Fabrication of Graphene/Molybdenum Disulfide Composites and Their Usage as Actuators for Electrochemical Sensors and Biosensors
Abstract
1. Introduction
2. Synthesis of Gr/2D MoS2 Composites
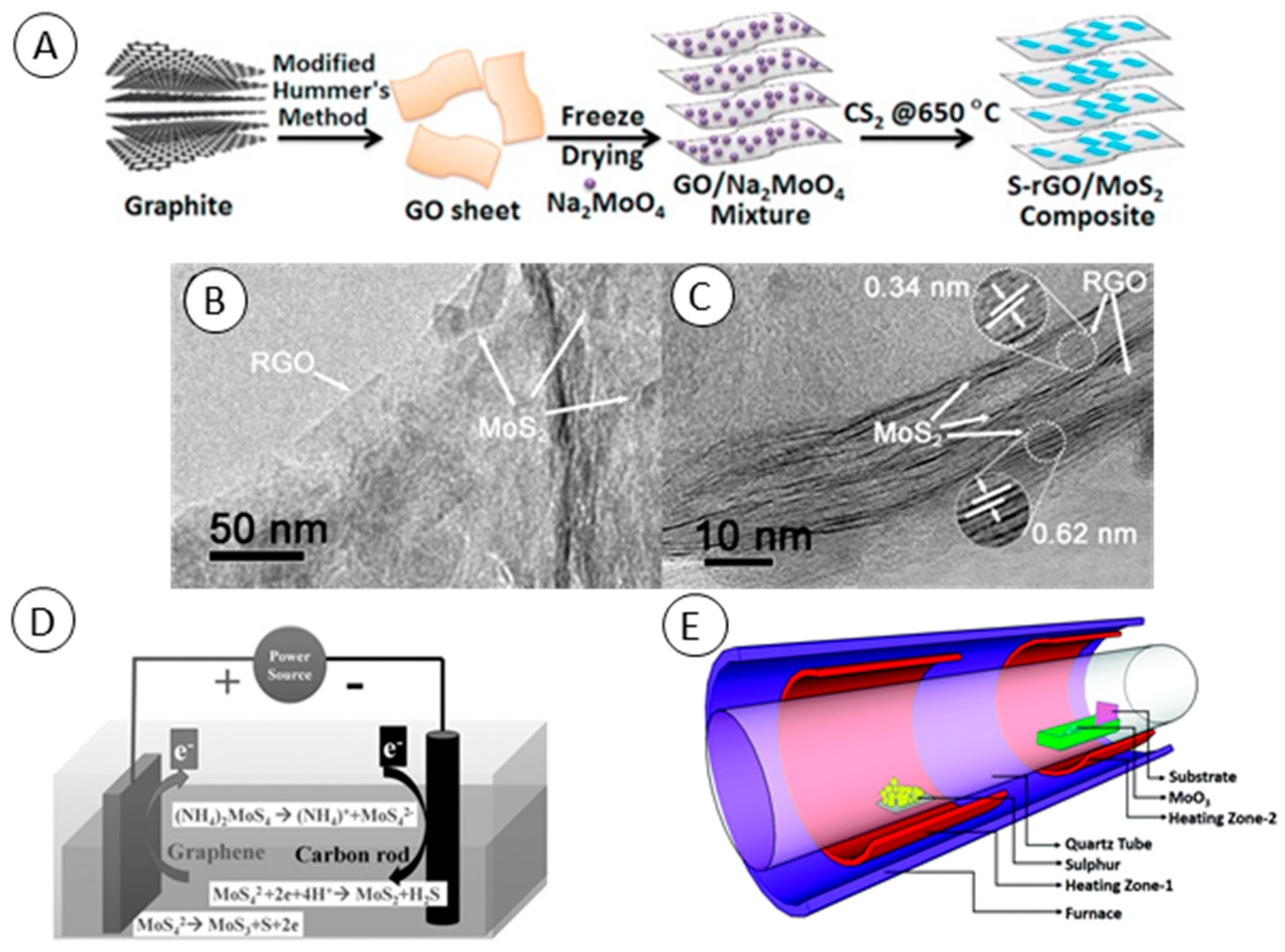
2.1. Hydrothermal and Solvothermal Synthesis
2.2. Thermal and Chemical Reduction
2.3. Microwave and Electrochemical Synthesis
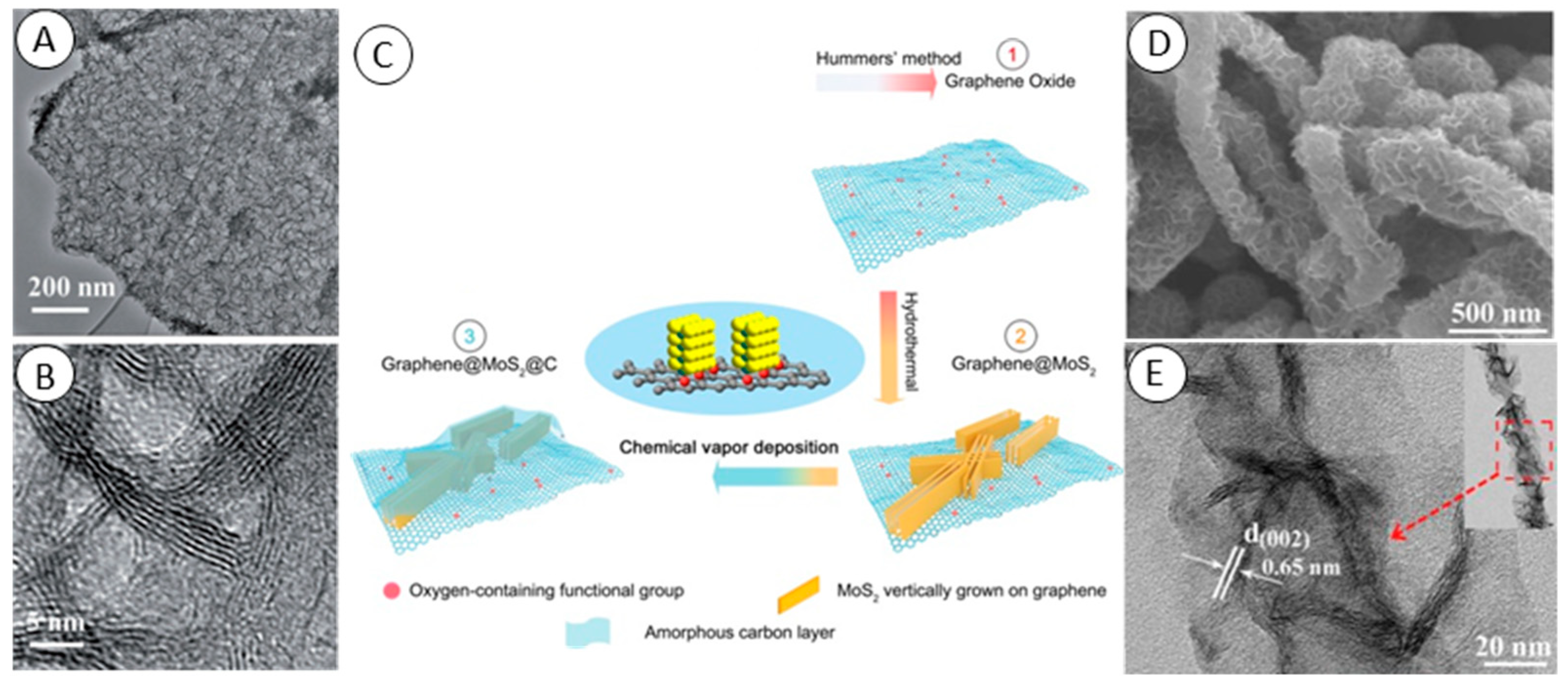
2.4. Chemical Vapor Deposition
2.5. Alternative Approaches
3. Applications of Gr/2D MoS2 Composites
3.1. Gr/2D MoS2 Composites in Electrochemical Sensors
3.2. Gr/2D MoS2 Composites in Electrochemical Biosensors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frindt, R.F. Optical absorption of a few unit-cell layers of MoS2. Phys. Rev. 1965, 140, A536–A539. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Robinson, J.A.; Dubey, M.; Terrones, H.; Terrones, M. Beyond Graphene: Progress in Novel Two-Dimensional Materials and van der Waals Solids. In Annual Review of Materials Research; Clarke, D.R., Ed.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 45, pp. 1–27. [Google Scholar]
- Xu, M.S.; Liang, T.; Shi, M.M.; Chen, H.Z. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Thevenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification—(Technical Report). Pure Appl. Chem. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.M.; Dong, S.J. Electrochemical Sensing and Biosensing Platform Based on Chemically Reduced Graphene Oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Wang, P.; Han, C.Y.; Zhou, F.Y.; Lu, J.S.; Han, X.G.; Wang, Z.W. Electrochemical determination of tert-butylhydroquinone and butylated hydroxyanisole at choline functionalized film supported graphene interface. Sens. Actuator B-Chem. 2016, 224, 885–891. [Google Scholar] [CrossRef]
- Jiang, B.Y.; Wang, M.; Chen, Y.; Xie, J.Q.; Xiang, Y. Highly sensitive electrochemical detection of cocaine on graphene/AuNP modified electrode via catalytic redox-recycling amplification. Biosens. Bioelectron. 2012, 32, 305–308. [Google Scholar] [CrossRef]
- Kudr, J.; Richtera, L.; Nejdl, L.; Xhaxhiu, K.; Vitek, P.; Rutkay-Nedecky, B.; Hynek, D.; Kopel, P.; Adam, V.; Kizek, R. Improved Electrochemical Detection of Zinc Ions Using Electrode Modified with Electrochemically Reduced Graphene Oxide. Materials 2016, 9, 31. [Google Scholar] [CrossRef]
- Zhang, W.S.; Zhang, P.P.; Su, Z.Q.; Wei, G. Synthesis and sensor applications of MoS2-based nanocomposites. Nanoscale 2015, 7, 18364–18378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Ma, T.C.; Ma, S.Y.; Liu, Y.J.; Tian, Y.P.; Wang, R.N.; Jiang, Y.B.; Hou, D.J.; Wang, J.L. Fluorometric determination of the antibiotic kanamycin by aptamer-induced FRET quenching and recovery between MoS2 nanosheets and carbon dots. Microchim. Acta 2017, 184, 203–210. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Tan, B.; Huang, Y.J.; Zhao, H.M.; Dang, X.M.; Chen, J.P.; Jain, R. MoS2 nanostructures for electrochemical sensing of multidisciplinary targets: A review. Trac-Trends Anal. Chem. 2018, 102, 75–90. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Ou, J.Z. Biosensors Based on Two-Dimensional MoS2. ACS Sens. 2016, 1, 5–16. [Google Scholar] [CrossRef]
- Wang, Y.H.; Huang, K.J.; Wu, X. Recent advances in transition-metal dichalcogenides based electrochemical biosensors: A review. Biosens. Bioelectron. 2017, 97, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.D.; Chuong, N.D.; Hien, H.V.; Kshetri, T.; Tuan, L.H.; Kim, N.H.; Lee, J.H. Recent advances in two-dimensional transition metal dichalcogenides-graphene heterostructured materials for electrochemical applications. Prog. Mater. Sci. 2018, 96, 51–85. [Google Scholar] [CrossRef]
- Yu, Z.T.; Ye, J.B.; Chen, W.X.; Xu, S.R.; Huang, F.H. Fabrication of few-layer molybdenum disulfide/reduced graphene oxide hybrids with enhanced lithium storage performance through a supramolecule-mediated hydrothermal route. Carbon 2017, 114, 125–133. [Google Scholar] [CrossRef]
- Huang, G.C.; Chen, T.; Chen, W.X.; Wang, Z.; Chang, K.; Ma, L.; Huang, F.H.; Chen, D.Y.; Lee, J.Y. Graphene-Like MoS2/Graphene Composites: Cationic Surfactant-Assisted Hydrothermal Synthesis and Electrochemical Reversible Storage of Lithium. Small 2013, 9, 3693–3703. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.R.; Hou, Y.; Ren, R.; Chen, J.H. Temperature-dependent Crystallization of MoS2 Nanoflakes on Graphene Nanosheets for Electrocatalysis. Nanoscale Res. Lett. 2017, 12, 479. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W.X. In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Yang, S.; Wang, F.X.; Zhuang, X.D.; Mullen, K.; Feng, X.L. Vertically Aligned MoS2 Nanosheets Patterned on Electrochemically Exfoliated Graphene for High-Performance Lithium and Sodium Storage. Adv. Energy Mater. 2018, 8, 1702254. [Google Scholar] [CrossRef]
- Teng, Y.Q.; Zhao, H.L.; Zhang, Z.J.; Zhao, L.N.; Zhang, Y.; Li, Z.L.; Xia, Q.; Du, Z.H.; Swierczek, K. MoS2 nanosheets vertically grown on reduced graphene oxide via oxygen bonds with carbon coating as ultrafast sodium ion batteries anodes. Carbon 2017, 119, 91–100. [Google Scholar] [CrossRef]
- Sun, H.Y.; Zhao, Y.Y.; Molhave, K. Graphene Oxide-Directed Tunable Assembly of MoS2 Ultrathin Nanosheets for Electrocatalytic Hydrogen Evolution. ChemistrySelect 2017, 2, 4696–4704. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, C.C.; Lin, L.X.; Wu, H.P.; Su, Y.W.; Zhang, X.B.; Liu, A.P. Controllable nanoscale engineering of vertically aligned MoS2 ultrathin nanosheets by nitrogen doping of 3D graphene hydrogel for improved electrocatalytic hydrogen evolution. Carbon 2017, 116, 223–231. [Google Scholar] [CrossRef]
- Zhu, C.B.; Mu, X.K.; van Aken, P.A.; Yu, Y.; Maier, J. Single-Layered Ultrasmall Nanoplates of MoS2 Embedded in Carbon Nanofibers with Excellent Electrochemical Performance for Lithium and Sodium Storage. Angew. Chem.-Int. Ed. 2014, 53, 2152–2156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Yu, X.Y.; Li, Z.; Paik, U.; Lou, X.W. Hierarchical MoS2 tubular structures internally wired by carbon nanotubes as a highly stable anode material for lithium-ion batteries. Sci. Adv. 2016, 2, e1600021. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Zhu, C.L.; Zhang, K.; Chen, Y.J.; Li, C.Y.; Gao, P.; Yang, P.P.; Ouyang, Q.Y. Three-dimensional hierarchical MoS2 nanoflake array/carbon cloth as high-performance flexible lithium-ion battery anodes. J. Mater. Chem. A 2014, 2, 4551–4557. [Google Scholar] [CrossRef]
- Li, A.N.; Hu, Y.Z.; Yu, M.P.; Liu, X.W.; Li, M.G. In situ growth of MoS2 on carbon nanofibers with enhanced electrochemical catalytic activity for the hydrogen evolution. Int. J. Hydrog. Energy 2017, 42, 9419–9427. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Kartick, B.; Choudhury, S.; Stamm, M. Thermally fabricated MoS2-graphene hybrids as high performance anode in lithium ion battery. Mater. Chem. Phys. 2016, 183, 383–391. [Google Scholar] [CrossRef]
- Koroteev, V.O.; Bulushev, D.A.; Chuvilin, A.L.; Okotrub, A.V.; Bulusheva, L.G. Nanometer-Sized MoS2 Clusters on Graphene Flakes for Catalytic Formic Acid Decomposition. ACS Catal. 2014, 4, 3950–3956. [Google Scholar] [CrossRef]
- Wang, Y.F.; Guo, Y.J.; Chen, W.T.; Luo, Q.; Lu, W.L.; Xu, P.; Chen, D.L.; Yin, X.; He, M. Sulfur-doped reduced graphene oxide/MoS2 composite with exposed active sites as efficient Pt-free counter electrode for dye-sensitized solar cell. Appl. Surf. Sci. 2018, 452, 232–238. [Google Scholar] [CrossRef]
- Li, J.L.; Qin, W.; Xie, J.P.; Lin, R.; Wang, Z.L.; Pan, L.K.; Mai, W.J. Rational design of MoS2-reduced graphene oxide sponges as free-standing anodes for sodium-ion batteries. Chem. Eng. J. 2018, 332, 260–266. [Google Scholar] [CrossRef]
- Jiang, L.F.; Lin, B.H.; Li, X.M.; Song, X.F.; Xia, H.; Li, L.; Zeng, H.B. Monolayer MoS2-Graphene Hybrid Aerogels with Controllable Porosity for Lithium-Ion Batteries with High Reversible Capacity. ACS Appl. Mater. Interfaces 2016, 8, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.M.; Luan, A.L.; Dai, C.C.; Li, M.; Yang, G.; Hou, W.H. Highly active free-standing and flexible MoS2/rGO sandwich-structured films for supercapacitor applications. Solid State Commun. 2019, 297, 45–49. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Chen, W.X.; Chang, K.; Ma, L.; Huang, G.C.; Chen, D.Y.; Lee, J.Y. CTAB-assisted synthesis of single-layer MoS2-graphene composites as anode materials of Li-ion batteries. J. Mater. Chem. A 2013, 1, 2202–2210. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, H.K.; Wei, W.; Meng, L.J. Advanced MoS2 and graphene heterostructures as high-performance anode for sodium-ion batteries. Nanotechnology 2019, 30, 104003. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.Y.; Dong, D.D.; Wen, F.S.; Zhao, J.; Zhang, X.Y.; Wang, L.M.; Liu, Z.Y. Microwave synthesized self-standing electrode of MoS2 nanosheets assembled on graphene foam for high-performance Li-Ion and Na-Ion batteries. J. Alloy. Compd. 2016, 660, 11–16. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, X.J.; Pan, L.K.; Qin, W.; Chen, T.Q.; Sun, Z. MoS2-reduced graphene oxide composites synthesized via a microwave-assisted method for visible-light photocatalytic degradation of methylene blue. Rsc Adv. 2014, 4, 9647–9651. [Google Scholar] [CrossRef]
- Li, S.L.; Min, H.H.; Xu, F.; Tong, L.; Chen, J.; Zhu, C.Y.; Sun, L.T. All electrochemical fabrication of MoS2/graphene counter electrodes for efficient dye-sensitized solar cells. RSC Adv. 2016, 6, 34546–34552. [Google Scholar] [CrossRef]
- Wan, X.; Chen, K.; Chen, Z.F.; Xie, F.Y.; Zeng, X.L.; Xie, W.G.; Chen, J.; Xu, J.B. Controlled Electrochemical Deposition of Large-Area MoS2 on Graphene for High-Responsivity Photodetectors. Adv. Funct. Mater. 2017, 27, 1603998. [Google Scholar] [CrossRef]
- Chen, X.P.; Zhang, L.L.; Chen, S.S. Large area CVD growth of graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Vabbina, P.; Choudhary, N.; Chowdhury, A.A.; Sinha, R.; Karabiyik, M.; Das, S.; Choi, W.; Pala, N. Highly Sensitive Wide Bandwidth Photodetector Based on Internal Photoemission in CVD Grown p-Type MoS2/Graphene Schottky Junction. ACS Appl. Mater. Interfaces 2015, 7, 15206–15213. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekar, P.; Periyanagounder, D.; Kulandaivel, J. Vertically aligned MoS2 nanosheets on graphene for highly stable electrocatalytic hydrogen evolution reactions. Nanoscale 2019, 11, 2439–2446. [Google Scholar] [CrossRef] [PubMed]
- Biroju, R.K.; Pal, S.; Sharma, R.; Giri, P.K.; Narayanan, T.N. Stacking sequence dependent photo-electrocatalytic performance of CVD grown MoS2/graphene van der Waals solids. Nanotechnology 2017, 28, 085101. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, C.; Chen, Z.L.; Shi, L.R.; Reddy, S.; Meng, H.; Ji, Q.Q.; Zhang, Y.F.; Liu, Z.F. Bioinspired synthesis of CVD graphene flakes and graphene-supported molybdenum sulfide catalysts for hydrogen evolution reaction. Nano Res. 2016, 9, 249–259. [Google Scholar] [CrossRef]
- Kumar, R.; Dias, W.; Rubira, R.J.G.; Alaferdov, A.V.; Vaz, A.R.; Singh, R.K.; Teixeira, S.R.; Constantino, C.J.L.; Moshkalev, S.A. Simple and Fast Approach for Synthesis of Reduced Graphene Oxide-MoS(2)Hybrids for Room Temperature Gas Detection. IEEE Trans. Electron Devices 2018, 65, 3943–3949. [Google Scholar] [CrossRef]
- Venkatesan, A.; Rathi, S.; Lee, I.Y.; Park, J.; Lim, D.; Kang, M.; Joh, H.I.; Kim, G.H.; Kannan, E.S. Molybdenum disulfide nanoparticles decorated reduced graphene oxide: Highly sensitive and selective hydrogen sensor. Nanotechnology 2017, 28, 365501. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.W.; Ma, Z.F. A glassy carbon electrode modified with a nanocomposite consisting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim. Acta 2016, 183, 257–263. [Google Scholar] [CrossRef]
- Huang, K.J.; Wang, L.; Li, J.; Liu, Y.M. Electrochemical sensing based on layered MoS2-graphene composites. Sens. Actuator B-Chem. 2013, 178, 671–677. [Google Scholar] [CrossRef]
- He, B.S.; Yan, D.D. Au/ERGO nanoparticles supported on Cu-based metal-organic framework as a novel sensor for sensitive determination of nitrite. Food Control 2019, 103, 70–77. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, C.; Yang, Y.P.; Dun, X.J.; Gao, J.M.; Jin, X.J. A novel electrochemical sensor based on CuO/H-C3N4/rGO nanocomposite for efficient electrochemical sensing nitrite. J. Alloy. Compd. 2019, 798, 764–772. [Google Scholar] [CrossRef]
- Suma, B.P.; Adarakatti, P.S.; Kempahanumakkagari, S.K.; Malingappa, P. A new polyoxometalate/rGO/Pani composite modified electrode for electrochemical sensing of nitrite and its application to food and environmental samples. Mater. Chem. Phys. 2019, 229, 269–278. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.Y.; Zhou, F.Y.; Yang, G.H.; Qu, L.L.; Miao, X.M. Development of a paper-based, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochem. Commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Zhao, Z.T.; Liu, J.; Shi, J.F.; Li, G.; Li, P.W.; Zhang, W.D.; Lian, K.; Zhuiykov, S. Synthesis and electrochemical properties of rGO-MoS2 heterostructures for highly sensitive nitrite detection. Ionics 2018, 24, 577–587. [Google Scholar] [CrossRef]
- Govindasamy, M.; Mani, V.; Chen, S.M.; Karthik, R.; Manibalan, K.; Umamaheswari, R. MoS2 Flowers Grown on Graphene/Carbon Nanotubes: A Versatile Substrate for Electrochemical Determination of Hydrogen Peroxide. Int. J. Electrochem. Sci. 2016, 11, 2954–2961. [Google Scholar] [CrossRef]
- Govindasamy, M.; Chen, S.M.; Mani, V.; Akilarasan, M.; Kogularasu, S.; Subramani, B. Nanocomposites composed of layered molybdenum disulfide and graphene for highly sensitive amperometric determination of methyl parathion. Microchim. Acta 2017, 184, 725–733. [Google Scholar] [CrossRef]
- Deo, R.P.; Wang, J.; Block, I.; Mulchandani, A.; Joshi, K.A.; Trojanowicz, M.; Scholz, F.; Chen, W.; Lin, Y.H. Determination of organophosphate pesticides at a carbon nanotube/organophosphorus hydrolase electrochemical biosensor. Anal. Chim. Acta 2005, 530, 185–189. [Google Scholar] [CrossRef]
- Kiransan, K.D.; Topcu, E. Free-standing and Flexible MoS2/rGO Paper Electrode for Amperometric Detection of Folic Acid. Electroanalysis 2018, 30, 810–818. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, T.; Bapurao, G.B.; Jo, J.; Oh, B.K.; Choi, J.W. Electrochemical H2O2 biosensor composed of myoglobin on MoS2 nanoparticle-graphene oxide hybrid structure. Biosens. Bioelectron. 2017, 93, 14–20. [Google Scholar] [CrossRef]
- Oohora, K.; Onoda, A.; Hayashi, T. Hemoproteins Reconstituted with Artificial Metal Complexes as Biohybrid Catalysts. Acc. Chem. Res. 2019, 52, 945–954. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, J.W.; Lim, J.; Mohammadniaei, M.; Bapurao, G.B.; Lee, T.; Choi, J.W. Electrochemical nitric oxide biosensor based on amine-modified MoS2/graphene oxide/myoglobin hybrid. Colloid Surf. B-Biointerfaces 2017, 159, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.J.; Sun, H.; Hou, S.F. Layered MoS2-graphene composites for biosensor applications with sensitive electrochemical performance. Anal. Methods 2016, 8, 3780–3787. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.J.; Su, X.; Duan, C.Y.; Guo, K.; Zhu, Z.F. Flower-like MoS2 Modified Reduced Graphene Oxide Nanocomposite: Synthesis and Application for Lithium-Ion Batteries and Mediator-Free Biosensor. J. Electrochem. Soc. 2015, 162, B312–B318. [Google Scholar] [CrossRef]
- Jeong, J.M.; Yang, M.; Kim, D.S.; Lee, T.J.; Choi, B.G.; Kim, D.H. High performance electrochemical glucose sensor based on three-dimensional MoS2/graphene aerogel. J. Colloid Interface Sci. 2017, 506, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Dong, S.; Gui, M.X.; Asif, M.; Wang, W.; Wang, F.; Liu, H.F. Graphene paper supported MoS2 nanocrystals monolayer with Cu submicron-buds: High-performance flexible platform for sensing in sweat. Anal. Biochem. 2018, 543, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Ni, Y.N.; Kokot, S. Electrochemical and bio-sensing platform based on a novel 3D Cu nano-flowers/layered MoS2 composite. Biosens. Bioelectron. 2016, 79, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, G.K.; Zhao, J.; Qiu, M.J.; Sun, P.; Fu, Y.F.; Han, D.X.; Cui, G.F. A portable micro glucose sensor based on copper-based nanocomposite structure. New J. Chem. 2019, 43, 7806–7813. [Google Scholar] [CrossRef]
- Vasilescu, I.; Eremia, S.A.V.; Kusko, M.; Radoi, A.; Vasile, E.; Radu, G.L. Molybdenum disulphide and graphene quantum dots as electrode modifiers for laccase biosensor. Biosens. Bioelectron. 2016, 75, 232–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Li, L.L.; Zhao, M.; Zhou, J.; Chen, Z.H.; Bai, L. An aptamer based voltammetric biosensor for endotoxins using a functionalized graphene and molybdenum disulfide composite as a new nanocarrier. Analyst 2019, 144, 1253–1259. [Google Scholar] [CrossRef]
- Geleta, G.S.; Zhao, Z.; Wang, Z.X. A novel reduced graphene oxide/molybdenum disulfide/polyaniline nanocomposite-based electrochemical aptasensor for detection of aflatoxin B-1. Analyst 2018, 143, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Chekin, F.; Bagga, K.; Subramanian, P.; Jijie, R.; Singh, S.K.; Kurungot, S.; Boukherroub, R.; Szunerits, S. Nucleic aptamer modified porous reduced graphene oxide/MoS2 based electrodes for viral detection: Application to human papillomavirus (HPV). Sens. Actuator B-Chem. 2018, 262, 991–1000. [Google Scholar] [CrossRef]
- Tian, L.; Qi, J.X.; Qian, K.; Oderinde, O.; Cai, Y.Y.; Yao, C.; Song, W.; Wang, Y.H. An ultrasensitive electrochemical cytosensor based on the magnetic field assisted binanozymes synergistic catalysis of Fe3O4 nanozyme and reduced graphene oxide/molybdenum disulfide nanozyme. Sens. Actuator B-Chem. 2018, 260, 676–684. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudr, J.; Adam, V.; Zitka, O. Fabrication of Graphene/Molybdenum Disulfide Composites and Their Usage as Actuators for Electrochemical Sensors and Biosensors. Molecules 2019, 24, 3374. https://doi.org/10.3390/molecules24183374
Kudr J, Adam V, Zitka O. Fabrication of Graphene/Molybdenum Disulfide Composites and Their Usage as Actuators for Electrochemical Sensors and Biosensors. Molecules. 2019; 24(18):3374. https://doi.org/10.3390/molecules24183374
Chicago/Turabian StyleKudr, Jiri, Vojtech Adam, and Ondrej Zitka. 2019. "Fabrication of Graphene/Molybdenum Disulfide Composites and Their Usage as Actuators for Electrochemical Sensors and Biosensors" Molecules 24, no. 18: 3374. https://doi.org/10.3390/molecules24183374
APA StyleKudr, J., Adam, V., & Zitka, O. (2019). Fabrication of Graphene/Molybdenum Disulfide Composites and Their Usage as Actuators for Electrochemical Sensors and Biosensors. Molecules, 24(18), 3374. https://doi.org/10.3390/molecules24183374