Dyes Adsorption Behavior of Fe3O4 Nanoparticles Functionalized Polyoxometalate Hybrid
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Descriptions
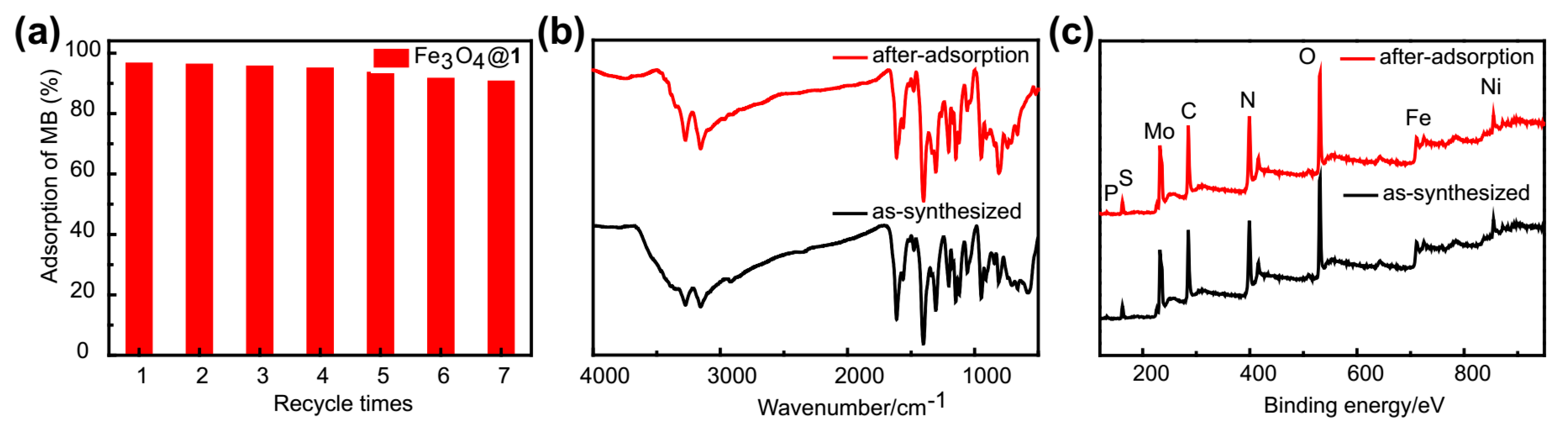
2.2. FTIR Spectra
2.3. UV-Vis Spectra
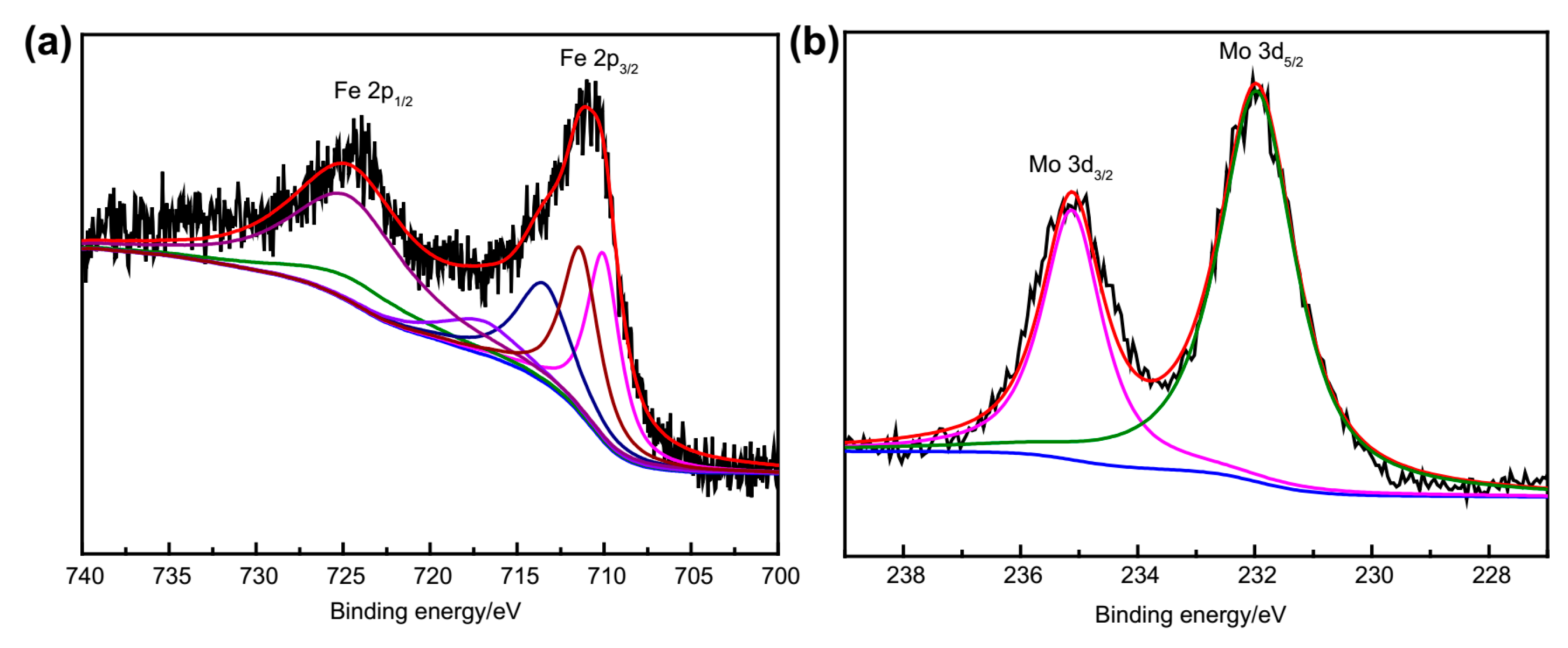
2.4. XPS Characterization
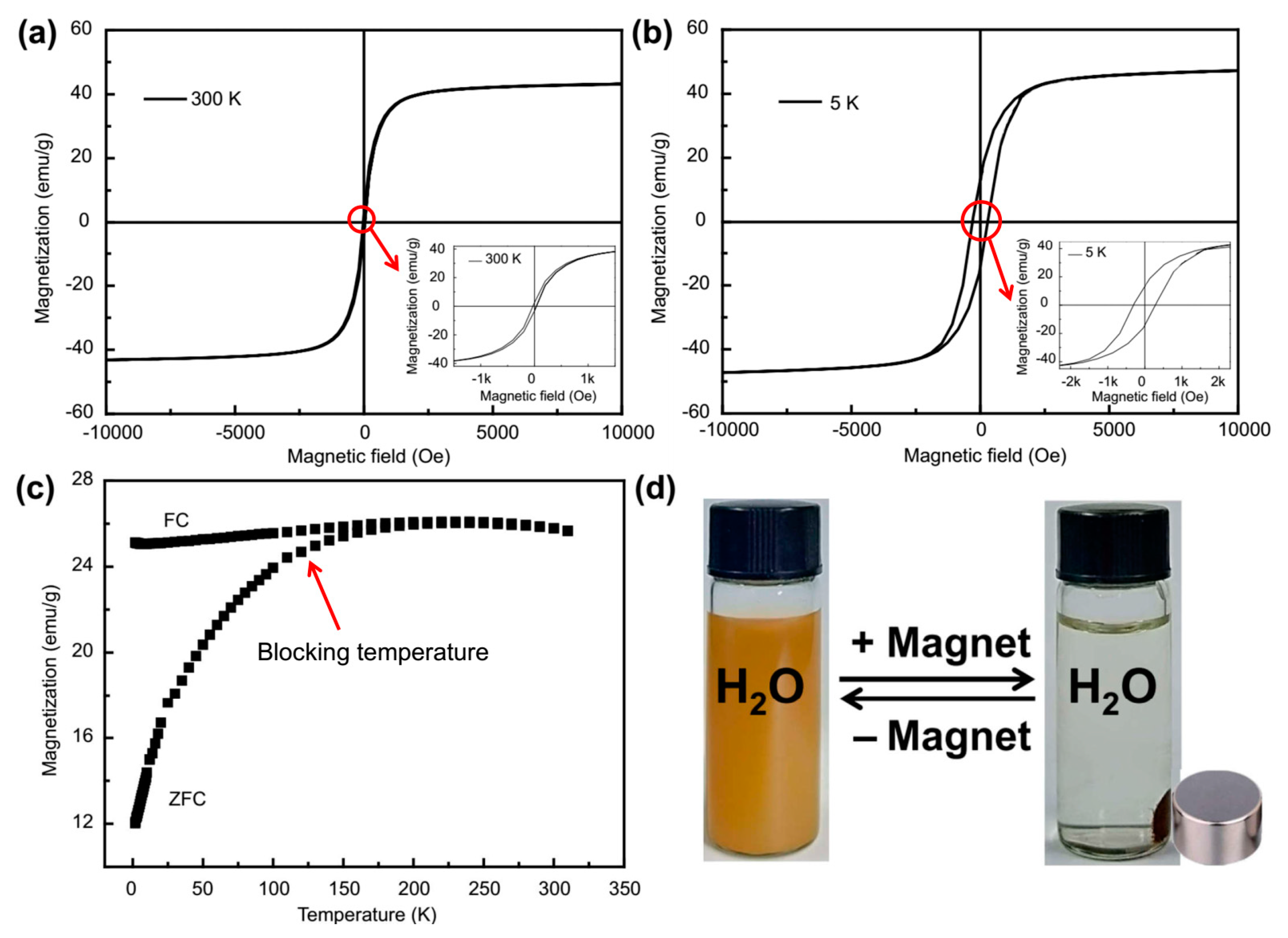
2.5. Magnetic Properties of Fe3O4@1
2.6. Morphology and Particle Size Analyses
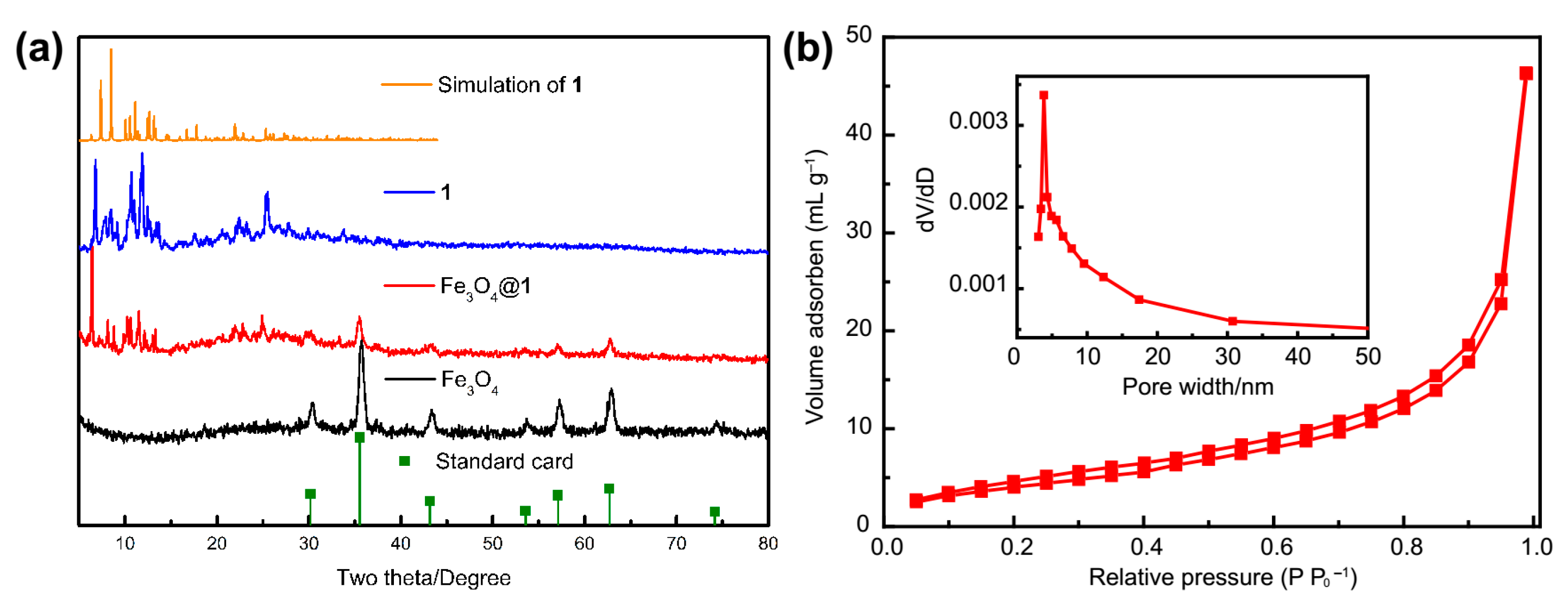
2.7. The XRD Patterns and BET Analysis
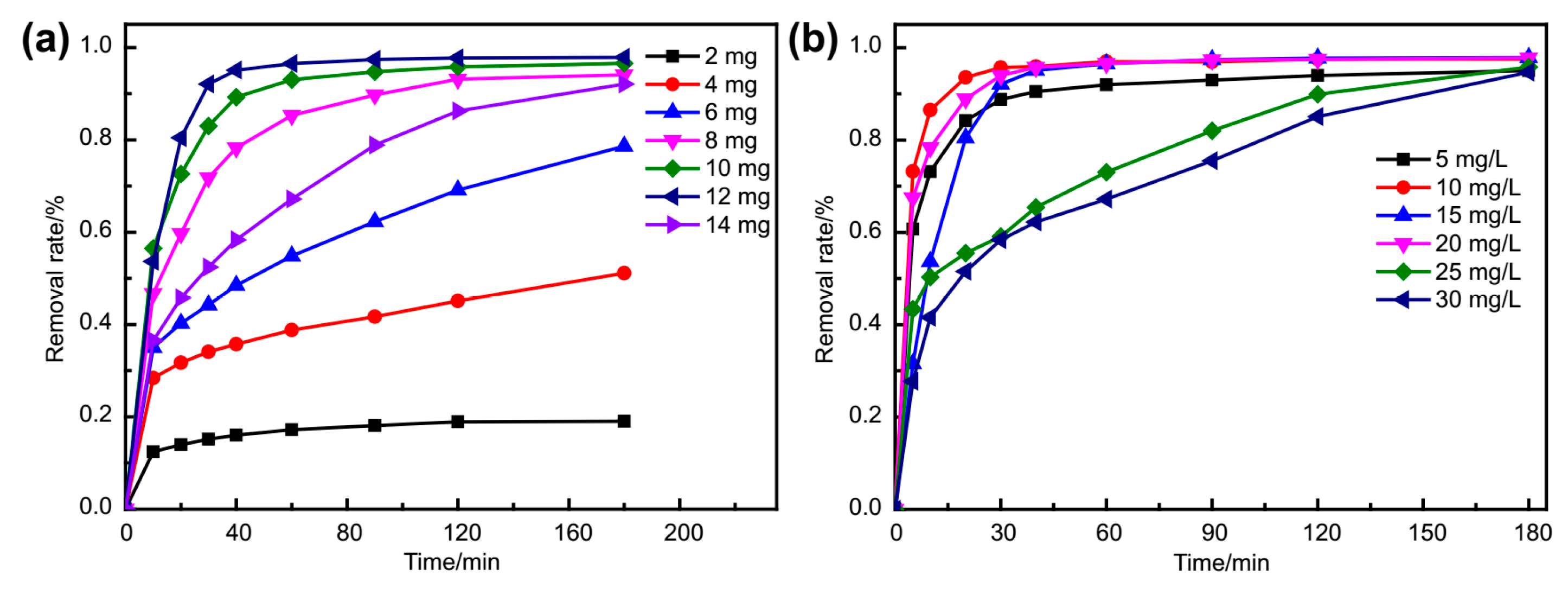
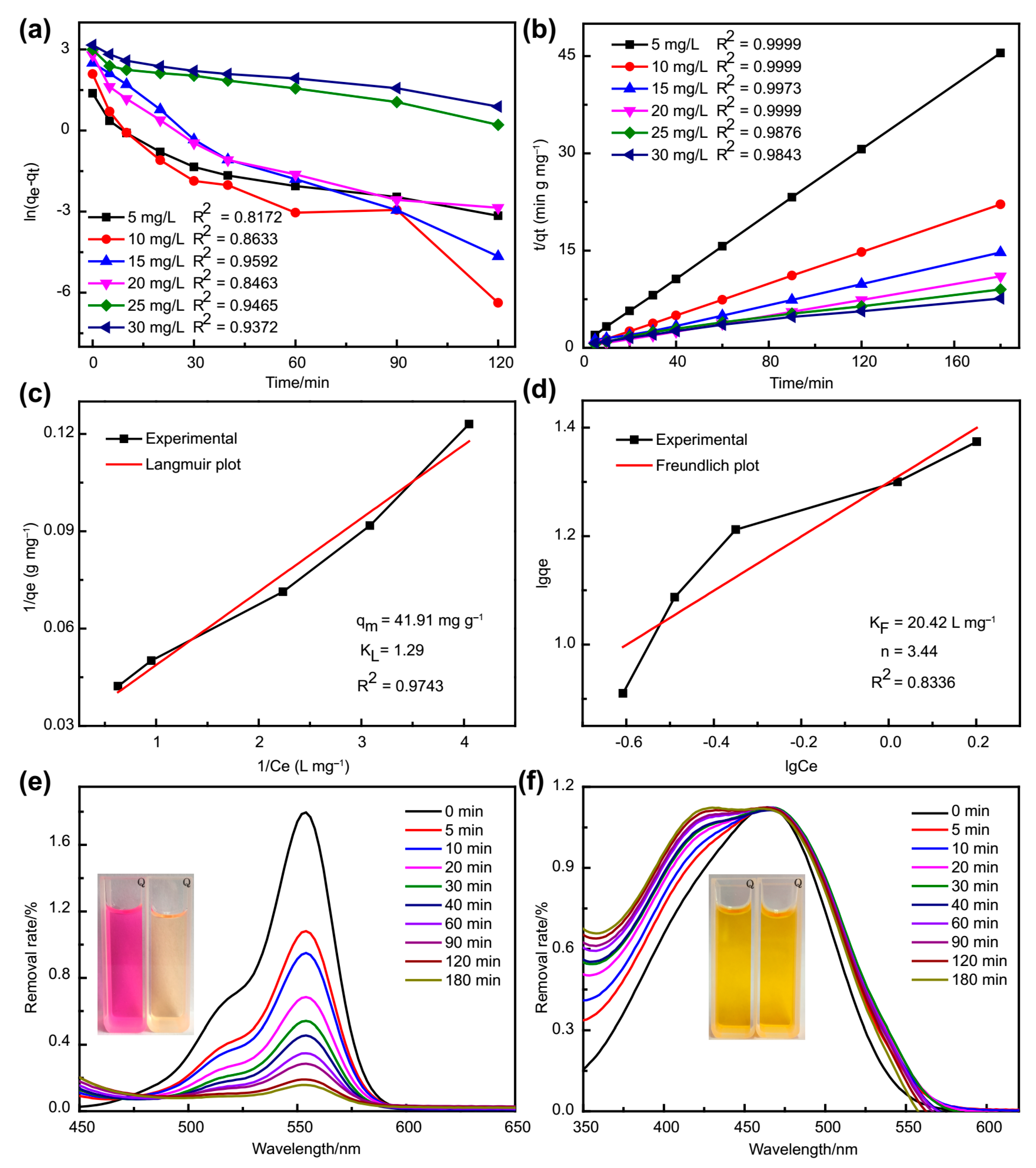
2.8. Dye Adsorption Experiments
3. Experiment
3.1. Materials and Methods
3.2. Preparations of [Ni(HL)2]2H2[P2Mo5O23]·2H2O (1)
3.3. Preparation of Nanocomposites Fe3O4@1
3.4. X-Ray Crystallographic Study
3.5. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Qu, M.N.; Ma, L.L.; Zhou, Y.C.; Zhao, Y.; Wang, J.X.; Zhang, Y.; Zhu, X.D.; Liu, X.R.; He, J.M. Durable and recyclable superhydrophilic-superoleophobic materials for efficient oil/water separation and water-soluble dyes removal. ACS Appl. Nano Mater. 2018, 1, 5197–5209. [Google Scholar] [CrossRef]
- Derami, H.G.; Jiang, Q.S.; Ghim, D.; Cao, S.S.; Chandar, Y.J.; Morrissey, J.J.; Jun, Y.S.; Singamaneni, S. A robust and scalable polydopamine/bacterial nanocellulose hybrid membrane for efficient wastewater treatment. ACS Appl. Nano Mater. 2019, 2, 1092–1101. [Google Scholar] [CrossRef]
- Florindo, C.; Lima, F.; Branco, L.C.; Marrucho, I.M. Hydrophobic deep eutectic solvents: A circular approach to water purification. ACS Sustain. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the use of metal-organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Oh, W.; Dong, Z.; Lim, T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development challenges and prospects. Appl. Catal. B: Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Rodriguez-Turienzo, L. Recovery of polyphenols onto porous carbons developed from exhausted grape pomace: A sustainable approach for the treatment of wine wastewaters. Water Res. 2018, 145, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B.; Gupta, V.; Rodriguez-Turienzo, L. Development of a green and sustainable clean up system from grape pomace for heavy metal remediation. J. Environ. Chem. Eng. 2016, 4, 4342–4353. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.X.; Bi, Y.P. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Nie, J.; Yang, J.H.; Qi, X.D.; Zhou, Z.W.; Wang, Y. Bio-inspired functionalization of microcrystalline cellulose aerogel with high adsorption performance toward dyes. Carbohyd. Polym. 2018, 198, 546–555. [Google Scholar] [CrossRef]
- Fu, J.W.; Chen, Z.H.; Wang, M.H.; Liu, S.J.; Zhang, J.H.; Zhang, J.N.; Han, R.P.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Yang, J.H.; Zhang, N.; Wang, Y.; Zhou, Z.W. Green synthesis of hybrid graphene oxide/microcrystalline cellulose aerogels and their use as superabsorbents. J. Hazard. Mater. 2017, 335, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B.; Gupta, V.; Sharma, P. Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: Effect of activation conditions. J. Colloid Interface Sci. 2017, 493, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Dolai, M.; Panja, A.; Ali, M. Hydrothermal synthesis of two supramolecular inorganic-organic hybrid phosphomolybdates based on Ni(II) and Co(II) ions: Structural diversity and heterogeneous catalytic activities. New J. Chem. 2016, 40, 6931–6938. [Google Scholar] [CrossRef]
- Li, J.; Zhao, H.Y.; Ma, C.G.; Han, Q.X.; Li, M.X.; Liu, H.L. Preparation of Fe3O4@polyoxometalates nanocomposites and their efficient adsorption of cationic dyes from aqueous solution. Nanomaterials 2019, 9, 649. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.M.; Ma, C.G.; Li, J.; Zhao, H.Y.; Chen, Q.Q.; Li, M.X.; Liu, H.L. A magnetic adsorbent for the removal of cationic dyes from wastewater. Nanomaterials 2018, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.H.; Tu, J.F.; Ding, S.M.; Wu, F.; Deng, N.S. Photodegradation of 17β-estradiol in water by UV-vis/Fe(III)/H2O2 system. J. Hazard. Mater. 2005, 127, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.B.G.; Krishna, P.M.; Kottam, N. Novelmetal-organic photocatalysts: Synthesis, characterization and decomposition of organic dyes. Spectrochim. Acta A. 2015, 137, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.M.; Liu, Y.; Xu, Y.C.; Li, R.J.; Chen, J.R.; Liao, B.Q.; Shen, L.G.; Lin, H.J. A conductive PVDF-Ni membrane with superior rejection, permeance and antifouling ability via electric assisted in-situ aeration for dye separation. J. Membr. Sci. 2019, 581, 401–412. [Google Scholar] [CrossRef]
- Chen, D.Y.; Huang, S.S.; Huang, R.T.; Zhang, Q.; Le, T.T.; Cheng, E.; Hu, Z.J.; Chen, Z.W. Convenient fabrication of Ni-doped SnO2 quantum dots with improved photodegradation performance for Rhodamine B. J. Alloys Compd. 2019, 788, 929–935. [Google Scholar] [CrossRef]
- Krishna, P.M.; Reddy, N.B.G.; Kottam, N.; Yallur, B.C.; Katreddi, H.R. Design and synthesis of metal complexes of (2E)-2-[(2E)-3-phenylprop-2-en-1-ylidene]hydrazinecarbothioamide and their photocatalytic degradation of methylene blue. Sci. World J. 2013, 2013, 828313. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Robyr, M.; Pulgarin, C.; Lavanchy, J.C.; Kiwi, J. A new perspective in the use of FeOx-TiO2 photocatalytic films: Indole degradation in the absence of Fe-leaching. J. Catal. 2016, 342, 184–192. [Google Scholar] [CrossRef]
- Li, Z.D.; Wang, H.L.; Wei, X.N.; Liu, X.Y.; Yang, Y.F.; Jiang, W.F. Preparation and photocatalytic performance of magnetic Fe3O4@TiO2 core-shell microspheres supported by silica aerogels from industrial fly ash. J. Alloys Compd. 2016, 659, 240–247. [Google Scholar] [CrossRef]
- Mangayayam, M.; Kiwi, J.; Giannakis, S.; Pulgarin, C.; Zivkovic, I.A.; Magrez, S.R. FeOx magnetization enhancing E. coli inactivation by orders of magnitude on Ag-TiO2 nanotubes under sunlight. Appl. Catal. B Environ. 2017, 202, 438–445. [Google Scholar] [CrossRef]
- Yavuz, E.; Tokalıoğlu, Ş.; Patat, Ş. Core-shell Fe3O4 polydopamine nanoparticles as sorbent for magnetic dispersive solid-phase extraction of copper from food samples. Food Chem. 2018, 263, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, J.P.; Jia, J.G.; Ma, P.T.; Zhang, D.D.; Wang, J.P.; Niu, J.Y. Isopentatungstate-supported metal carbonyl derivative: Synthesis, characterization, and catalytic properties for alkene epoxidation. Dalton Trans. 2016, 45, 6726–6731. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Wang, Y.; Zhang, L.C.; Wang, J.P.; You, W.S.; Zhu, Z.M. Three molybdophosphates based on strandbergtype anions and Zn(II)-H2biim/H2O subunits: Syntheses, structures and catalytic properties. Dalton Trans. 2014, 43, 5840–5846. [Google Scholar] [CrossRef]
- Liang, Y.F.; Li, S.Z.; Yang, D.H.; Ma, P.T.; Niu, J.Y.; Wang, J.P. Controllable assembly of multicarboxylic acids functionalized heteropolyoxomolybdates and allochroic properties. J. Mater. Chem. C 2015, 3, 4632–4639. [Google Scholar] [CrossRef]
- Cîrcu, M.; Radu, T.; Porav, A.S.; Turcu, R. Surface functionalization of Fe3O4@SiO2 core-shell nanoparticles with vinylimidazole-rare earth complexes: Synthesis, physico-chemical properties and protein interaction effects. Appl. Surf. Sci. 2018, 453, 457–463. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Wu, C.X.; Feng, X.J.; Tan, H.Q.; Yan, L.K.; Liu, Y.Z.; Kang, H.; Wang, E.B.; Li, Y.G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Nadeem, K.; Kamran, M.; Javed, A.; Zeb, F.; Hussain, S.S.; Mumtaz, M.; Krenn, H.; Szabo, D.V.; Brossmann, U.; Mu, X.K. Role of surface spins on magnetization of Cr2O3 coated γ-Fe2O3 nanoparticles. Solid State Sci. 2018, 83, 43–48. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Chen, J.; Chen, Z.; Huang, X.; Tang, X. Controlled fabrication of uniform hollow core porous shell carbon spheres by the pyrolysis of core/shell polystyrene/cross-linked polyphosphazene composites. Chem. Commun. 2010, 46, 6563–6565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Xu, S. Hollow mesoporous structured molecularly imprinted polymers for highly sensitive and selective detection of estrogens from food samples. J. Chromatogr. A 2017, 1501, 10–17. [Google Scholar] [CrossRef] [PubMed]
- He, J.C.; Li, J.; Du, W.; Han, Q.X.; Wang, Z.L.; Li, M.X. A mesoporous metal-organic framework: Potential advances in selective dye adsorption. J. Alloy. Compd. 2018, 750, 360–367. [Google Scholar] [CrossRef]
- Arshadi, M.; SalimiVahid, F.; Salvacion, J.W.L.; Soleymanzadeh, M. Adsorption studies of methyl orange on an immobilized Mn-nanoparticle: Kinetic and thermodynamic. RSC Adv. 2014, 4, 16005–16017. [Google Scholar] [CrossRef]
- Zhang, B.; Luan, L.G.; Gao, R.T.; Li, F.; Li, Y.J.; Wu, T. Rapid and effective removal of Cr(VI) from aqueous solution using exfoliated LDH nanosheets. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 399–408. [Google Scholar] [CrossRef]
- Zhang, S.X.; Zhang, Y.Y.; Bi, G.M.; Liu, J.S.; Wang, Z.G.; Xu, Q.; Xu, H.; Li, X.Y. Mussel-inspired polydopamine biopolymer decorated with magnetic nanoparticles for multiple pollutants removal. J. Hazard. Mater. 2014, 270, 27–34. [Google Scholar] [CrossRef]
- Fodi, T.; Didaskalou, C.; Kupai, J.; Balogh, G.T.; Huszthy, P.; Szekely, G. Nanofiltration-enabled in situ solvent and reagent recycle for sustainable continuous-flow synthesis. ChemSusChem 2017, 10, 3435–3444. [Google Scholar] [CrossRef]
- Schaepertoens, M.; Didaskalou, C.; Kim, J.F.; Livingston, A.G.; Szekely, G. Solvent recycle with imperfect membranes: A semi-continuous workaround for diafiltration. J. Membr. Sci. 2016, 514, 646–658. [Google Scholar] [CrossRef]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polym. Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Wu, J.H.; Min, J.H.; Zhang, X.Y.; Kim, Y.K. Tunable synthesis and multifunctionalities of Fe3O4-ZnO hybrid core-shell nanocrystals. Mater. Res. Bull. 2013, 48, 551–558. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Cryst. 2015, F71, 3–18. [Google Scholar]
- Wu, H.J.; Gao, H.Y.; Yang, Q.X.; Zhang, H.L.; Wang, D.S.; Zhang, W.J.; Yang, X.F. Removal of typical organic contaminants with a recyclable calcined chitosan-supported layered double hydroxide adsorbent: Kinetics and equilibrium isotherms. J. Chem. Eng. Data 2018, 63, 159–168. [Google Scholar] [CrossRef]
- Choi, B.H.; Jung, K.W.; Choi, J.W.; Lee, S.H.; Lee, Y.J.; Ahn, K.H. Facile one-pot synthesis of imidazole-functionalized poly-(vinylbenzylchloride) and its study on the applicability of synthetic dye removal from aqueous system. J. Clean. Prod. 2017, 168, 510–518. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and Fe3O4@1 are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Si, C.; Zhao, H.; Meng, Q.; Chang, B.; Li, M.; Liu, H. Dyes Adsorption Behavior of Fe3O4 Nanoparticles Functionalized Polyoxometalate Hybrid. Molecules 2019, 24, 3128. https://doi.org/10.3390/molecules24173128
Li J, Si C, Zhao H, Meng Q, Chang B, Li M, Liu H. Dyes Adsorption Behavior of Fe3O4 Nanoparticles Functionalized Polyoxometalate Hybrid. Molecules. 2019; 24(17):3128. https://doi.org/10.3390/molecules24173128
Chicago/Turabian StyleLi, Jie, Chen Si, Haiyan Zhao, Qingxi Meng, Bowen Chang, Mingxue Li, and Hongling Liu. 2019. "Dyes Adsorption Behavior of Fe3O4 Nanoparticles Functionalized Polyoxometalate Hybrid" Molecules 24, no. 17: 3128. https://doi.org/10.3390/molecules24173128
APA StyleLi, J., Si, C., Zhao, H., Meng, Q., Chang, B., Li, M., & Liu, H. (2019). Dyes Adsorption Behavior of Fe3O4 Nanoparticles Functionalized Polyoxometalate Hybrid. Molecules, 24(17), 3128. https://doi.org/10.3390/molecules24173128





