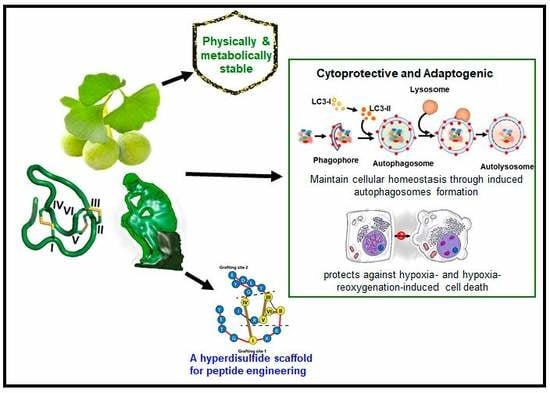
LIR Motif-Containing Hyperdisulfide β-Ginkgotide is Cytoprotective, Adaptogenic, and Scaffold-Ready
Abstract
:1. Introduction
2. Results
2.1. β-Ginkgotide: Synthesis and Biophysical Characterization
2.1.1. Chemical Synthesis and Oxidative Folding of β-Ginkgotide
2.1.2. β-Ginkgotide Secondary Structure and Stability
2.2. Intrinsic Functions of β-Ginkgotide
2.2.1. β-Ginkgotide Contains the LIR Motif and Induces Autophagosomes Formation
2.2.2. β-Ginkgotide Protects Hypoxia-Induced Cell Death
2.2.3. β-Ginkgotide Protects Cells from Hypoxia-Reoxygenation Injury
2.2.4. β-Ginkgotide in Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Synthesis and Oxidative Folding of β-Ginkgotide β-gB1
4.3. Circular Dichroism Spectroscopy
4.4. Enzymatic Stability
4.5. Cell Culture
4.6. Hypoxia and Hypoxia-Reoxygenation Model
4.7. MTT Assay
4.8. LDH Assay
4.9. Western Blot Analysis
4.10. Bioinformatics Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hruby, V.J. Designing peptide receptor agonists and antagonists. Nat. Rev. Drug Discov. 2002, 1, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Haskell-Luevano, C.; Shenderovich, M.D.; Sharma, S.D.; Nikiforovich, G.V.; Hadley, M.E.; Hruby, V.J. Design, synthesis, biology, and conformations of bicyclic alpha-melanotropin analogues. J. Med. Chem. 1995, 38, 1736–1750. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, T.K.; Hruby, V.J.; Darman, P.S.; Hadley, M.E. [half-Cys4,half-Cys10]-alpha-Melanocyte-stimulating hormone: A cyclic alpha-melanotropin exhibiting superagonist biological activity. Proc. Natl. Acad. Sci. USA 1982, 79, 1751–1755. [Google Scholar] [CrossRef]
- Cai, M.; Hruby, V.J. Design of cyclized selective melanotropins. Biopolymers 2016, 106, 876–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.S.; Graves, B.; Guerlavais, V.; Tovar, C.; Packman, K.; To, K.-H.; Olson, K.A.; Kesavan, K.; Gangurde, P.; Mukherjee, A.; et al. Stapled α−helical peptide drug development: A potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl. Acad. Sci. USA 2013, 110, E3445–E3454. [Google Scholar] [CrossRef] [PubMed]
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef] [PubMed]
- Jennings, C.; West, J.; Waine, C.; Craik, D.; Anderson, M. Biosynthesis and insecticidal properties of plant cyclotides: The cyclic knotted proteins from Oldenlandia affinis. Proc. Natl. Acad. Sci. USA 2001, 98, 10614–10619. [Google Scholar] [CrossRef]
- Hetrick, K.J.; van der Donk, W.A. Ribosomally synthesized and post-translationally modified peptide natural product discovery in the genomic era. Curr. Opin. Chem. Biol. 2017, 38, 36–44. [Google Scholar] [CrossRef]
- Olivera, B.; Gray, W.; Zeikus, R.; McIntosh, J.; Varga, J.; Rivier, J.; de Santos, V.; Cruz, L. Peptide neurotoxins from fish-hunting cone snails. Science 1985, 230, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Goudet, C.; Chi, C.-W.; Tytgat, J. An overview of toxins and genes from the venom of the Asian scorpion Buthus martensi Karsch. Toxicon 2002, 40, 1239–1258. [Google Scholar] [CrossRef]
- Shafee, T.M.A.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent evolution of defensin sequence, structure and function. Cell. Mol. Life Sci. 2017, 74, 663–682. [Google Scholar] [CrossRef] [PubMed]
- Kini, S.G.; Wong, K.H.; Tan, W.L.; Xiao, T.; Tam, J.P. Morintides: Cargo-free chitin-binding peptides from Moringa oleifera. BMC Plant. Biol. 2017, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Kumari, G.; Serra, A.; Shin, J.; Nguyen, P.Q.; Sze, S.K.; Yoon, H.S.; Tam, J.P. Cysteine-Rich Peptide Family with Unusual Disulfide Connectivity from Jasminum sambac. J. Nat. Prod. 2015, 78, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.K.; Zhang, S.; Nguyen, N.T.; Nguyen, P.Q.; Chiu, M.S.; Hardjojo, A.; Tam, J.P. Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the Fabaceae family. J. Biol. Chem. 2011, 286, 24275–24287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.; Luu, T.T.; Bai, Y.; Nguyen, G.K.; Pervushin, K.; Tam, J.P. Allotides: Proline-Rich Cystine Knot α-Amylase Inhibitors from Allamanda cathartica. J. Nat. Prod. 2015, 78, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Tan, W.L.; Serra, A.; Xiao, T.; Sze, S.K.; Yang, D.; Tam, J.P. Ginkgotides: Proline-Rich Hevein-Like Peptides from Gymnosperm Ginkgo biloba. Front. Plant Sci. 2016, 7, 1639. [Google Scholar] [CrossRef]
- Wong, K.H.; Tan, W.L.; Xiao, T.; Tam, J.P. beta-Ginkgotides: Hyperdisulfide-constrained peptides from Ginkgo biloba. Sci. Rep. 2017, 7, 6140. [Google Scholar] [CrossRef]
- Huang, J.; Wong, K.H.; Tay, S.V.; Serra, A.; Sze, S.K.; Tam, J.P. Astratides: Insulin-Modulating, Insecticidal, and Antifungal Cysteine-Rich Peptides from Astragalus membranaceus. J. Nat. Prod. 2019, 82, 194–204. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, L.; Huang, J.; Serra, A.; Yang, H.; Tam, J.P. Potentides: Novel Cysteine-Rich Peptides with Unusual Disulfide Connectivity from Potentilla anserina. ChemBioChem 2019. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B. Ginkgo biloba: A review of quality, safety, and efficacy. Nutr. Clin. Care 2001, 4, 140–147. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, J.P.; Nguyen, G.K.T.; Loo, S.; Wang, S.; Yang, D.; Kam, A. Ginsentides: Cysteine and Glycine-rich Peptides from the Ginseng Family with Unusual Disulfide Connectivity. Sci. Rep. 2018, 8, 16201. [Google Scholar] [CrossRef] [PubMed]
- Gouw, M.; Michael, S.; Samano-Sanchez, H.; Kumar, M.; Zeke, A.; Lang, B.; Bely, B.; Chemes, L.B.; Davey, N.E.; Deng, Z.; et al. The eukaryotic linear motif resource - 2018 update. Nucleic. Acids Res. 2018, 46, D428–D434. [Google Scholar] [CrossRef] [PubMed]
- Birgisdottir, A.B.; Lamark, T.; Johansen, T. The LIR motif - crucial for selective autophagy. J. Cell Sci. 2013, 126, 3237–3247. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Kirisako, T.; Ichimura, Y.; Okada, H.; Kabeya, Y.; Mizushima, N.; Yoshimori, T.; Ohsumi, M.; Takao, T.; Noda, T.; Ohsumi, Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 2000, 151, 263–276. [Google Scholar] [CrossRef]
- Weidberg, H.; Shpilka, T.; Shvets, E.; Abada, A.; Shimron, F.; Elazar, Z. LC3 and GATE-16 N termini mediate membrane fusion processes required for autophagosome biogenesis. Dev. Cell 2011, 20, 444–454. [Google Scholar] [CrossRef]
- Turer, A.T.; Hill, J.A. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am. J. Cardiol. 2010, 106, 360–368. [Google Scholar] [CrossRef]
- Li, X.; Arslan, F.; Ren, Y.; Adav, S.S.; Poh, K.K.; Sorokin, V.; Lee, C.N.; de Kleijn, D.; Lim, S.K.; Sze, S.K. Metabolic adaptation to a disruption in oxygen supply during myocardial ischemia and reperfusion is underpinned by temporal and quantitative changes in the cardiac proteome. J. Proteome Res. 2012, 11, 2331–2346. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Agani, F.; Feldser, D.; Lyer, N.; Kotch, L.; Laughner, E.; Yu, A. Hypoxia, HIF-1, and the Pathophysiologi of Common Human Diseases. In Oxygen Sensing: Molecule to Man; Lahiri, S., Prabhakar, N.R., Forster, R.E., Eds.; Springer US: Boston, MA, USA, 2002; pp. 123–130. [Google Scholar]
- Aziz, T.A.; Hussain, S.A.; Mahwi, T.O.; Ahmed, Z.A. Efficacy and safety of Ginkgo biloba extract as an “add-on” treatment to metformin for patients with metabolic syndrome: A pilot clinical study. Ther. Clin. Risk Manag. 2018, 14, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Canevelli, M.; Adali, N.; Kelaiditi, E.; Cantet, C.; Ousset, P.J.; Cesari, M. Effects of Gingko biloba supplementation in Alzheimer’s disease patients receiving cholinesterase inhibitors: Data from the ICTUS study. Phytomedicine 2014, 21, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, M.; Yang, Y.; Li, Y.; Asplund, K. Ginkgo biloba for acute ischaemic stroke. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2018, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Jacomin, A.-C.; Samavedam, S.; Promponas, V.; Nezis, I.P. iLIR database: A web resource for LIR motif-containing proteins in eukaryotes. Autophagy 2016, 12, 1945–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, K.; Lim, K. Why is autophagy important in human diseases? Exp Mol Med 2012, 44, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013, 19, 983–997. [Google Scholar] [CrossRef]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Ryter, S.W.; Cloonan, S.M.; Choi, A.M. Autophagy: A critical regulator of cellular metabolism and homeostasis. Mol. Cells 2013, 36, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. 2010, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, H.; Semba, H.; Takeda, N. The Roles of Hypoxia Signaling in the Pathogenesis of Cardiovascular Diseases. J. Atheroscler Thromb 2017, 24, 88–894. [Google Scholar] [CrossRef] [PubMed]
- Merelli, A.; Rodriguez, J.C.G.; Folch, J.; Regueiro, M.R.; Camins, A.; Lazarowski, A. Understanding the Role of Hypoxia Inducible Factor During Neurodegeneration for New Therapeutics Opportunities. Curr. Neuropharmacol. 2018, 16, 1484–1498. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic potential of intermittent hypoxia: A matter of dose. Am. J. Physiol Regul Integr Comp. Physiol 2014, 307, R1181–R1197. [Google Scholar] [CrossRef] [PubMed]
- Gallart-Palau, X.; Serra, A.; Hase, Y.; Tan, C.F.; Chen, C.P.; Kalaria, R.N.; Sze, S.K. Brain-derived and circulating vesicle profiles indicate neurovascular unit dysfunction in early Alzheimer’s disease. Brain Pathol. 2019. [Google Scholar] [CrossRef] [PubMed]






| Peptides | Cysteine motif 1 | No. of aa 2 | Cys (%) 3 |
|---|---|---|---|
| Scratch peptide | CICII••CIII••CIV••CVCVI | 12 | 50 |
| BtIIIA | CICII•••CIII••CIV••CVCVI | 13 | 46 |
| Mr3e | XCICII••••CIII•••CIV•CVCVIX | 14 | 43 |
| β-gB1 | X4CI••CIICIII••••••CIV••CVCVI | 16 | 38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, B.; Huang, J.; To, J.; Tam, J.P. LIR Motif-Containing Hyperdisulfide β-Ginkgotide is Cytoprotective, Adaptogenic, and Scaffold-Ready. Molecules 2019, 24, 2417. https://doi.org/10.3390/molecules24132417
Dutta B, Huang J, To J, Tam JP. LIR Motif-Containing Hyperdisulfide β-Ginkgotide is Cytoprotective, Adaptogenic, and Scaffold-Ready. Molecules. 2019; 24(13):2417. https://doi.org/10.3390/molecules24132417
Chicago/Turabian StyleDutta, Bamaprasad, Jiayi Huang, Janet To, and James P. Tam. 2019. "LIR Motif-Containing Hyperdisulfide β-Ginkgotide is Cytoprotective, Adaptogenic, and Scaffold-Ready" Molecules 24, no. 13: 2417. https://doi.org/10.3390/molecules24132417