Potent Vasodilator and Cellular Antioxidant Activity of Endemic Patagonian Calafate Berries (Berberis microphylla) with Nutraceutical Potential
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization, Identification and Relative Quantification of the Main Anthocyanins and Flavonols Extract Constituents
2.2. Comparative Studies between the Vascular Response Elicited by the Calafate Extract was Mimicked by the Main Calafate Extract Flavonoids; Effects in the Rat Arterial Mesenteric Bed Bioassay
2.3. Calafate Extract and Glycosylated Anthocyanins Antioxidant Properties
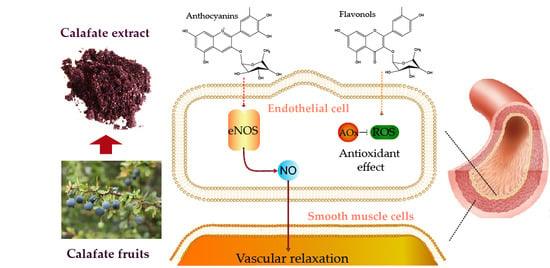
2.4. The Calafate Extract and Main Glycosylated Anthocyanins Elicit Endothelium and NO-Mediated Vasodilatations
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Calafate Berry Extract Preparation
4.3. Chemical Identification of the Extract by LC-MS
4.4. Quantification of the Principal Glycosylated Anthocyanins and Flavonols from the Calafate Extract
4.5. Vascular Reactivity Assays and Quantification of Vasodilator Responses
4.6. Denudation of the Endothelial Cell Layer of the Mesenteric Bed
4.7. eNOS Blockade by Structural L-Arginine Analogues Reduce the Extract-Induced Vasodilation
4.8. Determination of the Extract and Anthocyanins Cellular Antioxidant Activity (CAA) in Endothelial Cell Derived from the Mesenteric Bed
4.9. Antioxidant Activity Quantified by the DPPH-Scavenging Assay
4.10. NO Determinations and Quantification
4.11. Statistical Analysis
4.12. Chemical Compounds used in this Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romanucci, V.; D’Alonzo, D.; Guaragna, A.; Di Marino, C.; Davinelli, S.; Scapagnini, G.; Di Fabio, G.; Zarrelli, A.V. Bioactive Compounds of Aristotelia chilensis Stuntz and their Pharmacological Effects. Curr. Pharm. Biotechnol. 2016, 17, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J.S. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Dayoub, O.; Andriantsitohaina, R.; Clere, N.O. Pleiotropic beneficial effects of epigallocatechin gallate, quercetin and delphinidin on cardiovascular diseases associated with endothelial dysfunction. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 249–264. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of Action of Flavonoids as Anti-inflammatory Agents: A Review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2014, 22, 1132–1149. [Google Scholar] [CrossRef]
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C.H. First Web-Based Database on Total Phenolics and Oxygen Radical Absorbance Capacity (ORAC) of Fruits Produced and Consumed within the South Andes Region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Kennelly, E.J.; Simirgiotis, M. Anthocyanins and antioxidant capacities of six Chilean berries by HPLC–HR-ESI-ToF-MS. Food Chem. 2015, 176, 106–114. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and Antioxidant Activity of Calafate (Berberis microphylla) Fruits and Other Native Berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Ruiz, A.; Mardones, C.; Vergara, C.; Hermosín-Gutiérrez, I.; von Baer, D.; Hinrichsen, P.; Rodriguez, R.; Arribillaga, D.; Dominguez, E. Analysis of hydroxycinnamic acids derivatives in Calafate (Berberis microphylla G. Forst) berries by liquid chromatography with photodiode array and mass spectrometry detection. J. Chromatogr. A. 2013, 1281, 38–45. [Google Scholar] [CrossRef]
- Ruiz, A.; Zapata, M.; Sabando, C.; Bustamante, L.; von Baer, D.; Vergara, C.; Mardones, C. Flavonols, Alkaloids, and Antioxidant Capacity of Edible Wild Berberis Species from Patagonia. J. Agric. Food Chem. 2014, 62, 12407–12417. [Google Scholar] [CrossRef] [PubMed]
- Manosalva, L.; Mutis, A.; Urzúa, A.; Fajardo, V.; Quiroz, A. Antibacterial Activity of Alkaloid Fractions from Berberis microphylla G. Forst and Study of Synergism with Ampicillin and Cephalothin. Molecules 2016, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Vasquez, K.; Ovalle-Marin, A.; Fuentes, F.; Parra, C.; Quitral, V.; Jimenez, P.; Garcia-Diaz, D.F. Chilean Native Fruit Extracts Inhibit Inflammation Linked to the Pathogenic Interaction Between Adipocytes and Macrophages. J. Med. Food 2015, 18, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Vasquez, K.; Fuentes, F.; Ovalle-Marin, A.; Parra-Ruiz, C.; Zamora, O.; Pino, M.T.; Quitral, V.; Jimenez, P.; Garcia, L.; et al. Extracts of Chilean native fruits inhibit oxidative stress, inflammation and insulin-resistance linked to the pathogenic interaction between adipocytes and macrophages. J. Funct. Foods 2016, 27, 69–83. [Google Scholar] [CrossRef]
- Calfio, C.; Mateluna, C.; Huidobro-Toro, J.P. Patagonia Calafate (Berberis microphylla) berry extracts have a potent cellular antioxidant profile and elicits endothelium-dependent vascular relaxation. Basic Clin. Pharmacol. Toxicol. 2017, 121, 1–98. [Google Scholar]
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Padilla, E.; Ruiz, E.; Redondo, S.; Gordillo-Moscoso, A.; Slowing, K.; Tejerina, T. Relationship between vasodilation capacity and phenolic content of Spanish wines. Eur. J. Pharmacol. 2005, 517, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Aspects Med. 2010, 31, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Leung, S.W.S.; Yeung, D.K.Y.; Hu, L.H.; Chen, G.H.; Che, C.M.; Man, R.Y.K. Structure–activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry 2007, 68, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Auger, C.; Schini-Kerth, V. Activation of eNOS by Polyphenol-rich Products and Polyphenolic Compounds. Curr. Pharm. Des. 2014, 20, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- Schini-Kerth, V.B.; Auger, C.; Étienne-Selloum, N.; Chataigneau, T. Polyphenol-Induced Endothelium-Dependent Relaxations. Adv. Pharmacol. 2010, 60, 133–175. [Google Scholar] [CrossRef]
- Wan, H.; Liu, D.; Yu, X.; Sun, H.; Li, Y. A Caco-2 cell-based quantitative antioxidant activity assay for antioxidants. Food Chem. 2015, 175, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Structure−Activity Relationships of Flavonoids in the Cellular Antioxidant Activity Assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef]
- Gasparotto Junior, A.; dos Reis Piornedo, R.; Assreuy, J.; Da Silva-Santos, J.E. Nitric oxide and Kir6.1 potassium channel mediate isoquercitrin-induced endothelium-dependent and independent vasodilation in the mesenteric arterial bed of rats. Eur. J. Pharmacol. 2016, 788, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.L.E.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef]
- Hou, X.; Liu, Y.; Niu, L.; Cui, L.; Zhang, M. Enhancement of voltage-gated K+ channels and depression of voltage-gated Ca2+ channels are involved in quercetin-induced vasorelaxation in rat coronary artery. Planta Med. 2014, 80, 465–472. [Google Scholar] [CrossRef]
- Pérez-Vizcaíno, F.; Ibarra, M.; Cogolludo, A.L.; Duarte, J.; Zaragozá-Arnáez, F.; Moreno, L.; Lopez-Lopez, G.; Tamargo, J. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J. Pharmacol. Exp. Ther. 2002, 302, 66–72. [Google Scholar] [CrossRef]
- Maestro, A.; Terdoslavich, M.; Vanzo, A.; Kuku, A.; Tramer, F.; Nicolin, V.; Passamonti, S. Expression of bilitranslocase in the vascular endothelium and its function as a flavonoid transporter. Cardiovasc. Res. 2010, 85, 175–183. [Google Scholar] [CrossRef]
- Ziberna, L.; Lunder, M.; Tramer, F.; Drevenšek, G.; Passamonti, S. The endothelial plasma membrane transporter bilitranslocase mediates rat aortic vasodilation induced by anthocyanins. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 68–74. [Google Scholar] [CrossRef]
- Cunningham, P.; Afzal-Ahmed, I.; Naftalin, R.J. Docking Studies Show That D-Glucose and Quercetin Slide through the Transporter GLUT1. J. Biol. Chem. 2006, 281, 5797–5803. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Walle, U. The beta-D-glucoside and sodium-dependent glucose transporter 1 (SGLT1)-inhibitor phloridzin is transported by both SGLT1 and multidrug resistance-associated proteins 1/2. Drug Metab. Dispos. 2003, 31, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, M.; Tesse, A.; Martínez, M.C.; Rognan, D.; Arnal, J.-F.; Andriantsitohaina, R. Estrogen receptor alpha as a key target of red wine polyphenols action on the endothelium. PLoS ONE 2010, 5, e8554. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; McCarthy, D.; Burton-Freeman, B.M. Effect of Black Currant Anthocyanins on the Activation of Endothelial Nitric Oxide Synthase (eNOS) in Vitro in Human Endothelial Cells. J. Agric. Food Chem. 2011, 59, 8616–8624. [Google Scholar] [CrossRef] [PubMed]
- Donoso, M.V.; Faundez, H.; Rosa, G.; Fournier, A.; Edvinsson, L.; Huidobro-Toro, J.P. Pharmacological Characterization of the ET A Receptor in the Vascular Smooth Muscle Comparing its Analogous Distribution in the Rat Mesenteric Artery and in the Arterial Mesenteric Bed. Peptides 1996, 17, 1145–1153. [Google Scholar] [CrossRef]
- Figueroa, X.F.; Poblete, I.; Fernandez, R.; Pedemonte, C.; Cortes, V.; Huidobro-Toro, J.P. NO production and eNOS phosphorylation induced by epinephrine through the activation of -adrenoceptors. AJP Hear. Circ. Physiol. 2009, 297, H134–H143. [Google Scholar] [CrossRef] [PubMed]
- Poblete, I.M.; Orliac, M.L.; Briones, R.; Adler-Graschinsky, E.; Huidobro-Toro, J.P. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J. Physiol. 2005, 568, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Boric, M.P.; Figueroa, X.F.; Donoso, M.V.; Paredes, A.; Poblete, I.; Huidobro-Toro, J.P. Rise in endothelium-derived NO after stimulation of rat perivascular sympathetic mesenteric nerves. Am. J. Physiol. 1999, 277, H1027–H1035. [Google Scholar] [CrossRef] [PubMed]
- Donoso, M.V.; Hernández, F.; Villalón, T.; Acuña-Castillo, C.; Huidobro-Toro, J.P. Pharmacological dissection of the cellular mechanisms associated to the spontaneous and the mechanically stimulated ATP release by mesentery endothelial cells: Roles of thrombin and TRPV. Purinergic Signalling. 2018, 14, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (Calafate extract and flavonoids) are available from the authors. |





| Peak a | Retention Time (min) | Molecular Ion | Product Ions | Analyte | ||
|---|---|---|---|---|---|---|
| Anthocyanins Identified | ||||||
| 1 | 2.0 | 641.2 | 479 | 317 | Petunidin-O-hexoside-O-hexoside | |
| 2 | 2.8 | 627.1 | 464.9 | 303 | Delphinidin-O-hexoside-O-hexoside | |
| 3 | 3.1 | 611.2 | 449 | 303 | Delphinidin-rhamnosylhexoside | |
| 4 | 3.7 | 641.6 | 479 | 317.2 | Petunidin-O-hexoside-O-hexoside | |
| 4 | 3.9 | 595.4 | 449 | 286.9 | Cyanidin-O-rhamnosylhexoside | |
| 5 | 4.7 | 787.2 | 625.1 | 479.1 | 317.2 | Petunidin-O-rhamnosylhexoside-O-hexoside |
| 5 | 5.0 | 448.7 | 286 | Cyanidin-O-hexoside | ||
| 6 | 5.5 | 655.2 | 493 | 331.2 | Malvidin-O-hexoside-O-hexoside | |
| 6 | 6.0 | 462.4 | 300.1 | 269 | Peonidin-O-hexoside | |
| 6 | 6.4 | 655.4 | 493 | 331.1 | Malvidin-O-hexoside-O-hexoside | |
| 7 b | 6.9 | 465.0 | 303 | Delphinidin-O-hexoside | ||
| 8 b | 7.8 | 611.5 | 465 | 303 | Delphinidin-O-rhamnosylhexoside | |
| 9 b | 9.6 | 449.2 | 286.9 | Cyanidin-O-hexoside | ||
| 10 b | 11.0 | 595.6 | 448.9 | 286.9 | Cyanidin-O-rhamnosylhexoside | |
| 11 b | 12.3 | 479.0 | 317 | Petunidin-O-hexoside | ||
| 12 | 13.7 | 625.1 | 478.9 | 317 | Petunidin-O-coumaroyl-O-hexoside | |
| Petunidin-O-rhamnosylhexoside | ||||||
| 13 b | 15.8 | 463.7 | 301 | Peonidin-O-hexoside | ||
| 14 | 17.4 | 464.0 | 300.9 | Peonidin-O-hexoside | ||
| 15 b | 18.9 | 493.1 | 331.1 | Malvidin-O-hexoside | ||
| 16 | 19.7 | 639.5 | 493 | 331.1 | Malvidin-O-rhamnosylhexoside | |
| Flavonols Identified | ||||||
| 1 | 12.0 | 477.4 | 314.8 | Isorhamnetin-O-hexoside | ||
| 2 | 13.1 | 623.3 | 314.9 | Isorhamnetin-O-rutinoside | ||
| 3 | 14.9 | 479.5 | 316.8 | Myricetin-O-hexoside | ||
| 4 | 19.0 | 464.0 | 300.7 | Quercetin-O-hexoside | ||
| 5 b | 19.8 | 463.1 | 300.7 | Quercetin-O-hexoside | ||
| 6 b | 20.7 | 609.4 | 300.7 | Quercetin-O-rutinoside | ||
| 7 | 22.9 | 505.6 | 462.9 | 300.8 | Quercetin-O-acetyl hexoside | |
| 8 | 24.2 | 505.5 | 462.9 | 300.7 | Quercetin-O-acetyl hexoside | |
| 9 b | 27.2 | 447.1 | 300.7 | Quercetin-O-rhamnoside | ||
| 10 | 28.7 | 537.5 | 374.9 | Biapigenin | ||
| 11 | 29.6 | 537.2 | 374.8 | Biapigenin | ||
| 12 b | 30.2 | 623.4 | 314.9 | Isorhamnetin-O-rutinoside | ||
| 13 | 30.8 | 478.5 | 314.8 | Isorhamnetin-O-hexoside | ||
| Peak | Chemical Standards | Chemical Substituents | mg/g Extract |
|---|---|---|---|
| Main Anthocyanins | |||
| 7 | D3G, Delphinidin-3-glucoside | R1: OH, R2: OH, R3: OH, R4: Glucose | 4.04 ± 0.02 |
| 8 | D3R, Delphinidin-3-rutinoside | R1: OH, R2: OH, R3: OH, R4: Rutinose | 1.23 ± 0.02 |
| 9 | C3G, Cyanidin-3-glucoside | R1: OH, R2: OH, R4: Glucose | 0.92 ± 0.01 |
| 10 | C3R, Cyanidin-3-rutinoside | R1: OH, R2: OH, R4: Rutinose | 0.44 ± 0.02 |
| 11 | P3G, Petunidin-3-glucoside | R1: OH, R2: OH: R3: OCH3, R4: Glucose | 3.81 ± 0.03 |
| 13 | Po3G, Peonidin-3-glucoside | R1: OCH3, R2: OH, R4: Glucose | 0.44 ± 0.02 |
| 15 | M3G, Malvidin-3-glucoside | R1: OCH3, R2: OH, R3: OCH3, R4: Glucose | 2.24 ± 0.04 |
| Main Flavonols | |||
| 1 | Q3Ga, Quercetin-3-galactoside | R1: OH, R2: Galactose | 0.84 ± 0.01 |
| 2 | Q3R, Quercetin-3-rutinoside | R1: OH, R2: Rutinose | 2.05 ± 0.02 |
| 3 | Q3Rha, Quercetin-3-rhamnoside | R1: OH, R2: Rhamnose | 1.53 ± 0.01 |
| 4 | I3R, Isorhamnetin-3-rutinoside | R1: OCH3, R2: Rutinose | 1.49 ± 0.02 |
| Concentration | Compounds | n | Vasodilatation (%) Mean ± SEM |
|---|---|---|---|
| 100 nM | Delphinidin-3-glucoside, D3G | 4 | 43.50 ± 8.0 |
| 100 nM | Petunidin-3-glucoside, P3G | 6 | 39.08 ± 2.9 |
| 100 nM | Malvidin-3-glucoside, M3G | 4 | 38.65 ± 3.5 |
| 10 µM | Quercetin-3-rhamnoside, Q3Rha | 4 | 30.83 ± 1.99 |
| 10 µM | Quercetin-3-rutinoside, Q3R | 4 | 14.83 ± 3.92 |
| 100 nM | Acetylcholine, Ach | 5 | 66.95 ± 8.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calfío, C.; Huidobro-Toro, J.P. Potent Vasodilator and Cellular Antioxidant Activity of Endemic Patagonian Calafate Berries (Berberis microphylla) with Nutraceutical Potential. Molecules 2019, 24, 2700. https://doi.org/10.3390/molecules24152700
Calfío C, Huidobro-Toro JP. Potent Vasodilator and Cellular Antioxidant Activity of Endemic Patagonian Calafate Berries (Berberis microphylla) with Nutraceutical Potential. Molecules. 2019; 24(15):2700. https://doi.org/10.3390/molecules24152700
Chicago/Turabian StyleCalfío, Camila, and Juan Pablo Huidobro-Toro. 2019. "Potent Vasodilator and Cellular Antioxidant Activity of Endemic Patagonian Calafate Berries (Berberis microphylla) with Nutraceutical Potential" Molecules 24, no. 15: 2700. https://doi.org/10.3390/molecules24152700
APA StyleCalfío, C., & Huidobro-Toro, J. P. (2019). Potent Vasodilator and Cellular Antioxidant Activity of Endemic Patagonian Calafate Berries (Berberis microphylla) with Nutraceutical Potential. Molecules, 24(15), 2700. https://doi.org/10.3390/molecules24152700