu, Y.; Liu, H.; Li, C. Self-Volatilization approach to mesoporous carbon nanotube/silver nanoparticle hybrids: The role of silver in boosting Li ion storage. ACS Nano 2016, 10, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Lorestani, F.; Shahnavaz, Z.; Mn, P.; Alias, Y.; Manan, N.S.A. One-step hydrothermal green synthesis of silver nanoparticle-carbon nanotube reduced-graphene oxide composite and its application as hydrogen peroxide sensor. Sens. Actuators B 2015, 208, 389–398. [Google Scholar] [CrossRef]
- Yua, A.; Wang, Q.X.; Yong, J.W.; Mahon, P.J.; Malherbe, F.; Wang, F.; Zhang, H.; Wang, J. Silver nanoparticle-carbon nanotube hybrid films: Preparation and electrochemical sensing. Electrochim. Acta 2012, 74, 111–116. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Z.L.; Zhao, B.; Sun, Y.; Xu, F.; Zhang, Y.; Wen, Z.; Yang, H.; Li, Z. Carbon nanotube decorated with silver nanoparticles via noncovalent interaction for a novel nonenzymatic sensor towards hydrogen peroxide reduction. J. Electroanal. Chem. 2011, 656, 29–33. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, H.C.; Qin, X.; Wang, X.; Zhao, Z.; Miao, Z.; Chen, L.; Shan, M.; Fang, Y.; Chen, Q. A novel nonenzymatic hydrogen peroxide sensor based on multi-wall carbon nanotube/silver nanoparticle nanohybrids modified gold electrode. Talanta 2009, 80, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Takeuchi, K.; Hiraoka, T.; Furuta, T.; Kasai, T.; Sun, X.; Kiang, C.H.; Dresselhaus, M.S. Stacking nature of graphene layers in carbon nanotubes and nanofibers. J. Phys. Chem. Solids. 1997, 58, 1707–1712. [Google Scholar] [CrossRef]
- Wiley, B.J.; Chen, Y.; McLellan, J.M.; Xiong, Y.; Li, Z.Y.; Ginger, D.; Xia, Y. Synthesis and optical properties of silver nanobars and nanorice. Nano Lett. 2007, 7, 1032–1036. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Kundu, M.; Sasidharan, M. Electrochemical detection of hydrogen peroxide based on silver nanoparticles via amplified electron transfer process. J Mater. Sci. 2018, 53, 8328–8338. [Google Scholar] [CrossRef]
- Qi, Y.; Bai, J.; Ding, X.; Zhang, H.M. Electrochemically Prepared Three-dimensional Porous Nitrogen-doped Graphene Modified Electrode for Non-enzymatic Detection of Hydrogen Peroxide. Electroanalysis 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Ma, B.; Konga, C.; Hu, X.X.; Liu, K.; Huang, Q.; Lv, J.; Lu, W.; Zhang, X.; Yang, Z.; Yang, S. A sensitive electrochemical nonenzymatic biosensor for the detection of H2O2 released from living cells based on ultrathin concave Ag nanosheets. Biosens. Bioelectron. 2018, 106, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Hou, J.G.; Wang, Z.H.; Zhang, T.; Xu, C. An ultrasensitive biosensor for superoxide anion based on hollow porous PtAg nanospheres. Biosens. Bioelectron. 2018, 117, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Chen, Y.; Niu, X.Y.; Pan, C.; Chen, H.; Chen, X. High-performance electrochemical biosensor for nonenzymatic H2O2 sensing based on Au@CCo3O4 heterostructures. Biosens. Bioelectron. 2018, 118, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, D.; Qin, X.; Miao, Z.; Takahashi, S.; Anzai, J.; Chen, Q. A non-enzymatic hydrogen peroxide sensor based on poly (vinyl alcohol)-multiwalled carbon nanotubes-platinum nanoparticles hybrids modified glassy carbon electrode. Electrochim. Acta 2012, 70, 266–271. [Google Scholar] [CrossRef]
- Rajabzade, H.; Daneshgar, P.; Tazikeh, E.; Mehrabian, R.Z. Functionalized carbon nanotubes with gold nanoparticles to fabricate a sensor for hydrogen peroxide determination. Eur. J. Chem. 2012, 9, 2540–2549. [Google Scholar] [CrossRef]
- Lu, W.; Chang, G.; Luo, Y.; Liao, F.; Sun, X. Method for effective immobilization of Ag nanoparticles/graphene oxide composites on single-stranded DNA modified gold electrode for enzymeless H2O2 detection. J. Mater. Sci. 2011, 46, 5260–5266. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Gao, R.; Hu, N.; Yang, Z.; Zhu, Q.; Chai, J.; Su, Y.; Zhang, L.; Zhang, Y. Paper-like graphene-Ag composite films with enhanced mechanical and electrical properties. Nanoscale Res. Lett. 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: The AgNPs-MWCNT nanocomposite are available from the authors. |

| Modify Electrode | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|
| PtNPs-MWCNTs | 2–3800 | 0.7 | [35] |
| AuNPs-MWCNT | 20–300 | 0.4 | [36] |
| AgNCs-GO | 20–10000 | 3.0 | [13] |
| Ag NPs-MWCNT | 50–17000 | 0.5 | [37] |
| AgNPs-MWCNT-rGO | 100–100000 | 0.9 | [24] |
| AgNPs-MWCNT | 1–1000 | 0.38 | This work |
| Samples | Added (μM) | Founded (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 5.0 | 4.5 | 90.2 | 1.75 |
| 2 | 100 | 92.8 | 92.8 | 0.47 |
| 3 | 500 | 483.8 | 96.8 | 0.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Hou, B.; Qian, L.; Zhang, X.; Liu, G. Non-Enzymatic Electrochemical Sensor Based on Sliver Nanoparticle-Decorated Carbon Nanotubes. Molecules 2019, 24, 3411. https://doi.org/10.3390/molecules24183411
Xu D, Hou B, Qian L, Zhang X, Liu G. Non-Enzymatic Electrochemical Sensor Based on Sliver Nanoparticle-Decorated Carbon Nanotubes. Molecules. 2019; 24(18):3411. https://doi.org/10.3390/molecules24183411
Chicago/Turabian StyleXu, Dongqing, Bingbing Hou, Lisheng Qian, Xueji Zhang, and Guodong Liu. 2019. "Non-Enzymatic Electrochemical Sensor Based on Sliver Nanoparticle-Decorated Carbon Nanotubes" Molecules 24, no. 18: 3411. https://doi.org/10.3390/molecules24183411