3.1. Characterization of MIL-53 (Fe) and MPC
The crystalline patterns of MIL-53 (Fe) and MPC materials are shown in
Figure S1 (see the
Supplementary Materials document file). As can be seen in
Figure S1a, three typical peaks presented at around 9.6
°(101), 18.6
° (002), and 28.1° (302), presenting for the MIL-53 (Fe) was mostly commensurate with our previous study and several recent works [
36,
37,
38]. This result indicated the successful synthesis of MIL-53 (Fe) via the solvothermal method.
Figure S1b displayed an emergent peak at around 45
° (110) and a narrow peak at around 35.5
° (100), confirming the presence of zero-valent Fe (JCPDS No. 65–4899) in the inherent structure of MPC [
39]. More specifically, the broadband between 20
° and 30
° may be attributable to graphitic carbon formed by the pyrolysis of MIL-53 (Fe) [
40]. The Raman spectra in
Figure S2 show more identification of MIL-53 (Fe) and MPC. Functional groups are shown in
Figure S2a: C-H (615, 875 cm
−1) and C-C bonds (1160 cm
−1) of benzene rings, O-C-O bonds (1450, 1504 cm
−1) of acid groups support the structural consolidation of MIL-53 (Fe), which is generated by Fe clusters and C
6H
4(COOH)
2 ligands [
24]. Meanwhile, the exposure of two bands (D and G) in
Figure S2b also indicated the existence of graphitic carbon in MPC.
To gain more understanding about the intrinsic structure of MIL-53 (Fe) and MPC, scanning electron microscope (SEM) and transmission electron microscopy (TEM) images can be solid evidence. As shown in
Figure 3a,b, MIL-53 (Fe) crystals were observed like microsphere (100–150 nm in diameter) with a highly smooth surface. By contrast, MPC morphology exposed a relatively defective and amorphous nature, along with the presence of dark spots collapsed in opaque regions. This phenomenon may be because of Fe nanoparticles under magnetic aggregation embedded in carbon [
24]. Moreover, the N
2 adsorption/desorption isotherm plots in
Figure S3 give more information about the porosity of MIL-53 (Fe) and MPC. It is evident that MIL-53 (Fe) had a non-porous structure with a Type IV (IUPAC)-like pattern, as shown in
Figure S3a, while a hysteresis loop at a higher-relative pressure (P/P
° > 0.5) assumed a Type II (IUPAC) pattern with a mesoporous structure. Generally, a huge distinction in structure was observed between MIL-53 (Fe) and MPC upon the effect of pyrolysis. MPC material with a more porous structure (surface area by Brunauer–Emmett–Teller (BET) was approximately 225 m
2/g) may provide a better advantage in the sorption of TCC. Therefore, we used MPC as an adsorbent for the TCC adsorption investigation.
3.2. Screening Study
In the screening study, the possible parameters consisted of the initial TCC concentration (A), the dose of MPC (B), initial pH levels (C), contact time (D), temperature (E), shaking speed (F), and Na
+ ionic strength (G), as shown in
Table 1. In addition, the parameters were represented at three levels, including low (−1), central (0), and high (+1) points (
Table 1). Thus, there were 16 preliminary experiments (entries 1–16,
Table 3) with a duplicate for each (n = 2). To assess the potential curvature, four central points were repeated (entries 17–20,
Table 3). The response (Y) was denoted for the removal percentage of TCC using the MPC as an adsorbent. Each experiment of TCC adsorption was separately conducted based on the guides of minimum-run resolution IV.
By establishing the two-level factorial interaction regression (2FI) with the DX10 program, the ANOVA table allows the observed and predicted data to be analyzed [
41,
42]. In detail, according to
Table 4, estimated effects and their coefficients for two models were listed to find out the significance of parameters at three states, including a significant positive effect at
P < 0.05, a significant negative effect at
P < 0.05, and not significant at
P < 0.05. Apparently, there were four parameters, concentration (A), dose (B), pH (C), and contact time (D), along with a response variable (Y), which were statistically significant, while the other three parameters, temperature (E), shaking speed (F), and Na
+ ionic strength (G), were not statistically significant (
Table 4). Therefore, the latter factors can be eliminated in the next investigation. Moreover, among the four significant parameters, concentration (A) was the only significantly negative effect at
P < 0.05, while the others, along with the response variable (Y), were the significantly positive effects, indicating that decreasing the concentration, and rising the dose (B), pH (C), and contact time (D) tended to improve the removal percentage of TCC. Moreover,
Figure S4 was also constructed to support the high compatibility of the 2FI model [
41]. While the residuals against runs plot suggested a random distribution without any patterns, as shown in
Figure S4a, the actual and predicted results were mostly distributed in a straight line, as shown in
Figure S4b.
The diagnostic plots also supported the evidence of the significance of the selected factors. For half-normal and normal probability plots for the seven factors (
Figure 4), statistically insignificant factors are those that have linear lines near effects, and statistically significant factors are those whose effects are considerably escaped from linear lines [
43,
44], which were determined to be concentration (A), dose (B), pH (C), and contact time (D). Meanwhile, a Pareto chart of the standardized effects (
Figure 5), and a residuals versus runs for the models (
Figure 3) shows that the t-values of effects (concentration, dosage, and pH) are higher than the “Bonferroni limit” and higher than those of other effects (contact time, temperature, shaking speed, and Na
+ ionic strength), suggesting that the most influential factors were concentration, dosage, and pH for the proposed 2FI model [
45,
46]. Based on the above analysis, the three most influential factors, TCC concentration (A), dose (B), and pH (C), were selected for the further optimization studies using a central composite design, while the other insignificant parameters were neglected.
3.3. Optimization Study
After eliminating four factors using the screening study, a central composite design analysis was used to make the experimental space with three factors, TCC concentration (A: 1.6–18.4 mg/L), MPC dose (B: 0.016–0.184 g/L), and pH (C: 2.6–9.4), as shown in
Table 2. By establishing the quadratic regression model, the second-order polynomial equation can be generated to evaluate the interactive effect of factors, and then optimize the conditions for the removal of TCC. Under optimized conditions, a confirmation test was performed to check the suitability of the proposed model. All steps for optimization study were taken as follows.
More specifically,
Table S2 lists the twenty-trial actual and predicted values for the TCC removal. The highest percentage of TCC removal was 93.0% (entry 3), while the lowest was only 53.0% (entry 14) and the average six trials were 79.8% (entries 15–20). The empirical relationships between the response (Y) and the significant factors (A, B, and C) were achieved from using a central composite design via the Design-Expert® Software [
47]:
Based on these inputs, significant coefficients such as P values, R
2 coefficients, and AP ratios can be determined from the ANOVA data (
Table 5) [
48,
49]. Generally, a quadratic model is considered statistically significant at a confidence level of 0.95, if it meets the following conditions as closely as possible: P values for models and factors are lower than 0.05, the coefficient of determination (R
2) is closer to 1.0, and the AP ratio is higher than 4.0 [
50]. By comparing the values obtained from
Table 5 with the above standards, it is evident that the proposed model was statistically significant with the confidence level at 95%.
Moreover, the residual analysis was also used to confirm the assumption of significance for the proposed model with the three factors (
Figures S5–S8). More specifically, the normal plot of residuals in
Figure S5a tends to be an “S-shape” rather than linear or a normal line. Nevertheless, Grace et al. indicated that this plot might supply a better analysis with a transformation of the response, meaning that the residuals have been divided by the estimated standard deviation of each particular residual [
51]. The predicted versus residuals plot in
Figure S5b shows a random scatter, hence, the variance is a constant against the residuals’ variables. This analysis was highly suitable for the residual plot in
Figure S6. Accordingly,
Figure S6a diagnosed a random distribution without any patterns or trends, while the actual and predicted data in
Figure S6b had high-compatibility because these points were randomly scattered along the 45-degree line. Also, Cook’s distance in
Figure S7a was used to record any changes made by the quadratic model in the case of deleting or omitting any data point [
52]. Although the Cook’s distance of two points was found to be larger than 1.0, which may lead to a lack of their accuracy, the others (18 data points) were lower than 1.0, meaning that omitting one of them is highly unlikely to vary the estimate of the regression coefficients [
53]. The Box-Cox plot for power transforms in
Figure S7b implies that the current transformation (blue line) was not in the range of the best lambda value (red line), indicating that the current transformation is not required [
54].
Figure S8a shows leverage versus run values at two sides with upper and lower the average leverage (0.5), but close to zero, revealing that the clustering of points may be acceptable [
51]. Meanwhile, DFFITS is a factor that allows for the determining of significant runs in
Figure S8b. Generally, most of the points were inside two limits (±2.12132), indicating an insignificant difference in fitness [
51]. Consequently, with the above analysis, the model for TCC removal over MPC reached a high-compatibility with the actual data at the 95% confidence level and could be used to assess the interaction among factors.
Three-dimensional (3D) response surface plots, which described the effect of two factors (another was kept at zero-level) on the removal percentage of TCC, are presented in
Figure 6. All of the figures show the significant interactions of the factors and led to changes in TCC removal efficiency.
In detail,
Figure 6a indicates the effect of concentration (A) and dose (B) on the removal of TCC over MPC. An increase in dose and a decrease in concentration is likely to enhance the overall removal yield. Meanwhile, pH is the most influential factor in
Figure 6b, with the best conditions for the removal of TCC being at a low level of pH. Indeed, it is observed that the optimum pH level was in the range from 2.6–6.0, while the effect of concentration is negligible. Liang et al. also reported the same effect of pH on the TCC removal efficiency by organic acid-coated magnetic nanoparticles [
55]. In the opposite trend,
Figure 6c shows that both dose and pH factors had a strong interaction together, and their resonance vales could bring the best removal efficiency. At a high dose (e.g., 0.184 g/L), and a low pH level (e.g., 4.0), nearly 100% of the TCC could be removed by MPC. For further analysis, the optimized conditions were recommended via a central composite design powered by the DX10, which listed the best options along with the respective desirability values to obtain the highest percentage of TCC removal. Indeed,
Table S3 lists three independent runs according to the proposed conditions. It is evident that all of the confirmation tests gave the actual results as equivalent to the proposed ones, with very low errors, again indicating the excellent compatibility between the proposed and the actual model [
56]. Nearly 100% of the TCC was eliminated under the optimized conditions (entries 1–3,
Table S3). These results proved the promising application of MPC for the separation of TCC from wastewater.
3.4. Proposed Adsorption Mechanism
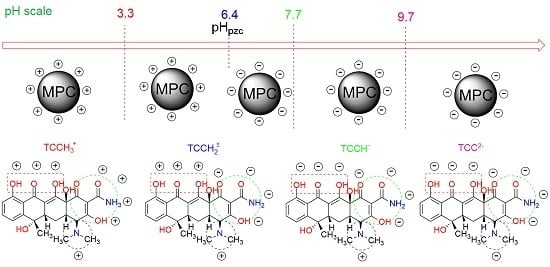
According to the screening (e.g., the Pareto chart) and optimization (e.g., response surfaces) studies, pH was the most influential factor affecting the adsorption of TCC, and thus, the investigation of pH solution was required to gain more insight into how the TCC molecules are absorbed on the MPC surface. Firstly, we fluctuated the pH values (adjusted by using HCl and NaOH solutions) in the wide range from 2 to 11 to observe the change in TCC adsorbed by MPC material, as shown in
Figure 7.
The results shown in
Figure 7a reveal that the adsorption capacity of the TCC antibiotic reached a peak of 82.4 mg/g at pH 4, while the lowest value was found to be 21.1 mg/g at pH 2. Generally, the adsorption of TCC tended to deplete, thereby increasing the pH solution from 3 to 10. It is also observed that this uptake seemed to be conducive in weak acidic media (3 ≤ pH ≤ 6) rather than in neutral or weak basic solutions (pH ≥ 7). These results were highly in-line with the optimized conditions obtained by the RSM model and confirmation tests (
Table S3), as well as with recent publications [
57,
58,
59].
To propose a plausible mechanism of how TCC molecules are adsorbed on the surface of MPC, we determined the effect of pH on the zeta potential of the adsorbent. According to the
Figure 7b, increasing the pH level of the solution would lead to a decrease in the magnitude of the zeta potential. Especially at around pH 6, the zeta potential of MPC reached a zero-value or an isoelectric point (IEP
MPC = 6), which means that the surface of the MPC tended to be negatively charged under the condition at pH > IEP
MPC = 6, and it charged positively at pH < 6. Adsorption of TCC on the surface of MPC may be contributed to by several factors, including electrostatic interaction, hydrogen bonds, and π–π interaction.
It was reported that TCC molecules have three values of acid dissociation constant with pKa
1 = 3.3, pKa
2 = 7.7, and pKa
3 = 9.7 (
Table S1). Notably, owing to the protonation or deprotonation process in water, it can exist in various states of ionic species at different pH points [
60,
61]. Kang et al. demonstrated that TCC molecules (H
2TCC) at pH values from 1 to 14 can present a wide range of ionic or neutral states as cations (H
3TCC
+), zwitterions (H
2TCC), and anions (HTCC
- and TCC
2−) [
62].
At pH < pKa
1 = 3.3, the surface of MPC becomes charged positively due to pH < IEP
MPC, thus TCC molecules are immediately protonated to form the cations (TCCH
3+), leading to an opposite charge between adsorbent and adsorbate [
63]. As a result, both components possibly appear in an “electrostatic repulsion” force [
64], preventing the contact between TCCH
3+ ions and MPC material, and finally, causing a considerable decrease in the adsorption capacity. Supporting this trend,
Figure 7a shows a low TCC adsorption capacity (21.1 mg/g) at pH 2, but when the pH values rose, the adsorbability of the MPC towards the TCC molecules increased noticeably. For example, the highest adsorption capacity (82.4 mg/g) in this case was found at pH 4. This phenomenon may be attributable to the fact that TCC molecules are partly deprotonated for the first ionization (TCCH
2±) at pKa
1 < pH < IEP
MPC, leading to the generation of an electrostatic attraction between the deprotonated TCC and the MPC surface and boosting the adsorption capacity, as reported by Liu et al. [
65]. Marzbali et al. also explained that when a negatively charged adsorbent surface increases gradually, the “electrostatic interaction” becomes stronger, resulting in intermolecular hydrogen bond forces, and increasing adsorption capacity [
63].
When the pH gradually reached IEP
MPC (pH 6), the surface of the MPC became more neutral, whereas there was no tetracycline ionization (zwitterions) at pH levels between 5 and 6, as reported by Kang et al [
62]. As a result, the effect of the “electrostatic interaction” tended to be negligible, causing a noticeable depletion in the TCC adsorption capacity. However, it was observed that the adsorption capacity still remained very high, for example, approximately 80 mg/g of TCC was adsorbed on the MPC at pH 6. Therefore, other main factors can play a crucial role in maintaining the adsorbability of MPC at the pH levels 5–6. Accordingly, both the TCC molecules and the MPC surface own π electrons on the benzene rings, generating the same type of intermolecular electron “donor–acceptor” interaction (or called the π–π interaction) as was reported by Esra et al. and Marzbali et al. [
63,
66]. The nature of this force relies on a non-hydrophobic interaction between electron-rich benzene rings in the TCC structure and the polarized aromatic rings in the carbonaceous adsorbent [
63], which are driving forces for the TCC antibiotic adsorption on MPC materials. Ghadim et al. also interpreted the same enhancement in adsorbability towards nonionic TCC at such a pH region, via the π-π interaction [
67].
Under basic solutions, there was a downward trend in the adsorption capacity, probably due to “electrostatic repulsion”, which was formed between the negatively charged MPC surface (pH > IEP
MPC) and the TCC anions (HTCC
- and TCC
2−) [
63]. This hypothesis was consolidated based on the results from
Figure 7b, which shows lower-capacity values than those at the acidic region.
3.5. Adsorption Kinetics
According to the screening study, the Pareto chart in
Figure 5 indicated that contact time is the fourth most influential factor among the seven surveyed factors, so the effect of contact time on the adsorption kinetic needs to be investigated. In addition, based on investigating the effect of pH in
Figure 7a, the solutions for the kinetic experiments were adjusted at an optimized pH 4. To begin, finding out the relationship between contact time and kinetic adsorption capacity can be achieved by placing the experiments at four concentrations (10, 20, 30, and 40 mg/L) and determining the TCC concentration by UV-Vis spectroscopy at regular periods.
In the present study, we selected the intervals (0, 10, 30, 60, 90, 120, 150, 180, and 240 min) under the constant temperature. According to
Figure 8a–d, boosting the contact time led to an increase in adsorption capacity for all plots. Moreover, larger adsorption capacities can be obtained under higher concentrations. Typically, the first 60-minute stage witnessed a sharp rise in the adsorption capacity. Next, the plots tended to reach a steadily and slowly increasing process, and finally, obtained an equilibrium-nature within 240 minutes. Therefore, further experiments could be conducted during this period. To describe the adsorption laws, we applied four non-linear kinetic models consisting of pseudo first-order, pseudo second-order, Elovich and Bangham equations.
Note that the mathematical description for these four models and error functions (R
2, MRE, and SSE) are available in the
Supplementary Materials document file. Theoretically, any model which obtained the standards: (1) most R
2 values close to 1 (adjusted R
2 > 0.9), and/or (2) MRE (%) values decrease to zero (MRE < 10%), and/or (3) obtain the lowest SSE values, is mathematically well-fitted, can reflect the relationship between the actual and the proposed data.
According to
Table 6, all nonlinear models obtained the excellence-of-fitness based on the adjusted determination of coefficients (adjusted R
2). Indeed, the adjusted R
2 values (0.9079–0.9996) reached close to 1.0, along with lower MRE (0.54–8.46) and SSE values. Pseudo first-order models may be unsuitable for describing the actual data because of their lowest adjusted R
2 (0.9079–0.9770), and greatest MRE (4.23–8.46) and SSE (410.98–1205.53) values. These results were highly agreeable with previous works [
55,
57,
68,
69,
70]. The others reflected better compatibility based on the error functions. However, the adsorption of TCC over MPC at various concentrations obeyed the Elovich model due to obtaining the highest adjusted R
2 (0.9839–0.9996)
, and the lowest MRE (0.54–3.85%) and SSE (11.71–72.06) among these kinetic models. These results were also commensurate with a recent study on the adsorption of TCC onto NaOH-activated carbon derived from macadamia-nut shells [
60]. Therefore, adsorption of TCC adheres to a heterogeneous mechanism, which is neither the desorption or interactions between adsorbed species at low surface coverage [
60]. Clearly, according to the Elovich equation, the adsorption rates (α = 536.46–3503.35 mg/g min) were far higher than the desorption rates (β = 0.05–0.12 g/mg), reflecting the absolute dominance of TCC adsorption over MPC.
3.6. Intraparticle Diffusion
To gain insight into the actual rate-controlling step for the TCC adsorption model, the Weber–Morris intraparticle diffusion equation, which assumes that the mechanism for TCC adsorption occurs in the bulk external-mass transfer, or the diffusion of TCC molecules through the micropores of MPC material and chemical reactions (adsorption/desorption) in heterogeneous phrases, could be adopted [
60]. Herein,
Figure 9 describes the intraparticle diffusion plots for TCC adsorption over MPC at various concentrations (10–40 mg/L)
According to the Weber–Morris plots at four concentrations (10–40 mg/L) in
Figure 9, the intraparticle diffusion of TCC over MPC could be divided into three stages. The first stage (0–30 min) is the most rapid adsorption stage, which may be due to the diffusion of the TCC molecules into the external surface or peripheral layers of the MPC materials. The high values of K
id,1 (11.0891–30.9549 mg/g min
1/2) in
Table 7 indicate the enormous rate of the adsorption process. The second stage describes the gradual adsorption during the next 60-min period (from 60 to 120 min) [
69]. The high R
2 values (0.9058–0.9891) obtained by the second stage of the Weber–Morris equation (
Table 7) demonstrated that the intraparticle diffusion at the second stage was the rate-limiting step. The final stage (150–240 min) tended to reach the equilibrium-nature with the slow intraparticle diffusion, mainly because of the shallow concentration of TCC remaining in the solution and a large amount of TCC molecules loaded on the micropores of the MPC. This stage had the lowest adsorption rates (K
id,3 = 0.5728–2.6756 mg/g min
1/2) (
Table 7) and its model intercepts were found to be as unequal as zero, indicating that the rate-limiting step was dominant not due to intraparticle diffusion solely [
71].
3.7. Adsorption Isotherms
The nonlinear isotherm models, including Langmuir, Freundlich, Temkin, and Dubinin-Radushkevich (D–R), could be used to better understand the adsorption mechanism and behaviors of TCC over MPC. Note that the mathematical description for their respective parameters and error functions (R
2, MRE, and SSE) are available in the
Supplementary Materials file. Moreover, based on the investigation of the effect of pH in
Figure 7a, the solutions for the isotherm experiments were adjusted to an optimized pH 4.
Figure 10 describes the effect of concentration (10–40 mg/L) on the equilibrium adsorption capacities (Q
e, mg/g), which increased the concentration resulting in an improvement in the Q
e values.
The isotherm parameters obtained from the equations were summarized in
Table 8. Naturally, all models showed excellent fitness, based on the adjusted R
2 (0.8982–0.9933), and the relatively low MRE (2.95–11.82%) and SSE (124.19–1900.35) values. According to the analysis of these error functions, the order-of-fitness for models is obeyed: Freundlich > Temkin > Langmuir > D–R, suggesting that the mechanism for TCC adsorption over MPC adhered to the Freundlich model, which assumes that multilayer adsorption behavior may be prevalent in this case. Moreover, the exponent value (1/n), which was determined by the Freundlich model, was found to be 0.37, and the R
L constant, which was determined by the Langmuir model, was found to be 0.15, suggesting that the adsorption of the TCC molecules over MPC was a favorable process.