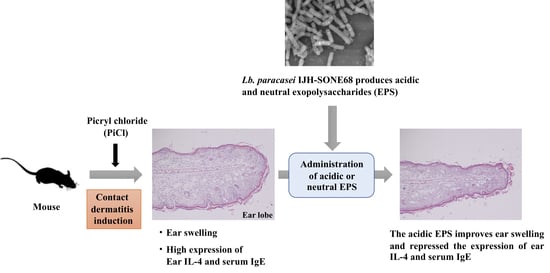
Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Improves the Picryl Chloride-Induced Contact Dermatitis
Abstract
1. Introduction
2. Results
2.1. Preventive Effect of IJH-SONE68-Derived EPS Observed on Delayed-Type Allergy Mouse Model
2.2. Deference in the Expression Level of Inflammatory Cytokines
2.3. Relation Between the Serum Immunoglobulin E (IgE) Level and Ear Inflammation
2.4. Histological Changes in Auricula
2.5. Recovery from the Delayed-Type Allergy by Direct Application of the IJH-SONE68-Derived EPS to the Mouse Ear
3. Discussion
4. Materials and Methods
4.1. Media and Growth Conditions
4.2. Purification of EPSs
4.3. Experimental Animals
4.4. Evaluation of Anti-Allergic Inflammatory Activity on a PiCl-Induced Delayed-Type Allergy Mouse Model
4.5. RNA Extraction and qRT-PCR Analysis
4.6. Measurement of Serum IgE Level
4.7. Histological Analysis
4.8. Scanning Electron Microscopy (SEM) Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | exopolysaccharide |
| GlcNAc | N-acetylglucosamine |
| HE | hematoxylin-eosin |
| IFN | interferon |
| Ig | immunoglobulin |
| IL | interleukin |
| LAB | lactic acid bacterium |
| Lb. | Lactobacillus |
| MRS | de Man, Rogosa, and Sharpe |
| NMR | nuclear magnetic resonance |
| PBS | phosphate-buffered saline |
| PiCl | picryl chloride |
| qRT-PCR | quantitative reverse transcription PCR |
| SDM | semi-defined medium |
| SEM | scanning electron microscopy |
| SPF | specific pathogen free |
| TCA | trichloroacetic acid |
| Th | T helper |
| TLR | toll-like receptor |
References
- Sanders, M.E. Probiotics: Definition, source, selection, and uses. Clin. Infect. Dis. 2008, 46, S58–S61. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pang, H.; Zhang, H.; Cai, Y. Biodiversity of lactic acid bacteria. In Bacteria Fundamentals and Practice; Zhang, H., Cai, Y., Eds.; Springer: Heidelberg, Germany, 2014; pp. 103–203. [Google Scholar]
- Cotter, P.D.; Ross, P.R.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies; William Heinemann: London, UK, 1907. [Google Scholar]
- Meydani, S.N.; Ha, W.K. Immunologic effects of yogurt. Am. J. Clin. Nutr. 2000, 71, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Heyman, M. Effect of lactic acid bacteria on diarrheal diseases. J. Am. Coll. Nutr. 2000, 19, 137S–146S. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.L.; Stevenson, L.M.; Gill, H.S. Anti-allergy properties of fermented foods: An important immunoregulatory mechanism of lactic acid bacteria? Int. Immunopharmacol. 2001, 1, 891–901. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Nomura, K.; Oku, H.; Sugiyama, M. Improvement of constipation and liver function by plant-derived lactic acid bacteria: A double-blind, randomized trial. Nutrition 2010, 26, 367–374. [Google Scholar] [CrossRef]
- Zhao, X.; Higashikawa, F.; Noda, M.; Kawamura, Y.; Matoba, Y.; Kumagai, T.; Sugiyama, M. The obesity and fatty liver are reduced by plant-derived Pediococcus pentosaceus LP28 in high fat diet-induced obese mice. PLoS ONE 2012, 7, e30696. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Danshiitsoodol, N.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Antiobesity effect of Pediococcus pentosaceus LP28 on overweight subjects: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2016, 70, 582–587. [Google Scholar] [CrossRef]
- Noda, M.; Sugimoto, S.; Hayashi, I.; Danshiitsoodol, N.; Fukamachi, M.; Sugiyama, M. A novel structure of exopolysaccharide produced by a plant-derived lactic acid bacterium Lactobacillus paracasei IJH-SONE68. J. Biochem. 2018, 164, 87–92. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Wong, G.; Earle, C.A.; Xia, W. Interaction of low molecular weight hyaluronan with CD44 and toll-like receptors promotes the actin filament-associated protein 110-actin binding and MyD88-NFκB signaling leading to proinflammatory cytokine/chemokine production and breast tumor invasion. Cytoskeleton 2011, 68, 671–693. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in tissue injury and repair. Annu. Rev. Cell. Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic acid in inflammation and tissue regeneration. Wounds 2010, 28, 78–88. [Google Scholar]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, N.; Sakai, S.; Yamaguchi, Y.; Takenaka, H. Inhibitory effects of microalgae on the activation of hyaluronidase. J. Appl. Phycol. 2001, 13, 489–492. [Google Scholar] [CrossRef]
- Maeda, Y.; Yamamoto, M.; Masui, T.; Sugiyama, K.; Yokota, M.; Nakagomi, K.; Tanaka, H.; Takahashi, T.; Kobayashi, E. Inhibitory effect of tea extracts on hyaluronidase. Shokuhin Eiseigaku Zashi 1990, 31, 233–237. [Google Scholar] [CrossRef]
- Raffels, S.; Forney, J.E. The role of the wax of the tubercle bacillus in establishing delayed hypersensitivity; hypersensitivity to a simple chemical substance, picryl chloride. J. Exp. Med. 1948, 88, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, M.; Majewska-Szczepanik, M.; Wong, F.S.; Kowalczyk, P.; Pasare, C.; Wen, L. Regulation of contact sensitivity in non-obese diabetic (NOD) mice by innate immunity. Contact Dermatitis. 2018, 79, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Satou, N.; Ishihara, K.; Hiratsuka, M.; Tanaka, H.; Endo, Y.; Saito, S.; Iwakura, Y.; Leonard, W.J.; Hirasawa, N. Induction of thymic stromal lymphopoietin production by xylene and exacerbation of picryl chloride-induced allergic inflammation in mice. Int. Arch. Allergy Immunol. 2012, 157, 194–201. [Google Scholar] [CrossRef]
- Okubo, K.; Kurono, Y.; Ichimura, K.; Enomoto, T.; Okamoto, Y.; Kawauchi, H.; Suzaki, H.; Fujieda, S.; Masuyama, K.; Japanese Society of Allergology. Japanese guidelines for allergic rhinitis 2017. Allergol. Int. 2017, 66, 205–219. [Google Scholar] [CrossRef]
- Arai, S.; Iwabuchi, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Hachimura, S. Orally administered heat-killed Lactobacillus paracasei MCC1849 enhances antigen-specific IgA secretion and induces follicular helper T cells in mice. PLoS ONE 2018, 13, e0199018. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.C.; Hsu, C.H. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr. Allergy Immunol. 2005, 16, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, S.; Okamoto, Y.; Okawa, T.; Hisamitsu, M.; Chazono, H.; Kobayashi, K.; Sakurai, D.; Horiguchi, S.; Hanazawa, T. Effects of daily intake of Lactobacillus paracasei strain KW3110 on Japanese cedar pollinosis. Allergy Asthma Proc. 2009, 30, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Cruse, J.M.; Lewis, R.E. Atlas of Immunology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Saulnier, M.; Huang, S.; Aguet, M.; Ryffel, B. Role of interferon-gamma in contact allergy assessed in interferon-gamma receptor-deficient mice. Toxicology 1995, 102, 301–312. [Google Scholar] [CrossRef]
- Reeve, V.E.; Bosnic, M.; Nishimura, N. Interferon-gamma is involved in photoimmunoprotection by UVA (320–400 nm) radiation in mice. J. Investig. Dermatol. 1999, 112, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S.; Satoskar, A.R.; Bluethmann, H.; David, J.R.; McEwen, B.S. Stress-induced enhancement of skin immune function: A role for gamma interferon. Proc. Natl. Acad. Sci. USA 2000, 97, 2846–2851. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Komiyama, Y.; Narumi, S.; Sudo, K.; Horai, R.; Tagawa, Y.; Sekikawa, K.; Matsushima, K.; Asano, M.; Iwakura, Y. IL-1-induced tumor necrosis factor-alpha elicits inflammatory cell infiltration in the skin by inducing IFN-gamma-inducible protein 10 in the elicitation phase of the contact allergy response. Int. Immunol. 2003, 15, 251–260. [Google Scholar] [CrossRef][Green Version]
- Yokozeki, H.; Ghoreishi, M.; Takagawa, S.; Takayama, K.; Satoh, T.; Katayama, I.; Takeda, K.; Akira, S.; Nishioka, K. Signal transducer and activator of transcription 6 is essential in the induction of contact allergy. J. Exp. Med. 2000, 19, 995–1004. [Google Scholar] [CrossRef]
- Thepen, T.; Langeveld-Wildschut, E.G.; Bihari, I.C.; van Wichen, D.F.; van Reijsen, F.C.; Mudde, G.C.; Bruijnzeel-Koomen, C.A. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1 response in situ: An immunocytochemical study. J. Allergy Clin. Immunol. 1996, 97, 828–837. [Google Scholar] [CrossRef]
- Matsushita, A.; Seike, M.; Hagiwara, T.; Sato, A.; Ohtsu, H. Close relationship between T helper (Th)17 and Th2 response in murine allergic contact dermatitis. Clin. Exp. Dermatol. 2014, 39, 924–931. [Google Scholar] [CrossRef]
- Liu, F.T.; Goodarzi, H.; Chen, H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011, 41, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Sato, A.; Goto, A.; Nakamura, M.; Ogawa, M.; Chiba, Y.; Hemmi, J.; Kano, H.; Takeda, K.; Okumura, K.; et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2016, 99, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Tohno, M.; Shimizu, T.; Ueda, W.; Anzawa, D.H.; Aso, H.; Nishimura, J.; Kawai, Y.; Saito, Y.; Saito, T.; Kitazawa, H. Molecular cloning of porcine RP105/MD-1 involved in recognition of extracellular phosphopolysaccharides from Lactococcus lactis ssp. cremoris. Mol. Immunol. 2007, 44, 2566–2577. [Google Scholar] [CrossRef]
- Mothes, N.; Heinzkill, M.; Drachenberg, K.J.; Sperr, W.R.; Krauth, M.T.; Majlesi, Y.; Semper, H.; Valent, P.; Niederberger, V.; Kraft, D.; et al. Allergen-specific immunotherapy with a monophosphoryl lipid A-adjuvanted vaccine: Reduced seasonally boosted immunoglobulin E production and inhibition of basophil histamine release by therapy-induced blocking antibodies. Clin. Exp. Allergy 2003, 33, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.W.; Marshall, J.S.; Ulrich, J.T. A Th1-inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy vaccines. Int. Arch. Allergy Immunol. 2001, 126, 135–139. [Google Scholar] [CrossRef]
- Gotoh, Y.; Suzuki, S.; Amako, M.; Kitamura, S.; Toda, T. Effect of orally administered exopolysaccharides produced by Lactococcus lactis subsp. cremoris FC on a mouse model of dermatitis induced by repeated exposure to 2,4,6-trinitro-1-chlorobenzene. J. Funct. Foods 2017, 35, 43–50. [Google Scholar] [CrossRef]
- Kano, H.; Kita, J.; Makino, S.; Ikegami, S.; Itoh, H. Oral administration of Lactobacillus delbrueckii subspecies bulgaricus OLL1073R-1 suppresses inflammation by decreasing interleukin-6 responses in a murine model of atopic dermatitis. J. Dairy Sci. 2013, 96, 3525–3534. [Google Scholar] [CrossRef]
- Masuda, Y.; Takahashi, T.; Yoshida, K.; Nishitani, Y.; Mizuno, M.; Mizoguchi, H. Anti-allergic effect of lactic acid bacteria isolated from seed mash used for brewing sake is not dependent on the total IgE levels. J. Biosci. Bioeng. 2012, 114, 292–296. [Google Scholar] [CrossRef]
- Panthavee, W.; Noda, M.; Danshiitsoodol, N.; Kumagai, T.; Sugiyama, M. Characterization of exopolysaccharides produced by thermophilic lactic acid bacteria isolated from tropical fruits of Thailand. Biol. Pharm. Bull. 2017, 40, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Shiraga, M.; Kumagai, T.; Danshiitsoodol, N.; Sugiyama, M. Characterization of the SN35N strain-specific exopolysaccharide encoded in the whole circular genome of a plant-derived Lactobacillus plantarum. Biol. Pharm. Bull. 2018, 41, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Activation of hyaluronidase by metallic salts and compound 48/80, and inhibitory effect of anti-allergic agents on hyaluronidase. Chem. Pharm. Bull. 1985, 33, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kuriyama, M.; Kosuge, E.; Ishihara, K.; Ito, K. Effects of saiboku-to (TJ-96) on the production of platelet-activating factor in human neutrophils. Ann. N. Y. Acad. Sci. 1993, 685, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; Goa, K.L.; Fitton, A.; Sorkin, E.M. Ketotifen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs 1990, 40, 412–448. [Google Scholar] [CrossRef]
- Schoch, C. Effects of ketotifen 0.025% and lodoxamide 0.1% on eosinophil infiltration into the guinea pig conjunctiva in a model of allergic conjunctivitis. J. Ocul. Pharmacol. Ther. 2003, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ruas-Madiedo, P.; Gueimonde, M.; Margolles, A.; de los Reyes-Gavilán, C.G.; Salminen, S. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J. Food Prot. 2006, 69, 2011–2015. [Google Scholar] [CrossRef]
- Vinderola, G.; Perdigón, G.; Duarte, J.; Farnworth, E.; Matar, C. Effect of the oral administration of the exopolysaccharide produce by Lactobacillus kefiranofaciens on the gut mucosal immunity. Cytokine 2006, 36, 254–260. [Google Scholar] [CrossRef]
- Furukawa, N.; Matsuoka, A.; Takahashi, T.; Yamanaka, Y. Anti-metastatic effect of kefir grain components on Lewis lung carcinoma and highly metastatic B16 melanoma in mice. J. Agric. Sci. Tokyo Univ. Agric. 2000, 45, 62–70. [Google Scholar]
- Maeda, H.; Zhu, X.; Omura, K.; Suzuki, S.; Kitamura, S. Effects of an exopolysaccharide (kefiran) on lipids, blood pressure, blood glucose, and constipation. Biofactors 2004, 22, 197–200. [Google Scholar] [CrossRef]
- Sengül, N.; Işık, S.; Aslım, B.; Uçar, G.; Demirbağ, A.E. The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig. Dis. Sci. 2011, 56, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, S.A.; Roberts, R.F. Development of a growth medium suitable for exopolysaccharide production by Lactobacillus delbrueckii ssp. bulgaricus RR. Int. J. Food Microbiol. 1998, 40, 87–92. [Google Scholar] [CrossRef]
- Kets, E.P.W.; Galinski, E.A.; de Bont, J.A.M. Carnitine: A novel compatible solute in Lactobacillus plantarum. Arch. Microbiol. 1994, 162, 243–248. [Google Scholar] [CrossRef]
- Yasutake, T.; Kumagai, T.; Inoue, A.; Kobayashi, K.; Noda, M.; Orikawa, A.; Matoba, Y.; Sugiyama, M. Characterization of the LP28 strain-specific exopolysaccharide biosynthetic gene cluster found in the whole circular genome of Pediococcus pentosaceus. Biochem. Biophys. Rep. 2016, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, M.; Sultana, N.; Hayashi, I.; Fukamachi, M.; Sugiyama, M. Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Improves the Picryl Chloride-Induced Contact Dermatitis. Molecules 2019, 24, 2970. https://doi.org/10.3390/molecules24162970
Noda M, Sultana N, Hayashi I, Fukamachi M, Sugiyama M. Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Improves the Picryl Chloride-Induced Contact Dermatitis. Molecules. 2019; 24(16):2970. https://doi.org/10.3390/molecules24162970
Chicago/Turabian StyleNoda, Masafumi, Nasrin Sultana, Ikue Hayashi, Mitsuhiro Fukamachi, and Masanori Sugiyama. 2019. "Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Improves the Picryl Chloride-Induced Contact Dermatitis" Molecules 24, no. 16: 2970. https://doi.org/10.3390/molecules24162970
APA StyleNoda, M., Sultana, N., Hayashi, I., Fukamachi, M., & Sugiyama, M. (2019). Exopolysaccharide Produced by Lactobacillus paracasei IJH-SONE68 Prevents and Improves the Picryl Chloride-Induced Contact Dermatitis. Molecules, 24(16), 2970. https://doi.org/10.3390/molecules24162970