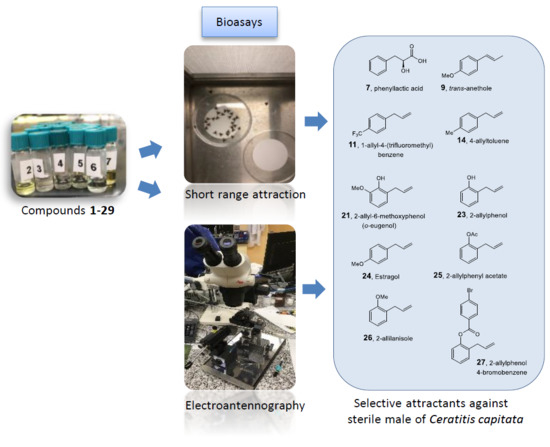
Laboratory Evaluation of Natural and Synthetic Aromatic Compounds as Potential Attractants for Male Mediterranean fruit Fly, Ceratitis capitata †
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Natural and Synthetic Compounds
Synthesis of 2-Allylphenol Derivatives 25–29
3.3. Insects
3.4. Short-Range Bioassays
3.5. Electroantennography (EAG) Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kendra, P.E.; Roda, A.L.; Montgomery, W.S.; Schnell, E.Q.; Niogret, J.; Epsky, N.D.; Heath, R.R. Gas chromatography for detection of citrus infestation by fruit fly larvae (Diptera: Tephritidae). Postharvest Biol. Technol. 2011, 59, 143–149. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Heath, R.R.; Papadopoulos, N.T.; Epsky, N.D.; Hendrichs, J. Field evaluation of Mediterranean fruit fly (Diptera: Tephritidae) female selective attractants for use in monitoring programs. J. Chem. Ecol. 1999, 92, 583–589. [Google Scholar] [CrossRef]
- Midgarden, D.; Ovalle, O.; Epsky, N.D.; Puche, H.; Kendra, P.E.; Rendon, P.; Heath, R.R. Capture of Mediterranean fruit flies (Diptera: Tephritidae) in dry traps baited with food-based attractant and Jackson traps baited with trimedlure during sterile male release in Guatemala. J. Econ. Entomol. 2004, 97, 2137–2143. [Google Scholar] [CrossRef]
- Puche, H.; Midgarden, D.G.; Ovalle, O.; Kendra, P.E.; Epsky, N.D.; Rendon, P.; Heath, R.R. Effect of elevation and host availability on distribution of sterile and wild Mediterranean fruit flies (Diptera: Tephritidae). Fla. Entomol. 2005, 88, 83–90. [Google Scholar] [CrossRef]
- Papadopoulos, N.T. Fruit fly invasion: Historical, biological, economical aspects and management. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies, 1st ed.; Shelly, T., Epsky, N.D., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer Publishing: New York, NY, USA, 2014; pp. 219–252. [Google Scholar]
- Szyniszewska, A.M.; Leppla, N.C.; Huang, Z.; Tatem, A.J. Analysis of seasonal risk for importation of the Mediterranean Fruit Fly, Ceratitis capitata (Diptera:Tephritidae), via air passenger traffic arriving in Florida and California. J. Econ. Entomol. 2016, 109, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- FAO-IAEA. Eradication of the Mediterranean Fruit Fly from the Dominican Republic Using Nuclear Technology. Available online: http://www.fao.org/3/ca0148en/CA0148EN.pdf (accessed on 13 May 2019).
- Yiridoe, E.K.; Bonti-Ankomah, S.; Ralph, C.; Martin, R.C. Comparison of consumer perceptions and preference toward organic versus conventionally produced foods: A review and update of the literature. Renew. Agric. Food Syst. 2005, 20, 193–205. [Google Scholar] [CrossRef]
- Johnson, J.A.; Walse, S.S.; Gerik, J.S. Status of alternatives for methyl bromide in the United States. Outlook Pest Manag. 2012, 23, 53–58. [Google Scholar] [CrossRef]
- Shelly, T.E.; Whittier, T.S.; Villalobos, E.M. Trimedlure affects mating success and mate attraction in male Mediterranean fruit flies. Entomol. Exp. Appl. 1996, 78, 181–185. [Google Scholar] [CrossRef]
- Jang, E.B.; Raw, A.S.; Carvalho, L.A. Field attraction of Mediterranean fruit fly, Ceratitis capitata (Wiedemann) to synthetic stereoselective enantiomers of the ceralure B1 isomer. J. Chem. Ecol. 2001, 27, 235–242. [Google Scholar] [CrossRef]
- Quilici, S.; Atiama-Nurbel, T.; Brevault, T. Plant odors as fruit fly attractants. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies, 1st ed.; Shelly, T., Epsky, N.D., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer Publishing: New York, NY, USA, 2014; pp. 119–144. [Google Scholar]
- Simanton, W.A. Studies of Mediterranean fruit fly lures in Florida. J. Econ. Entomol. 1958, 51, 679–682. [Google Scholar] [CrossRef]
- Niogret, J.; Montgomery, W.S.; Kendra, P.E.; Heath, R.R.; Epsky, N.D. Attraction and electroantennogram responses of Male Mediterranean fruit fly to volatile chemicals from Persea, Litchi and Ficus wood. J. Chem. Ecol. 2011, 37, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Niogret, J.; Gill, M.A.; Espinoza, H.R.; Kendra, P.E.; Epsky, N.D. Attraction and electroantennogram responses of male Mediterranean fruit fly (Diptera: Tephritidae) to six plant essential oils. J. Entomol. Zool. Stud. 2017, 5, 958–964. [Google Scholar]
- Epsky, N.F.; Niogret, J. Short range attraction of Ceratitis capitata (Diptera: Tephritidae) sterile males to six commercially available plant essential oils. Nat. Volatiles Essent. Oils 2017, 4, 1–7. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: West Sussex, UK, 2002. [Google Scholar]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Amaryllidaceae alkaloids: Absolute configuration and biological activity. Chirality 2017, 29, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Mubaiwa, B.; Mabank, T.; Karakoyun, C.; Cimmino, A.; Van Otterlo, W.A.L.; Green, I.R.; Evidente, A. Alkaloids isolated from indigenous South African Amaryllidaceae: Crinum buphanoides (Welw. ex Baker), Crinum graminicola (I. Verd.), Cyrtanthus mackenii (Hook. f) and Brunsvigia grandiflora (Lindl). S. Afr. J. Bot. 2018, 118, 88–191. [Google Scholar] [CrossRef]
- Masi, M.; Mubaiwa, B.; Cimmino, A.; Van Otterlo, W.A.L.; Green, I.R.; Evidente, A. First isolation of acetovanillone and piceol from Crinum buphanoides and Crinum graminicola (I. Verd.) Amaryllidaceae. S. Afr. J. Bot. 2018, 114, 37–39. [Google Scholar] [CrossRef]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Cimmino, A.; Evidente, A. Isolation of phytotoxic phenols and characterization of a new 5-hydroxymethyl-2-isopropoxyphenol from Dothiorella vidmadera, a causal agent of grapevine trunk disease. J. Agric. Food Chem. 2018, 66, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Pescitelli, G.; Cimmino, A.; Clement, S.; Peacock, B.; Evidente, A. Phytotoxic activity against Bromus tectorum for secondary metabolites of a seed-pathogenic Fusarium strain belonging to the F. tricinctum species complex. Nat. Prod. Res. 2017, 31, 2768–2777. [Google Scholar] [CrossRef]
- Gresser, M.J.; Wales, S.M.; Keller, P.A. The attempted stereoselective synthesis of chiral 2, 2′-biindoline. Tetrahedron 2010, 66, 6965–6976. [Google Scholar] [CrossRef]
- Denmark, S.E.; Werner, N.S. Cross-coupling of aromatic bromides with allylic silanolate salts. J. Am. Chem. Soc. 2008, 130, 16382–16393. [Google Scholar] [CrossRef]
- Lei, X.; Jalla, A.; Shama, M.A.A.; Stafford, J.M.; Cao, B. Chromatography-free and eco-friendly synthesis of aryl tosylates and mesylates. Synthesis 2015, 47, 2578–2585. [Google Scholar] [CrossRef]
- Shelly, T.E. Fruit fly alphabets. In Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies, 1st ed.; Shelly, T., Epsky, N.D., Jang, E.B., Reyes-Flores, J., Vargas, R., Eds.; Springer Publishing: New York, NY, USA, 2014; pp. 3–11. [Google Scholar]
- Baker, E.A.; Hayes, A.L.; Butler, R.C. Physicochemical properties of agrochemicals: Their effects on foliar penetration. Pest Manag. Sci. 1992, 34, 167–182. [Google Scholar] [CrossRef]
- Jewess, P.J.; Chamberlain, K.; Boogaard, A.B.; Devonshire, A.L.; Khambay, B.P. Insecticidal 2-hydroxy-3-alkyl-1,4-naphthoquinones: Correlation of inhibition of ubiquinol cytochrome c oxidoreductase (complex III) with insecticidal activity. Pest Manag. Sci. 2002, 58, 243–247. [Google Scholar] [CrossRef] [PubMed]
- ChemDraw Ultra version 18.0. Available online: https://www.perkinelmer.com/category/chemdraw (accessed on 11 May 2019).
- ChemAxon version 19.10. Available online: https://chemaxon.com/products/marvin (accessed on 11 May 2019).
- Lavermicocca, P.; Valerio, F.; Visconti, A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl. Environ. Microbiol. 2003, 69, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Sell, C. Chemistry of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications, 2nd ed.; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 121–150. [Google Scholar]
- King, J.R.; Knight, R.J. Occurrence and assay of estragole in the leaves of various avocado cultivars. J. Agric. Food Chem. 1987, 35, 842–844. [Google Scholar] [CrossRef]
- Yauk, Y.K.; Chagne, D.; Tomes, S.; Matich, A.J.; Wang, M.Y.; Chen, X.; Maddumage, R.; Hunt, M.B.; Rowan, D.D.; Atkinson, R.G. The O-methyltransferase gene MdoOMT1 is required for biosynthesis of methylated phenylpropenes in ripe apple fruit. Plant J. 2015, 82, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Abbate, L.; Tusa, N.; Fatta Del Bosco, S.; Tonia Strano, T.; Renda, A.; Ruberto, G. Genetic improvement of Citrus fruits: New somatic hybrids from Citrus sinensis (L.) Osb. and Citrus limon (L.) Burm. F. Food Res. Int. 2012, 48, 284–290. [Google Scholar] [CrossRef]
- Kendra, P.E.; Epsky, N.D.; Montgomery, W.S.; Heath, R.R. Response of Anastrepha suspensa (Diptera: Tephritidae) to terminal diamines in a food-based synthetic attractant. Environ. Entomol. 2008, 37, 1119–1125. [Google Scholar] [CrossRef]
- Kendra, P.E.; Vázquez, A.; Epsky, N.D.; Heath, R.R. Ammonia and carbon dioxide: Quantitation and electroantennogram responses of Caribbean fruit fly, Anastrepha suspensa (Diptera: Tephritidae). Environ. Entomol. 2005, 34, 569–575. [Google Scholar] [CrossRef]
- Franz, G. Genetic sexing strains amenable to large scale rearing as required for the sterile insect technique. In the Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 427–452. [Google Scholar]
- Dowell, R.V.; Siddiqui, I.A.; Meyer, F.; Spaugy, E.L. Mediterranean fruit fly preventative release programme in southern California. In Area-Wide Control of Fruit Flies and Other Insect Pests; Tan, K.H., Ed.; Penerbit Universiti Sains Malaysia: Pulau Pinang, Malaysia, 2000; pp. 369–375. [Google Scholar]
- McInnis, D.O.; Warthen, J.D., Jr. Mediterranean fruit fly (Diptera: Tephritidae): Laboratory bioassay for attraction of males to leaf or stem substances from Ficus and Litchi. J. Econ. Entomol. 1988, 81, 1637–1640. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Mateo, D.M.; Puche, H.; Epsky, N.D.; Heath, R.R. Effect of age on EAG response and attraction of female Anastrepha suspensa (Diptera: Tephritidae) to ammonia and carbon dioxide. Environ. Entomol. 2005, 34, 584–590. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Pruett, G.E.; Mayfield, A.E., III; MacKenzie, M.; Deyrup, M.A.; Bauchan, G.R.; Ploetz, R.C.; Epsky, N.D. North American Lauraceae: Terpenoid emissions, relative attraction, and boring preferences of redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2014, 9, e102086. [Google Scholar] [CrossRef] [PubMed]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Schnell, E.Q.; Deyrup, M.A.; Epsky, N.D. Evaluation of seven essential oils identifies cubeb oil as most effective attractant for detection of Xyleborus glabratus. J. Pest Sci. 2014, 87, 681–689. [Google Scholar] [CrossRef]
- Kendra, P.E.; Owens, D.; Montgomery, W.S.; Narvaez, T.I.; Bauchan, G.R.; Schnell, E.Q.; Tabanca, N.; Carrillo, D. α-Copaene is an attractant, synergistic with quercivorol, for improved detection of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae). PLoS ONE 2017, 12, e179416. [Google Scholar]
- SAS Institute. SAS System for Windows Release 9.4; SAS Institute: Cary, NC, USA, 2016. [Google Scholar]
- Abba, S.; Oluskin, J.; Dare, S.S.; Mohammed, Y.G.; Ajayi, A.M.; Okpanachi, A.O. Comparison of the attraction index of male and female Drosophila melanogaster to varying odorant substances. Curr. Research J. Biol. Sci. 2012, 4, 655–659. [Google Scholar]
- Box, G.E.P.; Hunter, W.G.; Hunter, J.S. Statistics for Experimenters. An Introduction to Design, Data Analysis, and Model Building; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |


| Compound | Log P Values £ | Number Responding |
|---|---|---|
| phenyllactic acid (7) | 1.16 | 32.6 ± 12.4 * |
| estragole (methyl chavicol, 4-allylanisole) (24) | 2.96 | 21.0 ± 9.0 * |
| 2-allyl-6-methoxyphenol (o-eugenol) (21) | 2.57 | 20.2 ± 4.3 * |
| 2-allylphenol (23) | 2.7 | 16.0 ± 3.8 * |
| 2-allylphenyl acetate (25) | 2.67 | 10.0 ± 8.7 |
| 4-allyltoluene (14) | 3.57 | 8.8 ± 8.3 |
| trans-anethole (9) | 2.91 | 6.0 ± 2.3 * |
| 2-allylphenyl 4-bromobenzoate (27) | 5.4 | 5.0 ± 2.3 * |
| 1-allyl-4-(trifluoromethyl)benzene (11) | 4.01 | 3.2 ± 1.3 * |
| 2-methoxy 4-propylphenol (19) | 2.84 | 3.0 ± 3.3 |
| 2-allylphenyl methanesulfonate (28) | 1.99 | 3.0 ± 0.7 |
| 4-hydroxybenzaldehyde (5) | 1.39 | 2.8 ± 2.6 |
| 2-allylphenyl 5-azidopentanoate (29) | 3.65 | 2.6 ± 1.3 |
| 1-allylbenzene (8) | 3.24 | 2.2 ± 1.1 |
| 4-allylphenol (chavicol) (12) | 2.7 | 2.2 ± 1.1 |
| tyrosol (3) | 1.35 | 2.0 ± 1.6 |
| resorcinol (4) | 1.26 | 2.0 ± 2.5 |
| 4-methylcatechol (6) | 1.74 | 1.6 ± 2.1 |
| 2-allyl-4,5-dimethoxyphenol (13) | 2.44 | 1.6 ± 0.5 * |
| allylbenzene (10) | 3.09 | 1.4 ± 0.9 |
| 4-allyl-1,2-dimethoxybenzene (= methyl eugenol) (20) | 2.83 | 1.4 ± 0.5 |
| 3-hydroxy acetophenone (16) | 0.96 | 1.2 ± 1.3 |
| 4-hydroxy-3-methoxymandelic acid (17) | 0.36 | 1.2 ± 1.3 |
| 2-allylanisole (26) | 2.96 | 1.2 ± 0.8 |
| 4-allyl-2,6-trimethoxybenzene (15) | 2.71 | 1.0 ± 0.7 |
| eugenol (4-allyl-2-methoxyphenol) (18) | 2.52 | 0.8 ± 0.8 |
| piceol (1) | 0.96 | 0.6 ± 0.9 |
| 4-allyl-2,6-dimethoxyphenol (22) | 2.44 | 0.6 ± 0.9 |
| acetovanillone (2) | 0.83 | 0.4 ± 0.5 |
| Compounds Tested | Number Responding to Each Compound in Bioassay | ||||||
|---|---|---|---|---|---|---|---|
| 7 | 21 | 23 | 24 | t | df | p | |
| 7 versus 21 | 2.1 ± 2.3 | 17.3 ± 4.4 | 10.75 | 16.6 | <0.0001 | ||
| 7 versus 23 | 3.2 ± 5.7 | 14.2 ± 4.3 | 5.3 | 22 | <0.0001 | ||
| 7 versus 24 | 2.4 ± 2.0 | 16.1 ± 5.6 | 7.97 | 13.7 | < 0.0001 | ||
| 21 versus 23 | 17.8 ± 6.0 | 7.8 ± 2.8 | 4.54 | 15.6 | 0.0001 | ||
| 21 versus 24 | 17.1 ± 5.7 | 7.8 ± 5.4 | 4.14 | 22 | 0.0004 | ||
| 23 versus 24 | 10.3 ± 3.2 | 10.4 ± 4.9 | 0.05 | 22 | 0.9809 | ||
© 2019 This work was produced by US government employees and is in the public domain in the US. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabanca, N.; Masi, M.; Epsky, N.D.; Nocera, P.; Cimmino, A.; Kendra, P.E.; Niogret, J.; Evidente, A. Laboratory Evaluation of Natural and Synthetic Aromatic Compounds as Potential Attractants for Male Mediterranean fruit Fly, Ceratitis capitata. Molecules 2019, 24, 2409. https://doi.org/10.3390/molecules24132409
Tabanca N, Masi M, Epsky ND, Nocera P, Cimmino A, Kendra PE, Niogret J, Evidente A. Laboratory Evaluation of Natural and Synthetic Aromatic Compounds as Potential Attractants for Male Mediterranean fruit Fly, Ceratitis capitata. Molecules. 2019; 24(13):2409. https://doi.org/10.3390/molecules24132409
Chicago/Turabian StyleTabanca, Nurhayat, Marco Masi, Nancy D. Epsky, Paola Nocera, Alessio Cimmino, Paul E. Kendra, Jerome Niogret, and Antonio Evidente. 2019. "Laboratory Evaluation of Natural and Synthetic Aromatic Compounds as Potential Attractants for Male Mediterranean fruit Fly, Ceratitis capitata" Molecules 24, no. 13: 2409. https://doi.org/10.3390/molecules24132409