Efficacy of Origanum syriacum Essential Oil against the Mosquito Vector Culex quinquefasciatus and the Gastrointestinal Parasite Anisakis simplex, with Insights on Acetylcholinesterase Inhibition
Abstract
:1. Introduction
2. Results
2.1. Essential Oil Extraction and Chemical Analysis
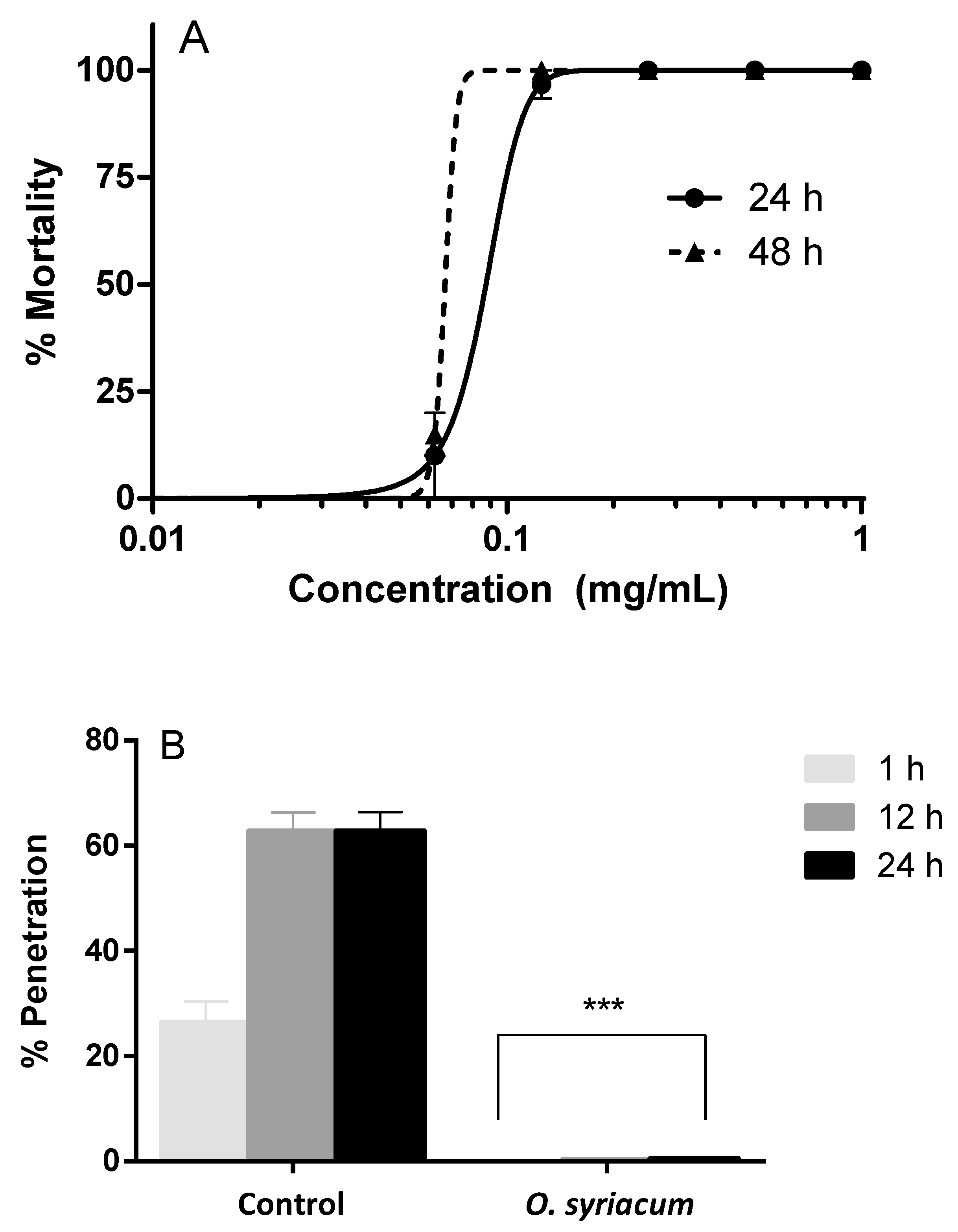
2.2. Anthelmintic Activity against A. simplex
2.3. Larvicidal, Tarsal, and Fumigation Activity on C. quinquefasciatus
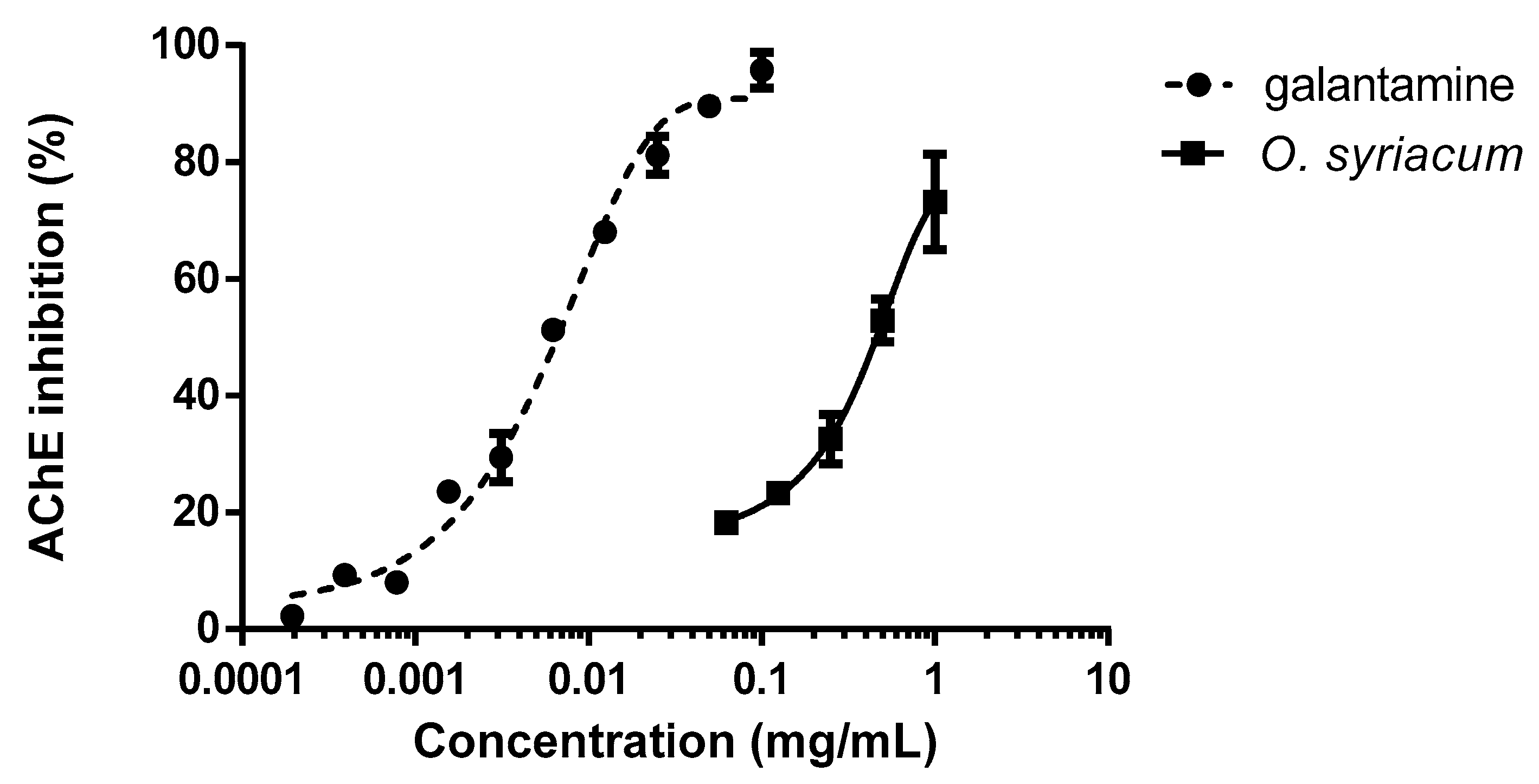
2.4. Inhibition of Acetylcholinesterase
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Isolation and Analysis of O. syriacum Essential Oil
4.3. Activities of O. syriacum Essential Oil against A. simplex
4.3.1. Isolation of A. simplex Larvae
4.3.2. Larvicidal Activity on A. simplex
4.3.3. Penetration Assays
4.4. Larvicidal Activity on C. quinquefasciatus
4.5. Tarsal Contact Test on C. quiquefasciatus Adults
4.6. Fumigation Test on C. quiquefasciatus Adults
4.7. Lethal Time Assessment on C. quiquefasciatus Adults
4.8. Inhibition of Acetylcholinesterase
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guardone, L.; Armani, A.; Nucera, D.; Costanzo, F.; Mattiucci, S.; Bruschi, F. Human anisakiasis in Italy: A retrospective epidemiological study over two decades. Parasite 2018, 25, 41. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Daschner, Á.; Perteguer, M.J.; Benito, A. Epidemiological scenario of anisakidosis in Spain based on associated hospitalizations: The tipping point of the iceberg. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kassai, T.; Del Campillo, M.C.; Euzeby, J.; Gaafar, S.; Hiepe, T.; Himonas, C.A. Standardized nomenclature of animal parasitic diseases (SNOAPAD). Vet. Parasitol. 1988, 29, 299–326. [Google Scholar] [CrossRef]
- Shimamura, Y.; Muwanwella, N.; Chandran, S.; Kandel, G.; Marcon, N. Common symptoms from an uncommon infection: Gastrointestinal anisakiasis. Can. J. Gastroenterol. Hepatol. 2016, 2016, 5176502. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Otranto, D.; Dantas-Torres, F. Vector-Borne Parasitic Zoonotic Infections in Humans. In Human Emerging and Re-emerging Infections; Singh, S.K., Ed.; John Wiley & Sons, Inc.: Chichester, UK, 2015; pp. 505–516. [Google Scholar] [CrossRef]
- Waltner-Toews, D. Zoonoses, One Health and complexity: Wicked problems and constructive conflict. Phil. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160171. [Google Scholar] [CrossRef]
- Petersen, E.; Wilson, M.E.; Touch, S.; McCloskey, B.; Mwaba, P.; Bates, M.; Dar, O.; Mattes, F.; Kidd, M.; Ippolito, G.; et al. Unexpected and rapid spread of Zika virus in the Americas—Implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic Games. Int. J. Infect. Dis. 2015, 44, 11–15. [Google Scholar] [CrossRef]
- Benelli, G.; Duggan, M.F. Management of arthropod vector data—Social and ecological dynamics facing the One Health perspective. Acta Trop. 2018, 182, 80–91. [Google Scholar] [CrossRef]
- WHO. Lymphatic Filariasis: Fact Sheet N°102; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Wilke, A.B.B.; Beier, J.C.; Benelli, G. Filariasis vector control down-played due to the belief the drugs will be enough—Not true! Entomol. Gen. 2019. [Google Scholar] [CrossRef]
- Benelli, G.; Romano, D. Mosquito vectors of Zika virus. Entomol. Gen. 2017, 36, 309–318. [Google Scholar] [CrossRef]
- Guedes, D.R.; Paiva, M.H.; Donato, M.M.; Barbosa, P.P.; Krokovsky, L.; dos SRocha, S.W.; La Saraiva, K.; Crespo, M.M.; Rezende, T.M.T.; Wallau, G.L.; et al. Zika virus replication in the mosquito Culex quinquefasciatus in Brazil. Emerg. Microbes Infect. 2017, 6, e69. [Google Scholar] [CrossRef] [PubMed]
- Van den Hurk, A.F.; Hall-Mendelin, S.; Jansen, C.C.; Higgs, S. Zika virus and Culex quinquefasciatus mosquitoes: A tenuous link. Lancet Infect. Dis. 2017, 17, 1014–1016. [Google Scholar] [CrossRef]
- Corbel, V.; N’guessan, R.; Brengues, C.; Chandre, F.; Djogbenou, L.; Martin, T.; Akogbéto, M.; Hougard, J.M.; Rowland, M. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007, 101, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Beier, J.C. Current vector control challenges in the fight against malaria. Acta Trop. 2017, 174, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A One Health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Paternoster, G.; Tomassone, L.; Favretto, A.; Balduzzi, G.; Tamba, M.; Chiari, M.; Lavazza, A.; Vogler, B. Evaluation of One Health practices to tackle zoonoses: The example of the integrated WNV surveillance in Northern Italy. In Proceedings of the 8th International Conference on Emerging Zoonoses focusing on Emerging and Transboundary Infectious Diseases, Manhattan, KS, USA, 7–10 May 2017; p. 28. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.C.; Isman, M.B.; Belmain, S.R. Pesticidal plants in Africa: A global vision of new biological control products from local uses. Ind. Crop. Prod. 2017, 110, 2–9. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Beyond mosquitoes—Essential oil toxicity and repellency against bloodsucking insects. Ind. Crops Prod. 2018, 117, 382–392. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef]
- Miresmailli, S.; Isman, M.B. Botanical insecticides inspired by plant—Herbivore chemical interactions. Trends Plant. Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef]
- Benelli, G. Managing mosquitoes and ticks in a rapidly changing world—Facts and trends. Saudi J. Biol. Sci. 2019. [Google Scholar] [CrossRef]
- AlShebly, M.M.; AlQahtani, F.S.; Govindarajan, M.; Gopinath, K.; Vijayan, P.; Benelli, G. Toxicity of ar-curcumene and epi-β-bisabolol from Hedychium larsenii (Zingiberaceae) essential oil on malaria, chikungunya and St. Louis encephalitis mosquito vectors. Ecotoxicol. Environ. Saf. 2017, 137, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, F.; Muscolino, D.; Beninati, C.; Giuffrida, A.; Panebianco, A. Activity of Thymus vulgaris essential oil against Anisakis larvae. Exp. Parasitol. 2014, 142, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rincón, C.; Langa, E.; Murillo, P.; Valero, M.S.; Berzosa, C.; López, V. Activity of tea tree (Melaleuca alternifolia) essential oil against L3 larvae of Anisakis simplex. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.C.; Navarro, M.C.; Martín-Sánchez, J.; Valero, A. Peppermint (Mentha piperita) and albendazole against anisakiasis in an animal model. Trop. Med. Int. Health 2014, 19, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mateos Pérez, M.; Navarro Moll, C.; Merino Espinosa, G.; Valero López, A. Evaluation of different Mediterranean essential oils as prophylactic agents in anisakidosis. Pharm Biol. 2017, 55, 456–461. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Ziino, G.; Giuffrida, A.; Marotta, S.M.; Lo Presti, V.; Chiofalo, V.; Panebianco, A. Activity of Tagetes minuta Linnaeus (Asteraceae) essential oil against L3 Anisakis larvae type 1. Asian Pac. J. Trop. Med. 2017, 10, 461–465. [Google Scholar] [CrossRef]
- López, V.; Cascella, M.; Benelli, G.; Maggi, F.; Gómez-Rincón, C. Green drugs in the fight against Anisakis simplex—Larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitol. Res. 2018, 117, 861–867. [Google Scholar] [CrossRef]
- Ietswaart, J.H. A Taxonomic Revision of the Genus Origanum (Labiatae); Leiden Botanical Series; Leiden University Press: Leiden, The Netherlands, 1980. [Google Scholar]
- Carlström, A. New species of Alyssum, Consolida, Origanum and Umblicus from the SE Aegean Sea. Willdenowia 1984, 14, 15–26. [Google Scholar]
- Danin, A. Two new species of Origanum (Labiatae) from Jordan. Willdenowia 1990, 19, 401–404. [Google Scholar]
- Danin, A.; Künne, I. Origanum jordanicum (Labiatae), a New Species from Jordan, and Notes on the Other Species of O. sect. Campanulaticalyx. Willdenowia 1996, 25, 601–611. [Google Scholar]
- Duman, H.; Aytaç, Z.; Ekici, M.; Karavelioğulları, F.A.; Dönmez, A.A.; Duran, A. Three New Species (Labiatae) From Turkey. Flora Mediter. 1996, 5, 221–228. [Google Scholar]
- Duman, H.; Başer, K.H.C.; Aytaç, Z. Two New Species and a New Hybrid from Anatolia. Turk. J. Bot. 1998, 22, 51–55. [Google Scholar]
- Dirmenci, T.; Yazıcı, T.; Özcan, T.; Çelenk, Ç.; Martin, E. A new species and a new natural hybrid of Origanum, L. (Lamiaceae) from the west of Turkey. Turk. J. Bot. 2018, 42, 73–90. [Google Scholar] [CrossRef]
- Greuter, W.; Burdet, H.M.; Long, G. Med-Checklist. Conserv. Jard. Bot. Ville Genkre 1986, 3, 308. [Google Scholar]
- Salah, S.M.; Jäger, A.K. Screening of traditionally used Lebanese herbs for neurological activities. J. Ethnopharmacol. 2005, 97, 145–149. [Google Scholar] [CrossRef] [PubMed]
- El Beyrouthy, M.; Arnold, N.A.; Annick, D.D.; Frederic, D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. Based Complement. Alternat. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Yaniv, Z.; Dafni, A.; Friedman, J.; Palevitch, D. Plants used for the treatment of diabetes in Israel. J. Ethnopharmacol. 1987, 19, 145–151. [Google Scholar] [CrossRef]
- Darwish, R.M.; Aburjai, T.A. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement. Altern. Med. 2010, 10, 9. [Google Scholar]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; de Cindio, B.; Houghton, P.J.; Menichini, F.; et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem. 2009, 117, 174–180. [Google Scholar] [CrossRef]
- El Gendy, A.N.; Leonardi, M.; Mugnaini, L.; Bertelloni, F.; Ebani, V.V.; Nardoni, S.; Mancianti, F.; Hendawy, S.; Omer, E.; Pistelli, L. Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai, Egypt. Ind. Crops Prod. 2015, 67, 201–207. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; El Gendy, A.E.N.G.; Sendra, E.; Fernandez-Lopez, J.; Abd El Razik, K.A.; Omer, E.A.; Pérez-Alvarez, J.A. Chemical composition and antioxidant and anti-Listeria activities of essential oils obtained from some Egyptian plants. J. Agric. Food Chem. 2010, 58, 9063–9070. [Google Scholar] [CrossRef] [PubMed]
- Lukas, B.; Samuel, R.; Novak, J. Oregano or marjoram? The enzyme γ-terpinene synthase affects chemotype formation in the genus Origanum. Isr. J. Plant. Sci. 2010, 58, 211–220. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kurkcuoglu, M.; Demirci, B.; Ozek, T. The essential oil of Origanum syriacum L. var. sinaicum (Boiss.) Ietswaart. Flavour. Fragr. J. 2003, 18, 98–99. [Google Scholar]
- Zein, S.; Awada, S.; Rachidi, S.; Hajj, A.; Krivoruschko, E.; Kanaan, H. Chemical analysis of essential oil from Lebanese wild and cultivated Origanum syriacum L. (Lamiaceae) before and after flowering. J. Med. Plants Res. 2011, 5, 379–387. [Google Scholar]
- Al Hafi, M.; El Beyrouthy, M.; Ouaini, N.; Stien, D.; Rutledge, D.; Chaillou, S. Chemical composition and antimicrobial activity of Origanum libanoticum, Origanum ehrenbergii, and Origanum syriacum growing wild in Lebanon. Chem. Biodivers. 2016, 13, 555–560. [Google Scholar] [CrossRef]
- Traboulsi, A.F.; Taoubi, K.; El-Haj, S.; Bessiere, J.M.; Rammal, S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest. Manag. Sci. 2002, 58, 491–495. [Google Scholar] [CrossRef]
- Kordali, S.; Emsen, B.; Yıldırım, E. Fumigant toxicity of essential oils from fifteen plant species against Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). Egypt. J. Biol. Pest. Co. 2013, 23, 241–246. [Google Scholar]
- Sener, O.; Arslan, M.; Demirel, N.; Uremis, I. Insecticidal effects of some essential oils against the confused flour beetle (Tribolium confusum du Val) (Col.: Tenebrinoidea) in stored wheat. Asian J. Chem. 2009, 21, 3995. [Google Scholar]
- Tunc, I.; Berger, B.M.; Erler, F.; Dağlı, F. Ovicidal activity of essential oils from five plants against two stored-product insects. J. Stored Prod. Res. 2000, 36, 161–168. [Google Scholar] [CrossRef]
- Oka, Y.; Nacar, S.; Putievsky, E.; Ravid, U.; Yaniv, Z.; Spiegel, Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 2000, 90, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.M.; Putievsky, E.; Lerner, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Iannarelli, R.; Petrelli, R.; Cappellacci, L.; Cianfaglione, K.; Afshar, F.; Nicoletti, M.; Canale, A.; Maggi, F. Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: Larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind. Crops Prod. 2017, 96, 186–195. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind. Crops Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crop. Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Bartolucci, F.; Canale, A.; Maggi, F. Origanum syriacum subsp. syriacum: From an ingredient of Lebanese ‘manoushe’to a source of effective and eco-friendly botanical insecticides. Ind. Crop. Prod. 2019, 134, 26–32. [Google Scholar]
- Romero, M.C.; Valero, A.; Martín-Sánchez, J.; Navarro-Moll, M.C. Activity of Matricaria chamomilla essential oil against anisakiasis. Phytomedicine 2012, 19, 520–523. [Google Scholar] [CrossRef]
- Audicana, M.T.; Kennedy, M.W. Anisakis simplex: From Obscure Infectious Worm to Inducer of Immune Hypersensitivity. Clin. Microbiol. Rev. 2008, 21, 360–379. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M.; Milos, M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res. 2007, 21, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crop. Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Pavela, R.; Zabka, M.; Bednar, J.; Tříska, J.; Vrchotová, N. New knowledge for yield, composition and insecticidal activity of essential oils obtained from the aerial parts or seeds of fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 2016, 83, 275–282. [Google Scholar] [CrossRef]
- Cheng, S.S.; Huang, C.G.; Chen, Y.J.; Yu, J.J.; Chen, W.J.; Chang, S.T. Chemical compositions and larvicidal activities of leaf essential oils from two Eucalyptus species. Bioresour. Technol. 2009, 100, 452–456. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal properties of Pimpinella anisum essential oils against the Culex quinquefasciatus and the non-target organism Daphnia magna. J. Asia Pac. Entomol. 2014, 17, 287–293. [Google Scholar] [CrossRef]
- Pavela, R. Encapsulation—A convenient way to extend the persistence of the effect of eco-friendly mosquito larvicides. Curr. Org. Chem. 2016, 20, 2674–2680. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G.; Pavoni, L.; Bonacucina, G.; Cespi, G.; Cianfaglione, K.; Bajalan, I.; Morshedloo, M.R.; Lupidi, G.; Romano, D.; et al. Microemulsions for delivery of Apiaceae essential oils—Towards highly effective and eco-friendly mosquito larvicides? Ind. Crops Prod. 2019, 129, 631–640. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as eco-friendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Everts, H.; Kappert, H.J.; Yeom, K.H.; Beynen, A.C. Dietary carvacrol lowers body weight gain but improves feed conversion in female broiler chickens. J. Appl. Poult. Res. 2003, 12, 394–399. [Google Scholar] [CrossRef]
- Mattila, H.R.; Otis, G.W.; Daley, J.; Schultz, T. Trials of apiguard, a thymol-based miticide part 2. Non-target effects on honey bees. Am. Bee J. 2000, 140, 68–70. [Google Scholar]
- George, D.R.; Sparagano, O.A.E.; Port, G.; Okello, E.; Shiei, R.S.; Guy, J.H. Repellence of plant essential oils to Dermanyssus gallinae and toxicity to the non-target invertebrate Tenebrio molitor. Vet. Parasitol. 2009, 162, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, M. Potential of Origanum compactum as a cercaricide in Morocco. Ann. Trop. Med. Parasitol. 2002, 96, 587–593. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl—Uptake in American cockroach ventral nerve cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Benelli, G. Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res. Vet. Sci. 2016, 104, 77–82. [Google Scholar] [CrossRef]
- Mouterde, P. Nouvelle Flore du Liban et de la Syrie; Dar El-Machreq: Beyrouth, Lebanon, 1984; Volume 3. [Google Scholar]
- Benelli, G.; Pavela, R.; Drenaggi, E.; Maggi, F. Insecticidal efficacy of the essential oil of jambú (Acmella oleracea (L.) R.K. Jansen) cultivated in central Italy against filariasis mosquito vectors, houseflies and moth pests. J. Ethnopharmacol. 2019, 229, 272–279. [Google Scholar] [CrossRef]
- WHO. Report of the Who Informal Consultation on the Evaluation and Testing of Insecticides; CTD/WHOPES/IC/96.1; WHO: Geneva, Switzerland, 1996. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University: London, UK, 1971; pp. 68–78. [Google Scholar]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Kavallieratos, N.G.; Cappellacci, L.; Petrelli, R.; Maggi, F.; Benelli, G. Rationale for developing novel mosquito larvicides based on isofuranodiene microemulsions. J. Pest Sci. 2019, 92, 909–921. [Google Scholar] [CrossRef]
Sample Availability: Samples of the O. syriacum essential oil are available from the authors. |
| Target Insect | Unit | LC50 | CI95 | LC90 | CI95 | χ2 |
|---|---|---|---|---|---|---|
| O. syriacum Essential Oil | ||||||
| C. quinquefasciatus third instar larvae | mg L−1 | 32.4 | 31.3–33.6 | 40.1 | 38.3–42.3 | 6.396 ns |
| C. quinquefasciatus adult females (tarsal toxicity test) | µg cm−2 | 28.1 | 25.9–30.3 | 46.9 | 42.1–54.1 | 4.698 ns |
| Carvacrol | ||||||
| C. quinquefasciatus third instar larvae | mg L−1 | 29.5 | 28.3–31.8 | 39.2 | 36.7–42.9 | 5.214 ns |
| C. quinquefasciatus adult females (tarsal toxicity test) | µg cm−2 | 25.5 | 21.2–27.3 | 35.8 | 32.7–41.5 | 3.251 ns |
| Positive Control, α-Cypermethrin | ||||||
| C. quinquefasciatus third instar larvae | mg L−1 | 0.0008 | 0.0006–0.0012 | 0.0025 | 0.0021–0.0032 | 5.235 ns |
| C. quinquefasciatus adults (tarsal toxicity test) | µg cm−2 | 1.22 | 0.95–1.38 | 2.18 | 2.01–2.26 | 3.245 ns |
| Treatment | LC50 (µL L−1) | CI95 | LC90 (µL L−1) | CI95 | χ2 |
|---|---|---|---|---|---|
| O. syriacum Essential Oil | |||||
| 1 h of exposure | 12.1 | 10.8–13.6 | 28.8 | 24.7–37.6 | 2.263 ns |
| 24 h of exposure | 1.3 | 1.3–1.5 | 2.2 | 1.9–2.6 | 1.159 ns |
| Carvacrol | |||||
| 1 h of exposure | 8.2 | 7.9–10.7 | 16.3 | 15.9–19.3 | 2.152 ns |
| 24 h of exposure | 0.8 | 0.7–1.1 | 1.5 | 1.3–1.8 | 2.313 ns |
| Parameter | LT50 (min) | CI95 | LT90 (min) | CI95 | χ 2 |
|---|---|---|---|---|---|
| Lethal time (LT50,90) for 20 µL L−1 | 66 | 62–69 | 103 | 97–109 | 2.239 ns |
| Lethal time (LT50,90) for 10 µL L−1 | 117 | 111–124 | 191 | 173–218 | 3.324 ns |
| Lethal time (LT50,90) for 5 µL L−1 | 201 | 185–222 | 408 | 343–537 | 4.957 ns |
| Lethal time (LT50,90) for 2.5 µL L−1 | 426 | 415–438 | 789 | 768–826 | 3.362 ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, V.; Pavela, R.; Gómez-Rincón, C.; Les, F.; Bartolucci, F.; Galiffa, V.; Petrelli, R.; Cappellacci, L.; Maggi, F.; Canale, A.; et al. Efficacy of Origanum syriacum Essential Oil against the Mosquito Vector Culex quinquefasciatus and the Gastrointestinal Parasite Anisakis simplex, with Insights on Acetylcholinesterase Inhibition. Molecules 2019, 24, 2563. https://doi.org/10.3390/molecules24142563
López V, Pavela R, Gómez-Rincón C, Les F, Bartolucci F, Galiffa V, Petrelli R, Cappellacci L, Maggi F, Canale A, et al. Efficacy of Origanum syriacum Essential Oil against the Mosquito Vector Culex quinquefasciatus and the Gastrointestinal Parasite Anisakis simplex, with Insights on Acetylcholinesterase Inhibition. Molecules. 2019; 24(14):2563. https://doi.org/10.3390/molecules24142563
Chicago/Turabian StyleLópez, Víctor, Roman Pavela, Carlota Gómez-Rincón, Francisco Les, Fabrizio Bartolucci, Veronica Galiffa, Riccardo Petrelli, Loredana Cappellacci, Filippo Maggi, Angelo Canale, and et al. 2019. "Efficacy of Origanum syriacum Essential Oil against the Mosquito Vector Culex quinquefasciatus and the Gastrointestinal Parasite Anisakis simplex, with Insights on Acetylcholinesterase Inhibition" Molecules 24, no. 14: 2563. https://doi.org/10.3390/molecules24142563











