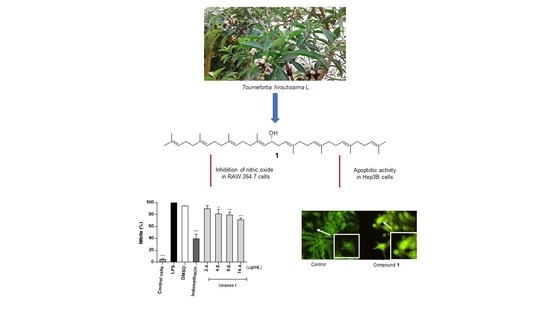
16-Hydroxy-Lycopersene, a Polyisoprenoid Alcohol Isolated from Tournefortia hirsutissima, Inhibits Nitric Oxide Production in RAW 264.7 Cells and Induces Apoptosis in Hep3B Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pharmacological Activity of Tournefortia hirsutissima
2.2. Purification and Characterization of Polyisoprenoid Alcohols
2.3. Effect of Compounds on NO Production in LPS-Stimulated RAW 264.7 Cells
2.4. Antiproliferative Activity of Compounds
2.5. Apoptosis Induced by Compound 1
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Preparation of Extracts
3.4. Isolation and Purification of Polyisoprenoid Alcohol Compounds 1–3
3.5. Preparation of (S)- and (R)-MTPA Esters of Compound 1 (1a and 1b)
3.6. Cell Viability of the RAW 264.7 Cells
3.7. Measurement of Nitrite Concentration
3.8. TPA-Induced Ear Edema in Mice
3.9. Antiproliferative Assay
3.10. Cell Cycle Analysis
3.11. Study of Cell Death by Annexin V-FITC/PI Staining
3.12. Cell Morphology Observation
3.13. RT-PCR
3.14. Caspase 3/7
3.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nisar, M.; He, J.; Ahmed, A.; Yang, Y.; Li, M.; Wan, C. Chemical Components and Biological Activities of the Genus Phyllanthus: A Review of the Recent Literature. Molecules 2018, 23, 2567. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Van Quan, N.; Xuan, T.D.; Tran, H.D.; Thuy, N.T.D.; Trang, L.T.; Huong, C.T.; Andriana, Y.; Tuyen, P.T. Antioxidant, α-Amylase and α-Glucosidase Inhibitory Activities and Potential Constituents of Canarium tramdenum Bark. Molecules 2019, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Aviello, G.; Borrelli, F.; Guida, F.; Romano, B.; Lewellyn, K.; De Chiaro, M.; Luongo, L.; Zjawiony, J.K.; Maione, S.; Izzo, A.A.; et al. Ultrapotent effects of salvinorin A, a hallucinogenic compound from Salvia divinorum, on LPS-stimulated murine macrophages and its anti-inflammatory action in vivo. J. Mol. Med. 2011, 89, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carranza, J.; Alvarez, L.; Marquina-Bahena, S.; Salas-Vidal, E.; Cuevas, V.; Jiménez, E.; Veloz, G.R.; Carraz, M.; González-Maya, L. Phenolic Compounds Isolated from Caesalpinia coriaria Induce S and G2/M Phase Cell Cycle Arrest Differentially and Trigger Cell Death by Interfering with Microtubule Dynamics in Cancer Cell Lines. Molecules 2017, 22, 666. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.; King, W.K. Tournefortia hirsutissima (Boraginaceae) New to the Flora of Texas. J. Bot. Res. Inst. Texas 2009, 3, 465–467. [Google Scholar]
- Maldonado-Almanza, B.J. Aprovechamiento de los Recursos Florísticos de la Sierra de Huautla, Morelos. Tesis de Maestría, Universidad Nacional Autónoma de México, Ciudad de México, Mexico, 1997. [Google Scholar]
- Andrade-Cetto, A.; Revilla-Monsalve, C.; Wiedenfeld, H. Hypoglycemic Effect of Tournefortia hirsutissima L., on n-Streptozotocin Diabetic Rats. J. Ethnopharmacol. 2007, 112, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Lans, C. A Ethnomedicines Used in Trinidad and Tobago for Urinary Problems and Diabetes Mellitus. J. Ethnobiol. Ethnomed. 2006, 2, 45. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilara, F.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.; Flores-Saenz, J. Study of the Anti-Hyperglycemic Effect of Plants Used as Antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- Hernández, M.Á.; Rojas, F.; Portillo, R.; Salgado, M.A.; Petranovskii, V.; Quiroz, K. Textural Properties of Hybrid Biomedical Materials Made from Extracts of Tournefortia hirsutissima L. Imbibed and Deposited on Mesoporous and Microporous Materials. J. Nanomater. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Skorupinska-Tudek, K.; Wojcik, J.; Swiezewska, E. Polyisoprenoid Alcohols—Recent Results of Structural Studies. Chem. Rec. 2008, 8, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Pronin, A.V.; Danilov, L.L.; Narovlyansky, A.N.; Sanin, A.V. Plant Polyisoprenoids and Control of Cholesterol Level. Arch. Immunol. Ther. Exp. (Warsz.) 2014, 62, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Qian, H.; Zhao, Y.; Liu, B.; Fu, C. Polyprenols from Taxus chinensis var. mairei Prevent the Development of CCl4-Induced liver fibrosis in Rats. J. Ethnopharmacol. 2012, 142, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Wang, C.-Z.; Kong, Z.-W. Antibacterial/Antifungal Activity and Synergistic Interactions between Polyprenols and Other Lipids Isolated from Ginkgo Biloba, L. Leaves. Molecules 2013, 18, 2166–2182. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Wang, C.; Ye, J.; Zhou, H.; Chen, H. Polyprenols of Ginkgo biloba Enhance Antibacterial Activity of Five Classes of Antibiotics. Biomed. Res. Int. 2016, 2016, 1–8. [Google Scholar]
- Yao, X.; Lu, B.; Lü, C.; Bai, Q.; Yan, D.; Wu, Y.; Hong, Z.; Xu, H. Solanesol Induces the Expression of heme oxygenase-1 via p38 and Akt and Suppresses the Production of Proinflammatory Cytokines in RAW264.7 Cells. Food Funct. 2017, 8, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yasugi, E.; Nakata, K.; Yokoyama, Y.; Kano, K.; Dohi, T.; Oshima, M. Dihydroheptaprenyl and Dihydrodecaprenyl Monophosphates Induce Apoptosis Mediated by Activation of Caspase-3-Like Protease. Biochim. Biophys. 1998, 1389, 132–140. [Google Scholar] [CrossRef]
- Dohi, T.; Yasugi, E.; Oshima, M. Activation of Mitogen Activated Protein Kinase in Dolichyl Phosphate-Induced Apoptosis in U937 Cells. Biochem. Biophys. Res. Commun. 1996, 224, 87–91. [Google Scholar] [CrossRef]
- Lee, M.; Hesek, D.; Zajíček, J.; Fisher, J.F.; Mobashery, S. Synthesis and Shift-Reagent-Assisted Full NMR Assignment of Bacterial (Z8,E2,ω)-Undecaprenol. Chem. Commun. 2017, 53, 12774–12777. [Google Scholar] [CrossRef]
- Lanzetta, R.; Monaco, P.; Previtera, L.; Simaldone, A. Polyprenols and Hydroxylated Lycopersenes from Myriophyllum verticillatum. Phytochemistry 1988, 27, 887–890. [Google Scholar] [CrossRef]
- Monaco, P.; Previtera, L.; Belardini, M. Polyprenols From the Leaves of Ouercus ilex Infected By Microsphaera alphitoides. J. Nat. Prod. 1983, 46, 174–177. [Google Scholar] [CrossRef]
- Ormerod, M.G.; Collins, M.K.L.; Rodriguez-Tarduchy, G.; Robertson, D. Apoptosis in Interleukin-3-dependent Haemopoietic Cells. Quantification by two Flow Cytometric Methods. J. Immunol. Methods 1992, 153, 57–65. [Google Scholar] [CrossRef]
- Lee, J.-S.; Ahn, J.-H.; Cho, Y.-J.; Kim, H.-Y.; Yang, Y.-I.; Lee, K.-T.; Jang, D.-S.; Choi, J.-H. α-Terthienylmethanol, Isolated from Eclipta prostrata, Induces Apoptosis by Generating Reactive Oxygen Species Via NADPH Oxidase in Human Endometrial Cancer Cells. J. Ethnopharmacol. 2015, 169, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-H.; Wang, G.-H.; Chou, T.-H.; Wang, S.-H.; Lin, R.-J.; Chan, L.-P.; So, E.C.; Sheu, J.-H. 5-epi-Sinuleptolide Induces Cell Cycle Arrest and Apoptosis Through Tumor Necrosis Factor/Mitochondria-Mediated Caspase Signaling Pathway in Human Skin Cancer Cells. Biochim. Biophys. Acta 2012, 1820, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.H.; Mahdavi, M.; Davoodi, J.; Tackallou, S.H.; Goudarzvand, M.; Neishabouri, S.H. Up Regulation of Bax and Down Regulation of Bcl2 During 3-NC Mediated Apoptosis in Human Cancer Cells. Cancer Cell Int. 2015, 15, 55. [Google Scholar] [CrossRef]
- Payá, M.; Ferrándiz, M.L.; Sanz, M.J.; Bustos, G.; Blasco, R.; Rios, J.L.; Alcaraz, M.J. Study of the Antioedema activity of some Seaweed and Sponge Extracts from the Mediterranean Coast in Mice. Phyther. Res. 1993, 7, 159–162. [Google Scholar] [CrossRef]
- Basu, A.; Saito, K.; Meyer, K.; Ray, R.B.; Friedman, S.L.; Chang, Y.H.; Ray, R. Stellate Cell Apoptosis by a Soluble Mediator from Immortalized Human Hepatocytes. Apoptosis 2006, 11, 1391–1400. [Google Scholar] [CrossRef]
- Kasibhatla, S. Acridine Orange/Ethidium Bromide (AO/EB) Staining to Detect Apoptosis. CSH Protoc. 2006, 2006, pdb.prot4493. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Compounds 1–3 are available from the authors. |








| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| CH3-1 | 1.69 | 25.89 | CH2-21 | 2.21–2.16 | 27.08 |
| C-2 | 131.15a | CH-22 | 5.26 | 124.73 | |
| CH-3 | 5.25 | 125.00b | C-23 | 135.22e | |
| CH2-4 | 2.22–2.16 | 27.29c | CH2-24 | 2.12 | 40.27d |
| CH2-5 | 2.12–2.08 | 40.27d | CH2-25 | 2.23–2.15 | 27.18g |
| C-6 | 135.05e | CH-26 | 5.30 | 124.82f | |
| CH-7 | 5.30 | 124.86f | C-27 | 135.28e | |
| CH2-8 | 2.23–2.15 | 27.30g | CH2-28 | 2.12–2.08 | 40.38d |
| CH2-9 | 2.12–2.08 | 40.23d | CH2-29 | 2.22–2.16 | 27.17c |
| C-10 | 135.09e | CH-30 | 5.25 | 124.98b | |
| CH-11 | 5.26 | 124.54 | C-31 | 131.17a | |
| CH2-12 | 2.19–2.15 | 26.91 | CH3-32 | 1.69 | 25.89 |
| CH2-13 | 2.04 | 40.03 | CH3-33 | 1.57 | 17.78 |
| C-14 | 137.29 | CH3-34 | 1.62 | 16.45h | |
| CH-15 | 5.36 | 129.04 | CH3-35 | 1.60 | 16.18h |
| CH-16 | 4.39 | 68.62 | CH3-36 | 1.61 | 16.76 |
| CH2-17 | 2.40, 2.26 | 37.13 | CH3-37 | 1.59 | 16.76 |
| CH-18 | 5.34 | 120.92 | CH3-38 | 1.60 | 16.18h |
| C-19 | 137.87 | CH3-39 | 1.62 | 16.45h | |
| CH2-20 | 2.11–2.09 | 40.25d | CH3-40 | 1.57 | 17.78 |
| Treatment | HeLa | PC3 | HepG2 | Hep3B | IHH |
|---|---|---|---|---|---|
| Th-H | N.D.b | N.D. | 21.6 ± 1.9 | 32.7 ± 0.9 | 77 ± 7 |
| Th-D | N.D. | N.D. | 33.6 ± 2.2 | > 100 | > 100 |
| Th-HA | N.D. | N.D. | 31 ± 1.4 | 36.1 ± 1.5 | 84 ± 10 |
| 1 | 37.3 ± 3.5 | 32.0 ± 4.4 | 26.6 ± 3.5 | 21.3 ± 3.5 | 88.8 ± 8.9 |
| 2 | 95.7 ± 12.0 | 74.2 ± 5.0 | 76.6 ± 6.0 | 50.3 ± 5.9 | 76.6 ± 6.0 |
| 3 | 84.1 ± 8.9 | 76.4 ± 6.6 | 74.2 ± 5.5 | 69.7 ± 5.5 | 74.1 ± 5.5 |
| Paclitaxela | 0.01 ± 0.002 | 0.0154 ± 0.0035 | 0.0065 ± 0.0007 | 0.008 ± 0.001 | 0.08 ± 0.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtado-Díaz, I.; Sánchez-Carranza, J.N.; Romero-Estrada, A.; González-Maya, L.; González-Christen, J.; Herrera-Ruiz, M.; Alvarez, L. 16-Hydroxy-Lycopersene, a Polyisoprenoid Alcohol Isolated from Tournefortia hirsutissima, Inhibits Nitric Oxide Production in RAW 264.7 Cells and Induces Apoptosis in Hep3B Cells. Molecules 2019, 24, 2366. https://doi.org/10.3390/molecules24132366
Hurtado-Díaz I, Sánchez-Carranza JN, Romero-Estrada A, González-Maya L, González-Christen J, Herrera-Ruiz M, Alvarez L. 16-Hydroxy-Lycopersene, a Polyisoprenoid Alcohol Isolated from Tournefortia hirsutissima, Inhibits Nitric Oxide Production in RAW 264.7 Cells and Induces Apoptosis in Hep3B Cells. Molecules. 2019; 24(13):2366. https://doi.org/10.3390/molecules24132366
Chicago/Turabian StyleHurtado-Díaz, Israel, Jessica Nayelli Sánchez-Carranza, Antonio Romero-Estrada, Leticia González-Maya, Judith González-Christen, Maribel Herrera-Ruiz, and Laura Alvarez. 2019. "16-Hydroxy-Lycopersene, a Polyisoprenoid Alcohol Isolated from Tournefortia hirsutissima, Inhibits Nitric Oxide Production in RAW 264.7 Cells and Induces Apoptosis in Hep3B Cells" Molecules 24, no. 13: 2366. https://doi.org/10.3390/molecules24132366