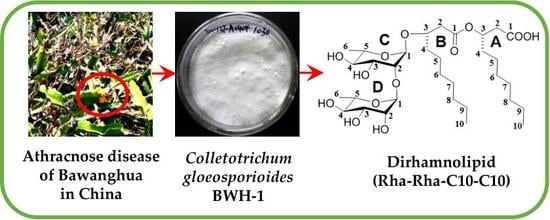
Dirhamnolipid Produced by the Pathogenic Fungus Colletotrichum gloeosporioides BWH-1 and Its Herbicidal Activity
Abstract
:1. Introduction
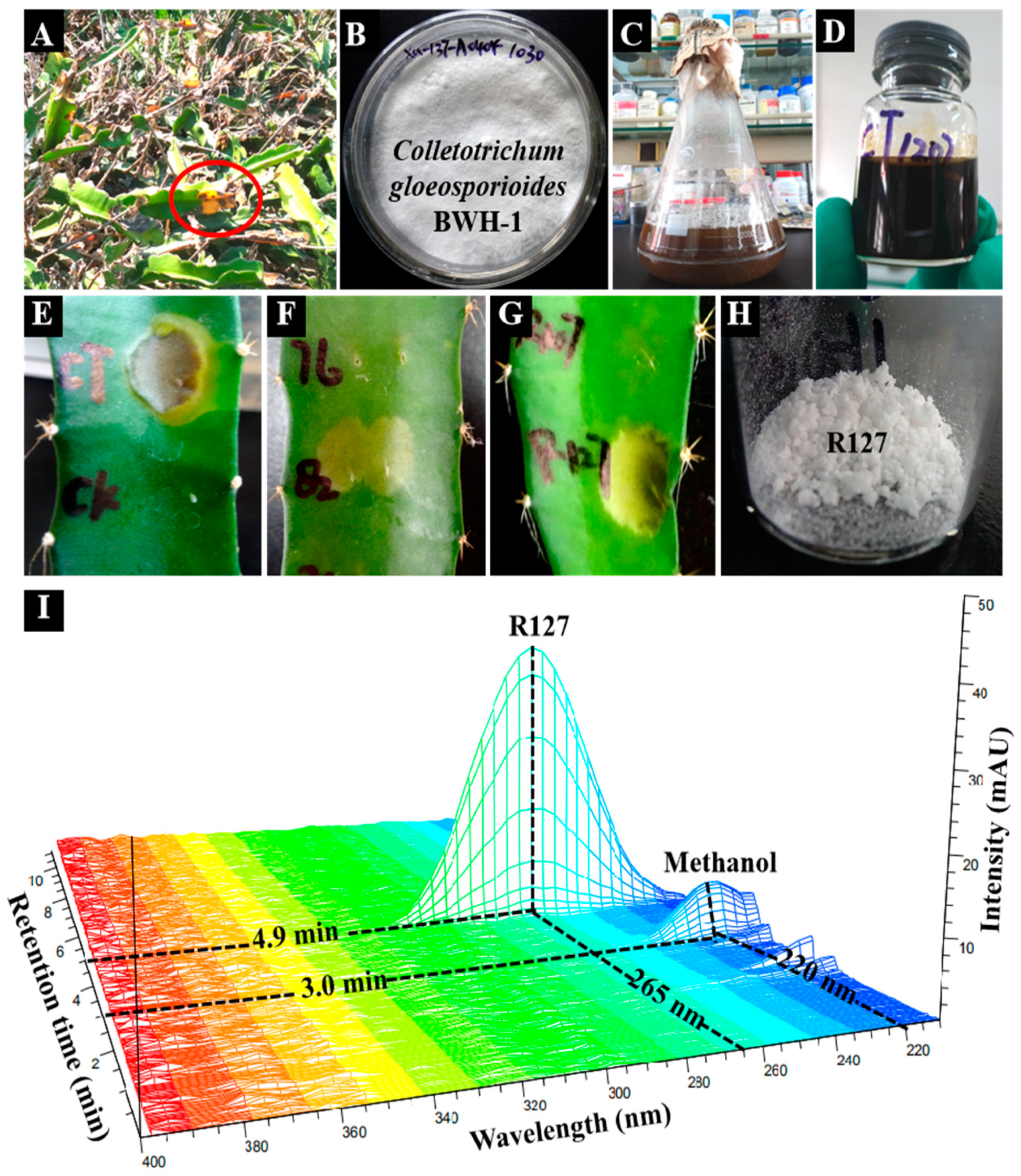
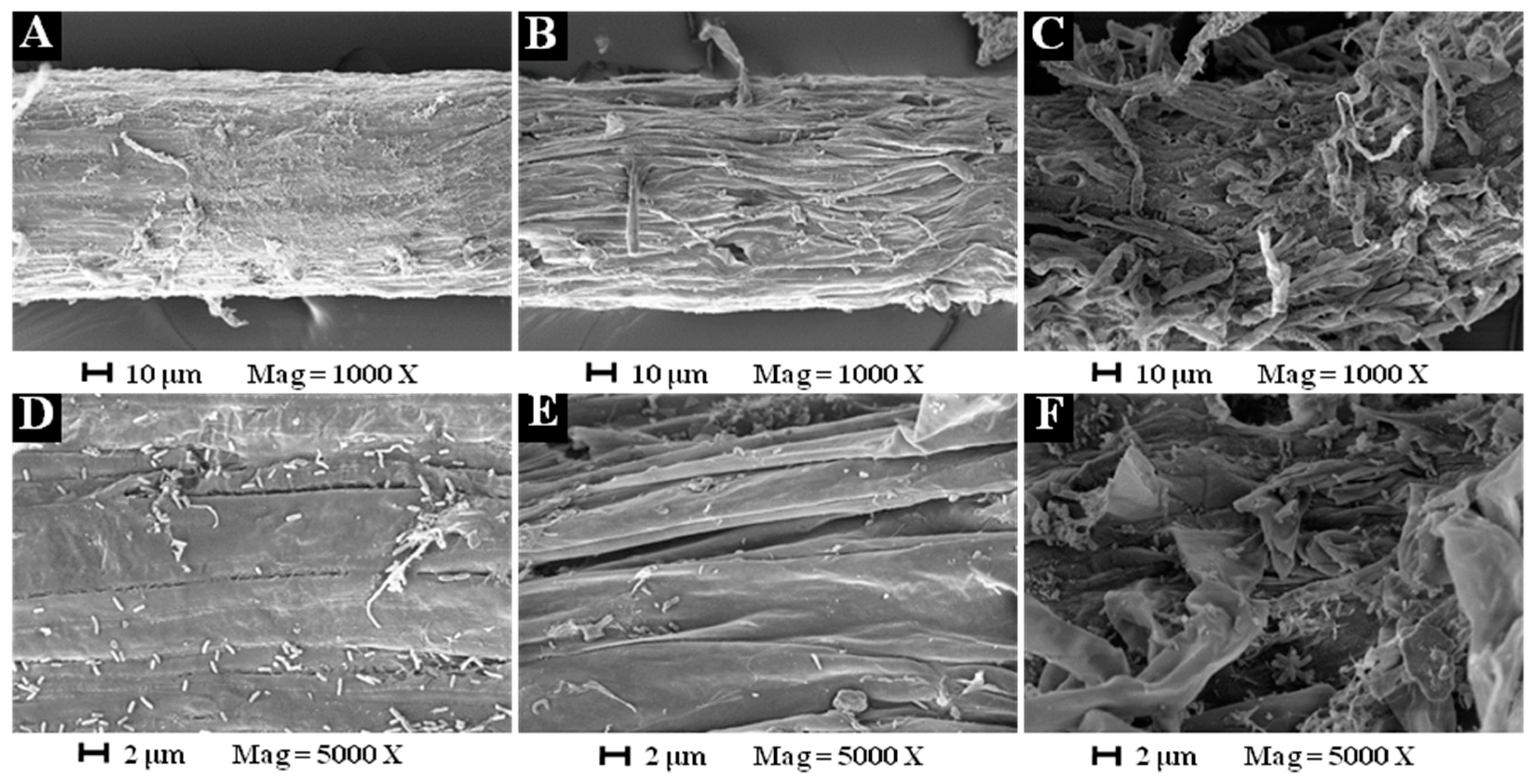
2. Results and Discussions
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strain and Liquid Culture
3.3. Bioactivity-guided Isolation and Purification
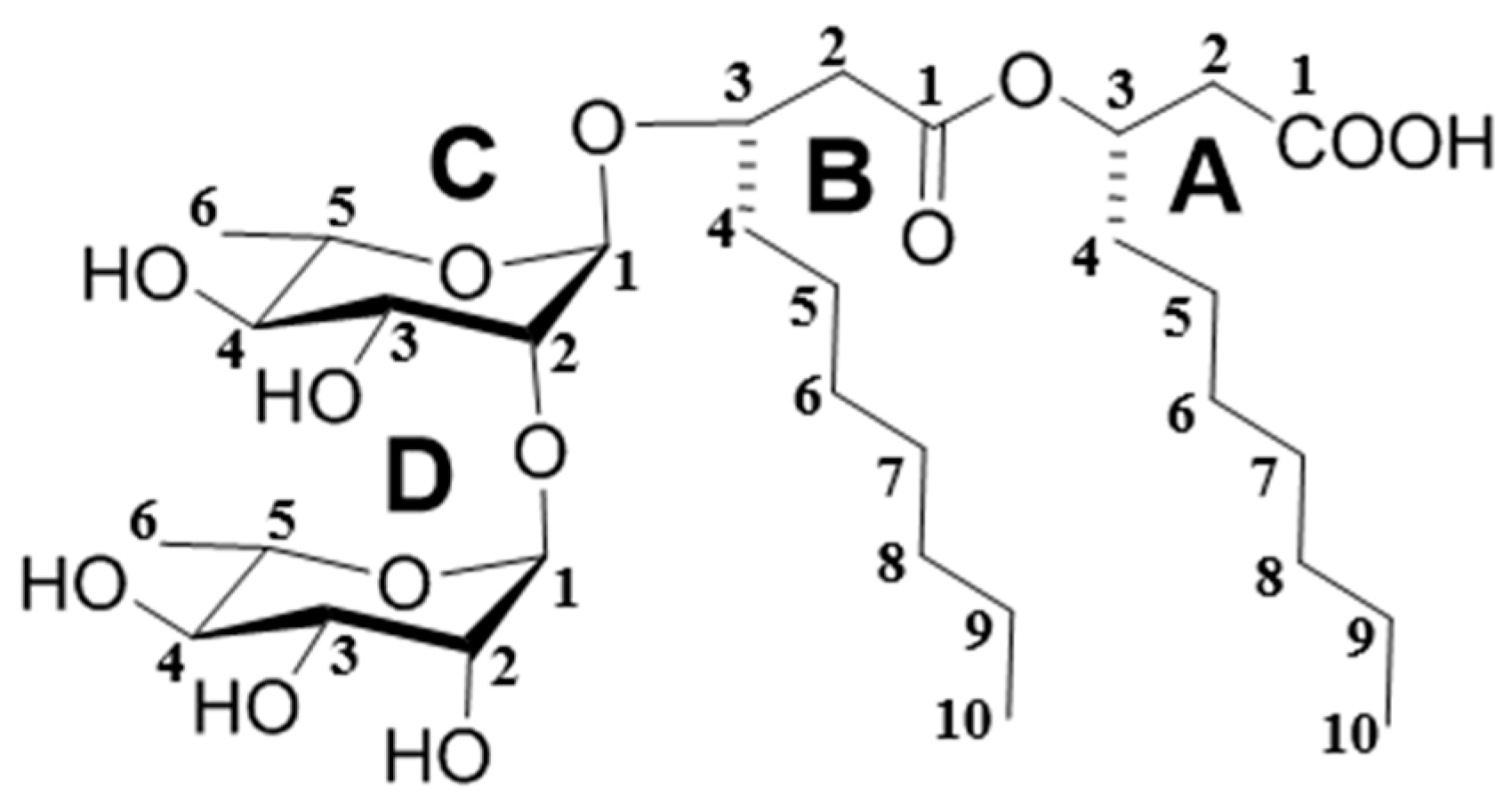
3.4. Structure Characterization
3.5. Evaluation of Herbicidal Activity
3.6. Evaluation of Synergistic Effect
3.7. Microscopy Observation
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Masi, M.; Freda, F.; Sangermano, F.; Calabro, V.; Cimmino, A.; Cristofaro, M.; Meyer, S.; Evidente, A. Radicinin, a fungal phytotoxin as a target-specific bioherbicide for invasive buffelgrass (Cenchrus ciliaris) control. Molecules 2019, 24, 1086. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ma, J.J.; Liu, B.; Huang, L.; Sang, X.Q.; Zhou, L.J. Herbicidal spectrum, absorption and transportation, and physiological effect on Bidens pilosa of the natural alkaloid berberine. J. Agric. Food Chem. 2017, 65, 6100–6113. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotox. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Phoulivong, S.; Lei, C.; Hang, C.; Mckenzie, E.H.C.; Abdelsalam, K.; Chukeatirote, E.; Hyde, K.D. Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers. 2010, 44, 33–43. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Boari, A.; Zonno, M.C.; Gorecki, M.; Pescitelli, G.; Tuzi, A.; Vurro, M.; Evidente, A. Colletopyrandione, a new phytotoxic tetrasubstituted indolylidenepyran-2,4-dione, and colletochlorins G and H, new tetrasubstituted chroman- and isochroman-3,5-diols isolated from Colletotrichum higginsianum. Tetrahedron 2017, 73, 6644–6650. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Boari, A.; Tuzi, A.; Zonno, M.C.; Baroncelli, R.; Vurro, M.; Evidente, A. Colletochlorins E and F, new phytotoxic tetrasubstituted pyran-2-one and dihydrobenzofuran, isolated from Colletotrichum higginsianum with potential herbicidal activity. J. Agric. Food Chem. 2017, 65, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Zonno, M.C.; Cimmino, A.; Reveglia, P.; Berestetskiy, A.; Boari, A.; Vurro, M.; Evidente, A. On the metabolites produced by Colletotrichum gloeosporioides a fungus proposed for the Ambrosia artemisiifolia biocontrol; spectroscopic data and absolute configuration assignment of colletochlorin A. Nat. Prod. Res. 2018, 32, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Yang, X.; Wang, X.R.; Zeng, Y.S.; Liao, M.D.; Chen, C.J.; Sun, S.; Jia, D.M. First report of anthracnose disease on young stems of Bawanghua (Hylocereus undatus) caused by Colletotrichum gloeosporioides in China. Plant Dis. 2014, 98, 991–992. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Z.; Poon, J.; Poon, S.K.; Chen, H.; Gao, J.B.; Kwan, P.; Fan, K.; Ling, Z.H. An improved independent component analysis model for 3D chromatogram separation and its solution by multi-areas genetic algorithm. BMC Bioinform. 2014, 15, S8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jansen, R.; Nimtz, M.; Johri, B.N.; Wray, V. Rhamnolipids from the rhizosphere bacterium Pseudomonas sp GRP(3) that reduces damping-off disease in chilli and tomato nurseries. J. Nat. Prod. 2007, 70, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.J.; Xu, S.X.; Guo, J.; Chen, Q.R.; Meng, Q.; Zheng, X.D. Biocontrol of post-harvest Alternaria alternata decay of cherry tomatoes with rhamnolipids and possible mechanisms of action. J. Sci. Food Agric. 2015, 95, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Toribio, J.; Escalante, A.E.; Soberon-Chavez, G. Rhamnolipids: Production in bacteria other than Pseudomonas aeruginosa. Eur. J. Lipid Sci. Technol. 2010, 112, 1082–1087. [Google Scholar] [CrossRef]
- Chen, J.W.; Wu, Q.H.; Hua, Y.; Chen, J.; Zhang, H.W.; Wang, H. Potential applications of biosurfactant rhamnolipids in agriculture and biomedicine. Appl. Microbiol. Biotechnol. 2017, 101, 8309–8319. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.C.; Lee, S.; Kim, J.C.; Yun, M.Y.; Kim, I.S. Insecticidal activity of rhamnolipid isolated from Pseudomonas sp EP-3 against green peach aphid (Myzus persicae). J. Agric. Food. Chem. 2011, 59, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, G.; Hoti, S.L.; Rao, H.S.P.; Vijjapu, S. Di-rhamnolipid is a mosquito pupicidal metabolite from Pseudomonas fluorescens (VCRC B426). Acta Trop. 2015, 148, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ottis, B.V.; Mattice, J.D.; Talbert, R.E. Determination of antagonism between cyhalofop-butyl and other rice (Oryza sativa) herbicides in barnyardgrass (Echinochloa crus-galli). J. Agric. Food Chem. 2005, 53, 4064–4068. [Google Scholar] [CrossRef]
- Kim, J.S.; Oh, J.I.; Kim, T.J.; Pyon, J.Y.; Cho, K.Y. Physiological basis of differential phytotoxic activity between fenoxaprop-P-ethyl and cyhalofop-butyl-treated barnyardgrass. Weed Biol. Manag. 2005, 5, 39–45. [Google Scholar] [CrossRef]
- Yen, T.B.; Chang, S.T. Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresour. Technol. 2008, 99, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, C.L.; Zhang, D.J.; Shu, B.; Xiao, J.; Xia, R.X. Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under sand culture. Sci. Hortic. 2013, 162, 100–105. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
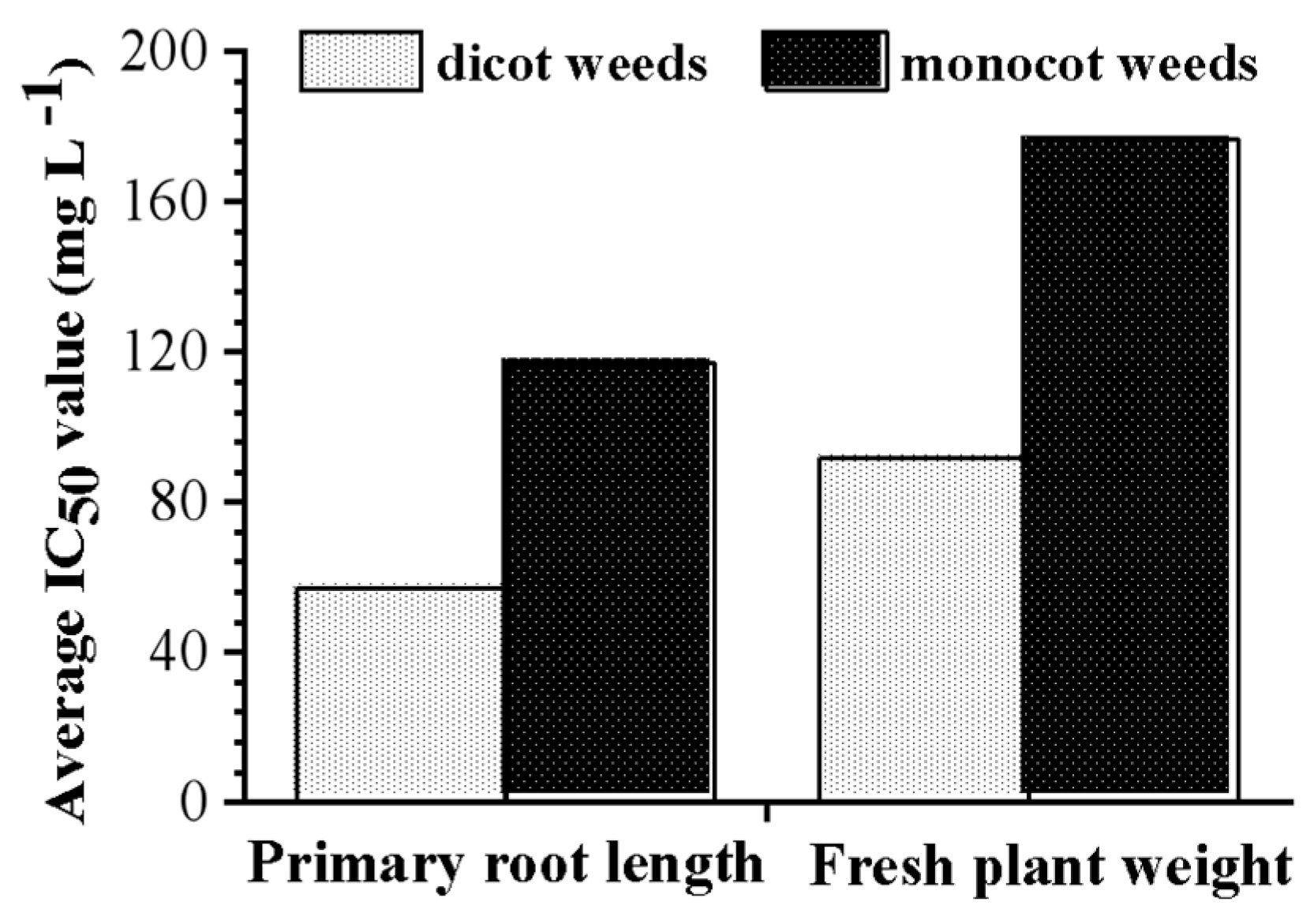

| Test Plants | Inhibition Rate (%) b | IC50 (mg L−1) c | |||||
|---|---|---|---|---|---|---|---|
| 50 mg L−1 | 100 mg L−1 | 250 mg L−1 | 500 mg L−1 | 1000 mg L−1 | |||
| Ageratum Conyzoides | L | 70.11 ± 0.02d | 85.78 ± 0.08c | 92.64 ± 0.14b | 100 ± 0.00a | 100 ± 0.00a | 28.91 |
| W | 48.99 ± 0.49c | 71.18 ± 0.85b | 73.34 ± 2.10b | 100 ± 0.00a | 100 ± 0.00a | 55.95 | |
| Celosia argentea | L | 52.17 ± 0.08c | 88.00 ± 0.02b | 100 ± 0.00a | 100 ± 0.00a | 100 ± 0.00a | 47.24 |
| W | 57.10 ± 0.10c | 69.11 ± 0.10b | 100 ± 0.00a | 100 ± 0.00a | 100 ± 0.00a | 50.07 | |
| Bidens pilosa | L | 51.94 ± 0.02d | 82.98 ± 0.02c | 92.67 ± 0.06b | 100 ± 0.00a | 100 ± 0.00a | 47.34 |
| W | 38.09 ± 0.06d | 55.68 ± 0.14c | 76.51 ± 0.18b | 100 ± 0.00a | 100 ± 0.00a | 79.56 | |
| Mikania micrantha | L | 40.59 ± 1.62d | 82.37 ± 0.71c | 94.27 ± 0.18b | 100 ± 0.00a | 100 ± 0.00a | 57.13 |
| W | 8.54 ± 2.86d | 63.11 ± 1.10c | 64.18 ± 0.81c | 87.04 ± 0.40b | 100 ± 0.00a | 123.72 | |
| Capsella bursa-pastoris | L | 51.48 ± 0.18d | 51.28 ± 0.06d | 70.87 ± 0.03c | 75.51 ± 0.10b | 92.85 ± 0.06a | 65.68 |
| W | 38.34 ± 1.14c | 41.64 ± 1.86c | 77.05 ± 0.57b | 78.25 ± 0.29b | 89.51 ± 0.19a | 98.25 | |
| Echinochloa crusgalli | L | 31.51 ± 0.11e | 58.21 ± 0.09d | 74.50 ± 0.04c | 86.16 ± 0.06b | 90.91 ± 0.03a | 88.77 |
| W | 27.08 ± 0.07d | 50.97 ± 0.06c | 68.16 ± 0.41b | 68.86 ± 0.26ab | 69.46 ± 0.47a | 136.03 | |
| Amaranthus retroflexus | L | 31.82 ± 0.10e | 52.53 ± 0.04d | 68.66 ± 0.03c | 96.26 ± 0.04b | 100 ± 0.00a | 95.29 |
| W | 21.21 ± 0.26e | 42.17 ± 0.10d | 64.96 ± 0.10c | 74.74 ± 0.10b | 100 ± 0.00a | 142.10 | |
| Alopecurus aequalis | L | 20.04 ± 0.34e | 45.74 ± 0.25d | 60.79 ± 0.11c | 76.62 ± 0.12b | 95.77 ± 0.01a | 145.23 |
| W | 24.09 ± 1.02d | 25.97 ± 0.63d | 56.90 ± 0.69c | 71.52 ± 0.18b | 76.46 ± 0.22a | 217.71 | |
| Oryza sativa | L | 13.20 ± 0.12d | 21.34 ± 0.04c | 38.58 ± 0.10b | 38.67 ± 0.12b | 54.13 ± 0.04a | 779.44 |
| W | 0.20 ± 0.04b | 0.18 ± 0.03b | 1.27 ± 0.90b | 4.70 ± 0.06a | 4.58 ± 0.33a | >1000 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Shi, M.; Tian, Y.; Zhao, P.; Niu, Y.; Liao, M. Dirhamnolipid Produced by the Pathogenic Fungus Colletotrichum gloeosporioides BWH-1 and Its Herbicidal Activity. Molecules 2019, 24, 2969. https://doi.org/10.3390/molecules24162969
Xu Z, Shi M, Tian Y, Zhao P, Niu Y, Liao M. Dirhamnolipid Produced by the Pathogenic Fungus Colletotrichum gloeosporioides BWH-1 and Its Herbicidal Activity. Molecules. 2019; 24(16):2969. https://doi.org/10.3390/molecules24162969
Chicago/Turabian StyleXu, Zhaolin, Mengying Shi, Yongqing Tian, Pengfei Zhao, Yifang Niu, and Meide Liao. 2019. "Dirhamnolipid Produced by the Pathogenic Fungus Colletotrichum gloeosporioides BWH-1 and Its Herbicidal Activity" Molecules 24, no. 16: 2969. https://doi.org/10.3390/molecules24162969
APA StyleXu, Z., Shi, M., Tian, Y., Zhao, P., Niu, Y., & Liao, M. (2019). Dirhamnolipid Produced by the Pathogenic Fungus Colletotrichum gloeosporioides BWH-1 and Its Herbicidal Activity. Molecules, 24(16), 2969. https://doi.org/10.3390/molecules24162969