Design, Synthesis, and Anticancer Effect Studies of Iridium(III) Polypyridyl Complexes against SGC-7901 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physical Measurements
2.2. Synthesis of Iridium(III) Complexes
2.2.1. Synthesis of [Ir(Hppy)2(NP)]PF6 (1)
2.2.2. Synthesis of [Ir(Hppy)2(NAP)]PF6 (2)
2.2.3. Synthesis of [Ir(Hppy)2(DAP)]PF6 (3)
2.3. Cell Culture
2.4. Oil–Water Partition Coefficient Determination
2.5. Cell Uptake
2.6. In Vitro Cytotoxicity Assay
2.7. Colony-Forming Assay
2.8. Analysis of Cell Invasion
2.9. Comet Assay
2.10. Apoptosis Analyses
2.11. Measurement of Reactive Oxygen Species
2.12. Determination of Intracellular Ca2+ Levels
2.13. Localization at the Mitochondria of the Complexes
2.14. Mitochondrial Membrane Potential Assay (∆Ψm)
2.15. ATP Quantification Assay
2.16. Cell Cycle Assay
2.17. Western Blot Analysis
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Determination of Lipophilicity
3.3. Cellular Uptake
3.4. Cytotoxic Activity In Vitro
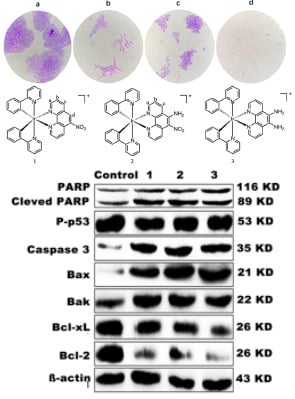
3.5. Inhibition of Colony Formation
3.6. Transwell Migration Assay
3.7. Comet Assay
3.8. Apoptosis Studies
3.9. Iridium(III) Complexes Prompt ROS Production
3.10. Detection of Intracellular Ca2+ Levels
3.11. Cellular Localization and Mitochondrial Damage Analysis
3.12. Determination of ATP
3.13. Cell Cycle Distribution
3.14. Western Blot Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuo, T.C.; Chiang, C.L.; Chang, C.H.; Liu, H.K.; Huang, G.S.; Huang, R.Y.J.; Lee, H.; Huang, C.S.; Wo, A.M. Modeling of cancer metastasis and drug resistance via biomimetic nano-cilia and microfluidics. Biomaterials 2014, 35, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.Q.; Lin, M.X.; Zhu, J.H.; Zhang, J.F.; Li, Y.Z.; Guo, Z.J. Platinum (II) compounds bearing bone-targeting group: Synthesis, crystal structure and antitumor activity. Chem. Commun. 2010, 46, 1212–1214. [Google Scholar] [CrossRef]
- Jakupec, M.A.; Galanski, M.; Arion, V.B.; Hartinger, C.G.; Keppler, B.K. Antitumour metal compounds: More than theme and variations. Dalton Trans. 2008, 14, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Giandomenico, C.M. Current status of platinum-based antitumor drugs. Chem. Rev. 1999, 99, 2451–2466. [Google Scholar] [CrossRef] [PubMed]
- Culy, C.R.; Clemett, D.; Wiseman, L.R. Oxaliplatin. A review of its pharmacological properties and clinical efficacy in metastatic colorectal cancer and its potential in other malignancies. Drugs 2000, 60, 895–924. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC tria. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef]
- Tan, J.; Wang, B.; Zhu, L. DNA binding, cytotoxicity, apoptotic inducing activity, and molecular modeling study of quercetin zinc(II) complex. Bioorgan. Med. Chem. 2009, 17, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Akladios, F.N.; Andrew, S.D.; Parkinson, C.J. Increased generation of intracellular reactive oxygen species initiates selective cytotoxicity against the MCF-7 cell line resultant from redox active combination therapy using copper–thiosemicarbazone complexes. J. Biol. Inorg. Chem. 2016, 21, 407–419. [Google Scholar] [CrossRef]
- Schmitt, F.; Govindaswamy, P.; Süss-Fink, G.; Ang, W.H.; Dyson, P.J. Ruthenium Porphyrin Compounds for Photodynamic Therapy of Cancer. J. Med. Chem. 2008, 51, 1811–1816. [Google Scholar] [CrossRef]
- Wan, D.; Tang, B.; Wang, Y.J.; Guo, B.H.; Yin, H.; Yi, Q.Y.; Liu, Y.J. Synthesis and anticancer properties of ruthenium (II) complexes as potent apoptosis inducers through mitochondrial disruption. Eur. J. Med. Chem. 2017, 13, 180–190. [Google Scholar] [CrossRef]
- Caruso, F.; Rossi, M.; Tanski, J.; Sartori, R.; Sariego, R.; Moya, S.; Diez, S.; Navarrete, E.; Cingolani, A.; Marchetti, F.; et al. Synthesis, structure, and antitumor activity of a novel tetranuclear titanium complex. J. Med. Chem. 2000, 43, 3665–3670. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.X.; Chen, M.H.; Lin, Y.N.; Zhang, H.; Tan, C.P.; Ji, L.N.; Mao, Z.W. Dual Functions of Cyclometalated iridium(III) complexes: Anti-metastasis and lysosome-damaged photodynamic therapy. ACS Appl. Mater. Interfaces 2017, 9, 42471–42481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Habtemariam, A.; Pizarro, A.M.; Fletcher, S.A.; Kisova, A.; Vrana, O.; Salassa, L.; Bruijnincx, P.C.A.; Clarkson, G.J.; Brabec, V.; et al. Organometallic half-sandwich iridium anticancer complexes. J. Med. Chem. 2011, 54, 3011–3026. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.; Chen, C.B.; Pan, C.Q.; Perrin, D.M.; Cho, Y.M.; Sigman, D.S. Optimizing the targeted chemical nuclease activity of 1,10-phenanthroline-copper by ligand modification. Bioconj. Chem. 1996, 7, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, P.; Satyanarayana, S. DNA binding and cleavage properties of certain ethylenediamine cobalt(III) complexes of modified 1,10-phenanthrolines. Polyhedron 2007, 26, 1686–1692. [Google Scholar] [CrossRef]
- Cao, J.J.; Tan, C.P.; Chen, M.H.; Wu, N.; Yao, D.Y.; Liu, X.G.; Ji, L.N.; Mao, Z.W. Targeting cancer cell metabolism with mitochondria-immobilized phosphorescent cyclometalated iridium (III) complexes. Chem. Sci. 2017, 8, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Wei, J.H.; Liu, Y.C.; Liu, M.; Gu, Y.Q.; Huang, K.B.; Wang, M.; Liang, H. High antitumor activity of 5,7-dihalo-8-quinolinolato cerium complexes. Eur. J. Med. Chem. 2013, 68, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Icsel, C.; Yilmaz, V.T.; Cevatemre, B.; Aygun, M.; Ulukaya, E. Structures and anticancer activity of chloride platinum (II) saccharinate complexes with mono-and dialkylphenyllphos-phines. J. Inorg. Biochem. 2019, 195, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.M.; Guo, H.M.; Yuan, X.L.; Li, X.H.; Ding, H.D.; Gao, P.; Zhao, C.X.; Wu, W.T.; Wu, W.H.; Zhao, J.Z. A highly selective off-on red-emitting phosphorescent thiol probe with large stokes shift and long luminescent lifetime. Org. Lett. 2010, 12, 2876–2879. [Google Scholar] [CrossRef] [PubMed]
- Bolger, J.; Gourdon, A.; Ishow, E.; Launay, J.P. Mononuclear and binuclear tetrapyrido[3,2-a:2′,3′-c:3ʺ,2′′′-h:2′′,3′′′-j]phenazine (tpphz)ruthenium and osmium complexes. Inorg. Chem. 1996, 35, 2937–2944. [Google Scholar] [CrossRef]
- Chen, X.; Sun, L.; Chen, Y.; Chen, X.L.; Wu, W.J.; Ji, L.N.; Chao, H. A fast and selective two-photon phosphorescent probe for the imaging of nitric oxide in mitochondria. Biomaterials 2015, 58, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Medvetz, D.A.; Stakleff, K.D.; Schreiber, T.; Custer, P.D.; Hindi, K.; Panzner, M.J.; Blanco, D.D.; Taschner, M.J.; Tessier, C.A.; Youngs, W.J. Ovarian cancer activity of cyclic amine and thiaether metal complexes. J. Med. Chem. 2007, 50, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.Y.; Wan, D.; Tang, B.; Wang, Y.J.; Zhang, W.Y.; Du, F.; He, M.; Liu, Y.J. Synthesis, characterization and anticancer activity in vitro and in vivo evaluation of an iridium (III) polypyridyl complex. Eur. J. Med. Chem. 2018, 145, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, E.; Pajak, K.; Jozwiak, K. Lipophilicity-methods of determination and its role in medicinal chemistry. Acta Pol. Pharm. 2013, 70, 3–18. [Google Scholar] [PubMed]
- Pierroz, V.; Joshi, T.; Leonidova, A.; Mari, G.; Schur, J.; Ott, I.; Spiccia, L.; Ferrari, S.; Gasser, G. Molecular and cellular characterization of the biological effects of ruthenium(II) complexes incorporating 2-pyridyl-2-pyrimidine-4-carboxylic acid. J. Am. Chem. Soc. 2012, 134, 20376–20387. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Lee, J.S.; Kim, Y.R.; Song, I.G.; Hong, E.K. Polysaccharide from inonotus obliquus inhibits migration and invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via downregulation of NF-κB signaling pathway. Oncol. Rep. 2014, 31, 2447–2453. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liang, C.H.; Lippard, S.J.; Natl, P. Steroid hormones induce HMG1 overexpression and sensitize breast cancer cells to cisplatin and carboplatin. Acad. Sci. 2000, 97, 5768–5772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inci, D.; Aydin, R.; Vatan, O.; Huriyet, H.; Zorlu, Y.; Cosut, B.; Cinkilic, N. Cu (II) tyrosinate complexes containing methyl substituted phenanthrolines: Synthesis, X-ray crystal structures, biomolecular interactions, antioxidant activity, ROS generation and cytotoxicity. Appl. Organomet. Chem. 2019, 33, e4652. [Google Scholar] [CrossRef]
- Zeng, C.C.; Jiang, G.B.; Lai, S.H.; Zhang, C.; Yin, H.; Tang, B.; Wan, D.; Liu, Y.J. Synthesis, characterization and anticancer activity studies of ruthenium(II) polypyridyl complexes on A549 cells. J. Photochem. Photobiol. B 2016, 161, 295–303. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Dai, R.H.; Ma, J.J.; Wang, S.Y.; Wei, X.H.; Wang, H.F. Photoinduced DNA damage and cytotoxicity by a triphenylamine-modified platinum-diimine complex. J. Inorg. Biochem. 2015, 143, 64–68. [Google Scholar] [CrossRef]
- He, X.D.; Tian, M.; Liu, X.C.; Tang, Y.H.; Shao, C.F.; Gong, P.W.; Liu, J.F.; Zhang, S.M.; Guo, L.H.; Liu, Z. Triphenylamine-Appended Half-Sandwich Iridium(III) Complexes and Their Biological Applications. Chem. Asian J. 2018, 13, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Verme, I.; Haanen, C.; Nakken, H.S.; Reutelingsperger, C. A novel assay for apoptosis Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. Immunol. J. Methods 1995, 18, 439–451. [Google Scholar] [CrossRef]
- Yu, S.J.; Zhang, L.P.; Liu, C.; Yang, J.; Zhang, J.H.; Huang, L. PACS2 is required for ox-LDL-induced endothelial cell apoptosis by regulating mitochondria-associated ER membrane formation and mitochondrial Ca2+ elevation. Exp. Cell Res. 2019, 379, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.L.; Zhang, L.; Ge, Y.K.; Lu, M.; Zheng, X.X. Redistribution of intracellular calcium and its effect on apoptosis in macrophages: Induction by oxidized LDL. Biomed. Pharmacother. 2009, 63, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.N.; Gonzales, A.L.; Pires, P.W.; Bruhl, A.; Leo, M.D.; Li, W.C.; Oulidi, A.; Boop, F.A.; Feng, Y.M.; Jaggar, J.H.; et al. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci. Signal. 2015, 8, ra2. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.Y.; Zhang, W.Y.; He, M.; Du, F.; Wang, X.Z.; Wang, Y.J.; Gu, Y.Y.; Bai, L.; Liu, Y.J. Anticancer and antibacterial activity in vitro evaluation of iridium(III) polypyridyl complexes. J. Biol. Inorg. Chem. 2019, 24, 151–169. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssiere, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Nayak, D.; Kumari, M.; Rajachandar, S.; Ashe, S.; Thathapudi, N.C.; Nayak, B. Biofilm Impeding AgNPs Target Skin Carcinoma by Inducing Mitochondrial Membrane Depolarization Mediated through ROS Production. ACS Appl. Mater. Interfaces 2016, 8, 28538–28553. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.X.; Cao, Q.; Hao, L.; Zhou, D.X.; Gan, Z.J.; Ji, L.N.; Mao, Z.W. Simultaneously inducing and tracking cancer cell metabolism repression by mitochondria-immobilized rhenium(I) complex. ACS Appl. Mater. Interfaces 2017, 9, 13900–13912. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Wang, Y.J.; Du, F.; He, M.; Gu, Y.Y.; Bai, L.; Yang, L.L.; Liu, Y.J. Evaluation of anticancer effect in vitro and in vivo of iridium(III) complexes on gastric carcinoma SGC-7901 cells. Eur. J. Med. Chem. 2019, 178, 401–416. [Google Scholar] [CrossRef]
- He, M.; Yi, Q.Y.; Zhang, W.Y.; Bai, L.; Du, F.; Gu, Y.Y.; Liu, Y.J.; Wei, P. Evaluation of anticancer activity in vitro and in vivo of iridium(III) polypyridyl complexes. New J. Chem. 2019, 43, 8566–8579. [Google Scholar] [CrossRef]
- Hao, H.L.; Liu, X.C.; Ge, X.X.; Zhao, Y.; Tian, X.; Ren, T.; Wang, Y.; Zhao, C.F.; Liu, Z. Half-sandwich iridium(III) complexes with α-picolinic acid frameworks and antitumor applications. J. Inorg. Biochem. 2019, 192, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Loo, G.V.; Saelens, X.; Gurp, M.V.; Macfarlane, M.; Martin, S.J.; Vandenabeele, P. The role of mitochondrial factors in apoptosis: A Russian roulette with more than one bullet. Cell Death Differ. 2002, 9, 1031–1042. [Google Scholar] [PubMed]
- Zhang, C.C.; Zhang, H.; Yu, Y.Y.; Wu, S.; Chen, F. Ratio fluorometric determination of ATP base on the reversion of fluorescence of calcein quenched by Eu(III) ion using carbon dots as reference. Talant 2019, 197, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Si, J.C.; Gao, Z.F.; Zhang, Y.; Lei, J.L.; Luo, H.Q.; Li, N.B. Highly selective and sensitive electrochemical biosensor for ATP based on the dual strategy integrating the cofactor-dependent enzymatic ligation reaction with self-cleaving DNA zyme-amplified electrochemical detection. Biosens. Bioelectron. 2015, 63, 14–20. [Google Scholar] [CrossRef]
- Wang, G.X.; Su, X.L.; Xu, Q.J.; Xu, G.Y.; Lin, J.H.; Luo, X.L. Antifouling aptasensor for the detection of adenosine triphosphate in biological media based on mixed self-assembled aptamer and zwitterionic peptide. Biosens. Bioelectron. 2018, 101, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997, 4, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Billecke, C.; Finniss, S.; Tahash, L.; Miller, C.; Mikkelsen, T.; Farrell, N.P.; Bogler, O. Polynuclear platinum anticancer drugs are more potent than cisplatin and induce cell cycle arrest in glioma. Neuro-Oncology 2006, 8, 215–226. [Google Scholar] [CrossRef]
- Turner, J.; Koumenis, C.; Kute, T.E.; Planalp, R.P.; Brechbiel, M.W.; Beardsley, D.; Cody, B.; Brown, K.D.; Torti, F.M.; Torti, S.V. Tachpyridine, a metal chelator, induces G2 cell-cycle arrest, activates checkpoint kinases, and sensitizes cells to ionizing radiation. Blood 2005, 106, 3191–3199. [Google Scholar] [CrossRef]
- Bica, L.; Meyerowitz, J.; Parker, S.J.; Caragounis, A.; Du, T.; Paterson, B.M.; Barnham, K.J.; Crouch, P.J.; White, A.R.; Donnely, P.S. Cell cycle arrest in cultured neuroblastoma cells exposed to a bis(thiosemicarbazonato) metal complex. Biometals 2011, 24, 117–133. [Google Scholar] [CrossRef]
- Xiao, W.Q.; Xu, Z.J.; Chang, S.K.; Li, B.; Yu, D.D.; Wu, H.Q.; Xie, Y.S.; Wang, Y.C.; Xie, B.Q.; Sun, X.; et al. Rafoxanide, an organohalogen drug, triggers apoptosis and cell cycle arrest in multiple myeloma by enhancing DNA damage responses and suppressing the p38 MAPK pathway. Cancer Lett. 2019, 444, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, G.; Calabrese, E.; Hideshima, T.; Ecsedy, J.; Perrone, G.; Mani, M.; Ikeda, H.; Bianchi, G.; Hu, Y.; Cirstea, D.; et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood 2010, 115, 5202–5213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.D.; Kong, X.; He, S.F.; Chen, J.X.; Sun, J.; Chen, B.B.; Zhao, J.W.; Mao, Z.W. Cyclometalated iridium(III)-guanidinium complexes as mitochondria-targeted anticancer agents. Eur. J. Med. Chem. 2017, 138, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Araki, T. Nephrol. Dial. Fluvastatin prevents development of arterial stiffness in haemodialysis patients with type 2 diabetes mellitus. Nephrol. Dial. Transplant. 2002, 17, 8–10. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 2 and 3 are available from the authors. |















| Complex | SGC-7901 | A549 | HeLa | Eca-109 | HepG2 | BEL-7402 | NIH 3T3 | logPo/w |
|---|---|---|---|---|---|---|---|---|
| NP | 18.4 ± 0.1 | 11.1 ± 0.7 | 18.1 ± 1.7 | ND | 16.7 ± 0.5 | 23.5 ± 1.1 | >200 | ND |
| NAP | 90.9 ± 7.4 | 42.7 ± 2.1 | 45.8 ± 4.0 | ND | 56.0 ± 1.9 | 21.1 ± 0.8 | 61.9 ± 1.9 | ND |
| DAP | 15.8 ± 2.8 | 5.0 ± 0.3 | 9.2 ± 1.0 | ND | 4.6 ± 0.8 | 37.4 ± 3.2 | 12.3 ± 0.8 | ND |
| [Ir(Hppy)2Cl]2 | 3.5 ± 0.1 | 3.3 ± 0.4 | 2.5 ± 0.2 | ND | 5.5 ± 0.3 | 8.4 ± 0.4 | 6.3 ± 0.3 | ND |
| 1 | 3.6 ± 0.1 | 14.3 ± 2.4 | 16.3 ± 0.9 | 20.3 ± 0.9 | 30.9 ± 0.7 | 23.0 ± 0.8 | 14.6 ± 1.3 | 1.01 |
| 2 | 14.1 ± 0.5 | 80.6 ± 1.4 | 52.6 ± 1.4 | 72.6 ± 5.3 | 99 ± 14.7 | 68.7 ± 1.5 | 23.0 ± 1.5 | 1.33 |
| 3 | 11.1 ± 1.3 | >100 | 59.7 ± 6.8 | 97.1 ± 0.2 | 95.0 ± 9.1 | 68.8 ± 4.6 | >100 | 0.78 |
| Cisplatin | 3.6 ± 0.5 | 7.5 ± 1.3 | 7.0 ± 1.0 | 11.2 ± 0.6 | 12.5 ± 1.5 | 10.8 ± 1.6 | ND | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.-X.; Gu, Y.-Y.; Wang, Y.-J.; Bai, L.; Du, F.; Zhang, W.-Y.; He, M.; Liu, Y.-J.; Chen, Y.-Z. Design, Synthesis, and Anticancer Effect Studies of Iridium(III) Polypyridyl Complexes against SGC-7901 Cells. Molecules 2019, 24, 3129. https://doi.org/10.3390/molecules24173129
Zhang L-X, Gu Y-Y, Wang Y-J, Bai L, Du F, Zhang W-Y, He M, Liu Y-J, Chen Y-Z. Design, Synthesis, and Anticancer Effect Studies of Iridium(III) Polypyridyl Complexes against SGC-7901 Cells. Molecules. 2019; 24(17):3129. https://doi.org/10.3390/molecules24173129
Chicago/Turabian StyleZhang, Li-Xia, Yi-Ying Gu, Yang-Jie Wang, Lan Bai, Fan Du, Wen-Yao Zhang, Miao He, Yun-Jun Liu, and Yan-Zhong Chen. 2019. "Design, Synthesis, and Anticancer Effect Studies of Iridium(III) Polypyridyl Complexes against SGC-7901 Cells" Molecules 24, no. 17: 3129. https://doi.org/10.3390/molecules24173129
APA StyleZhang, L.-X., Gu, Y.-Y., Wang, Y.-J., Bai, L., Du, F., Zhang, W.-Y., He, M., Liu, Y.-J., & Chen, Y.-Z. (2019). Design, Synthesis, and Anticancer Effect Studies of Iridium(III) Polypyridyl Complexes against SGC-7901 Cells. Molecules, 24(17), 3129. https://doi.org/10.3390/molecules24173129