Gas Permeability of Cellulose Aerogels with a Designed Dual Pore Space System
Abstract
:1. Introduction
2. Results and Discussion
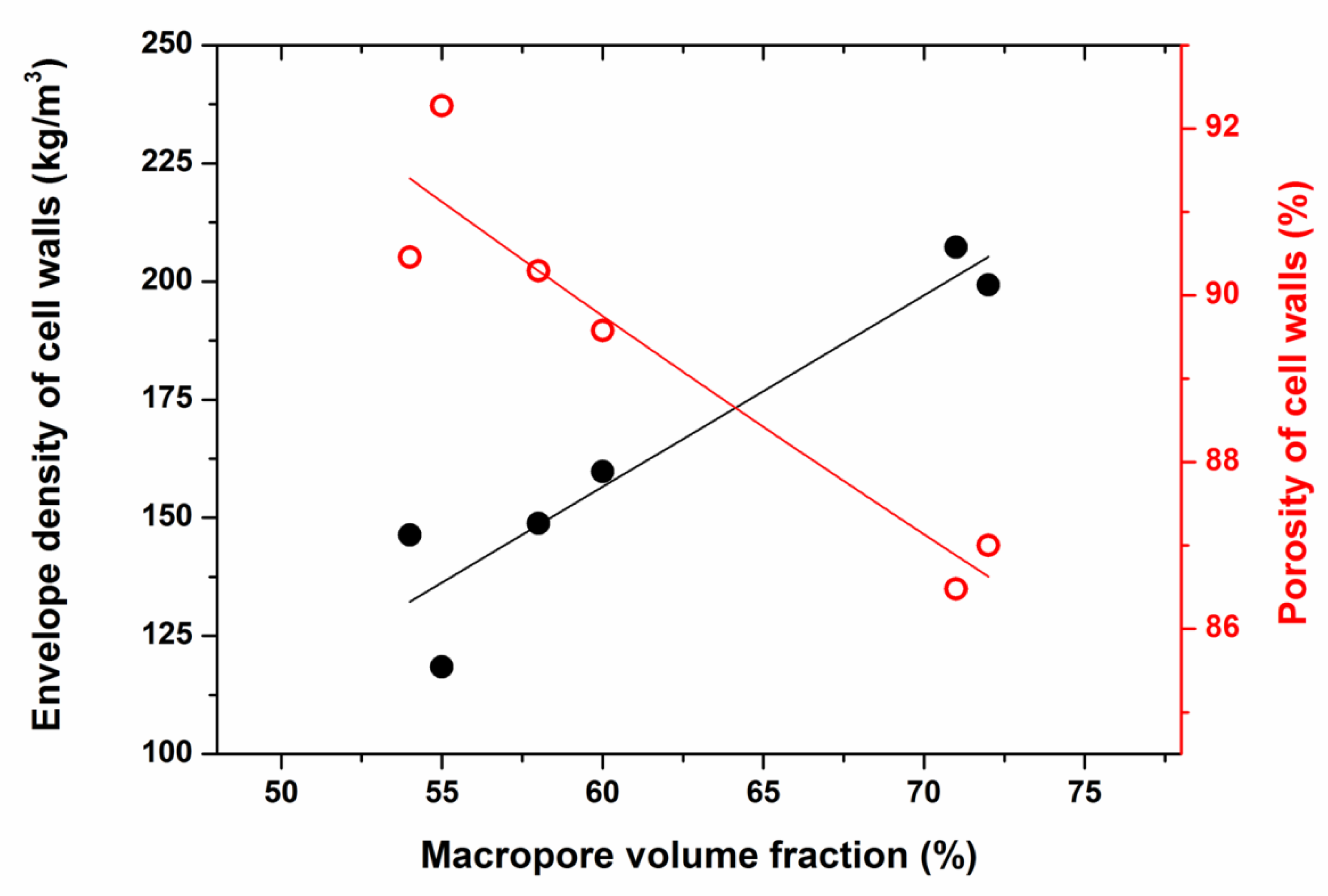
2.1. Physical Properties of Aerogels
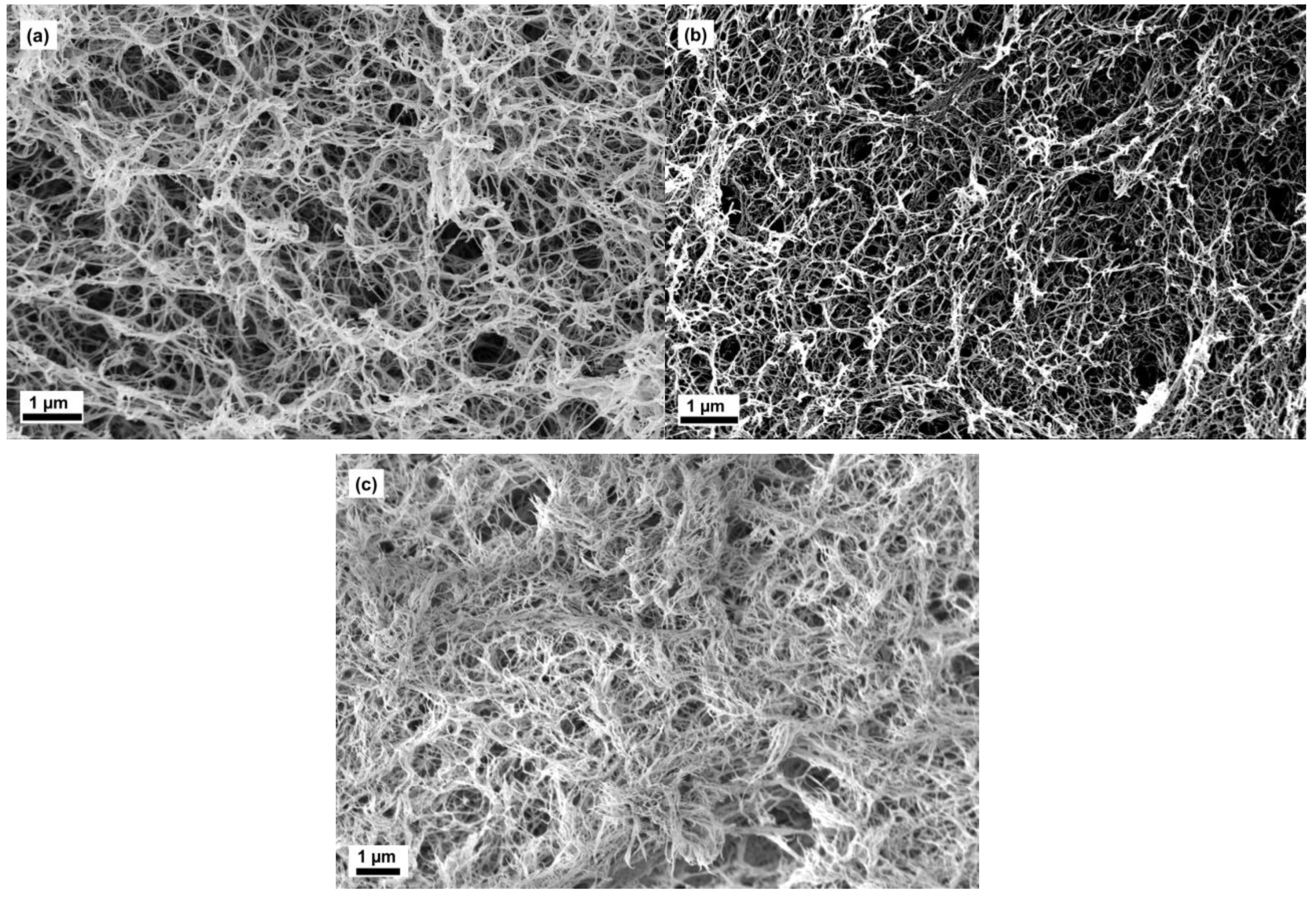
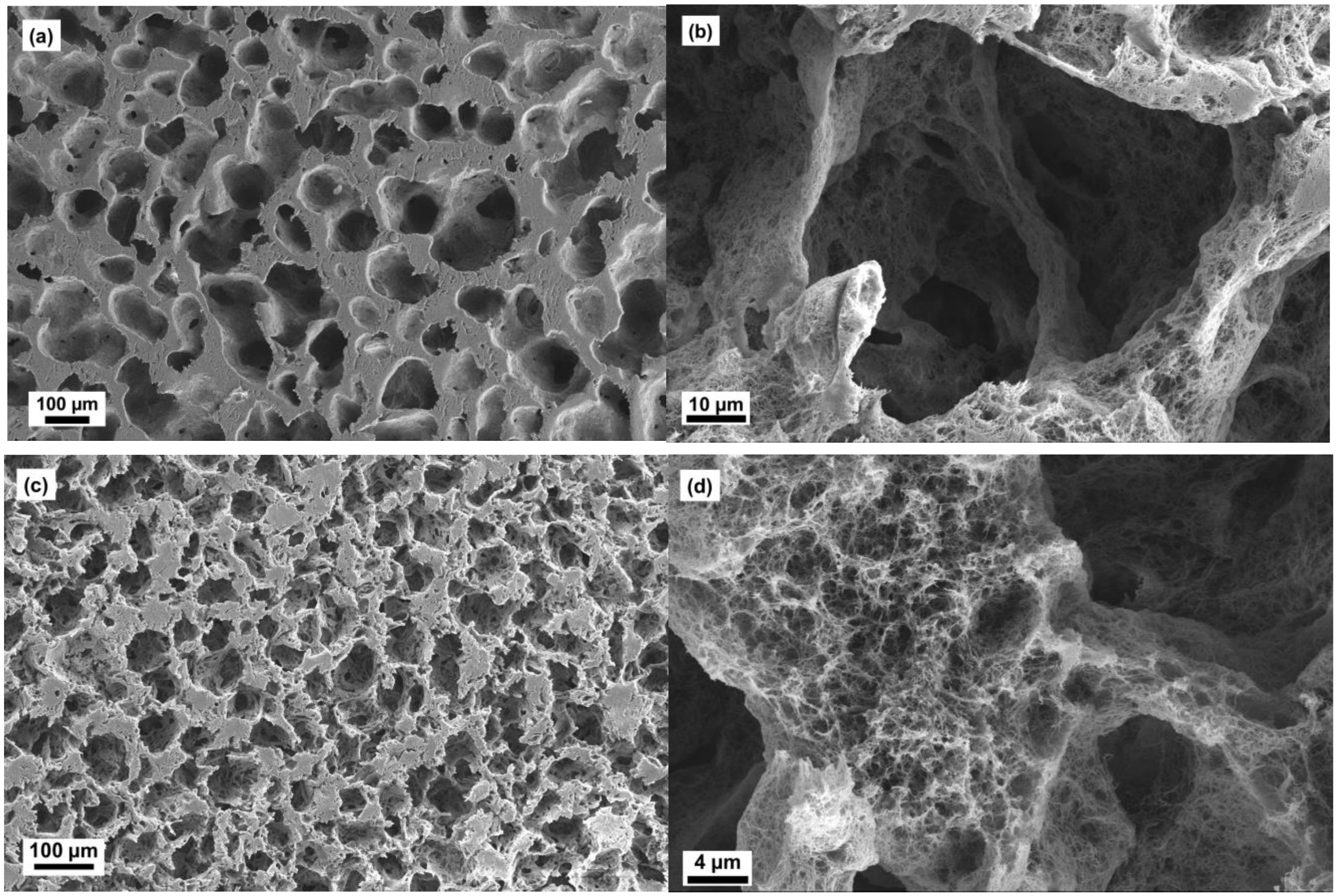
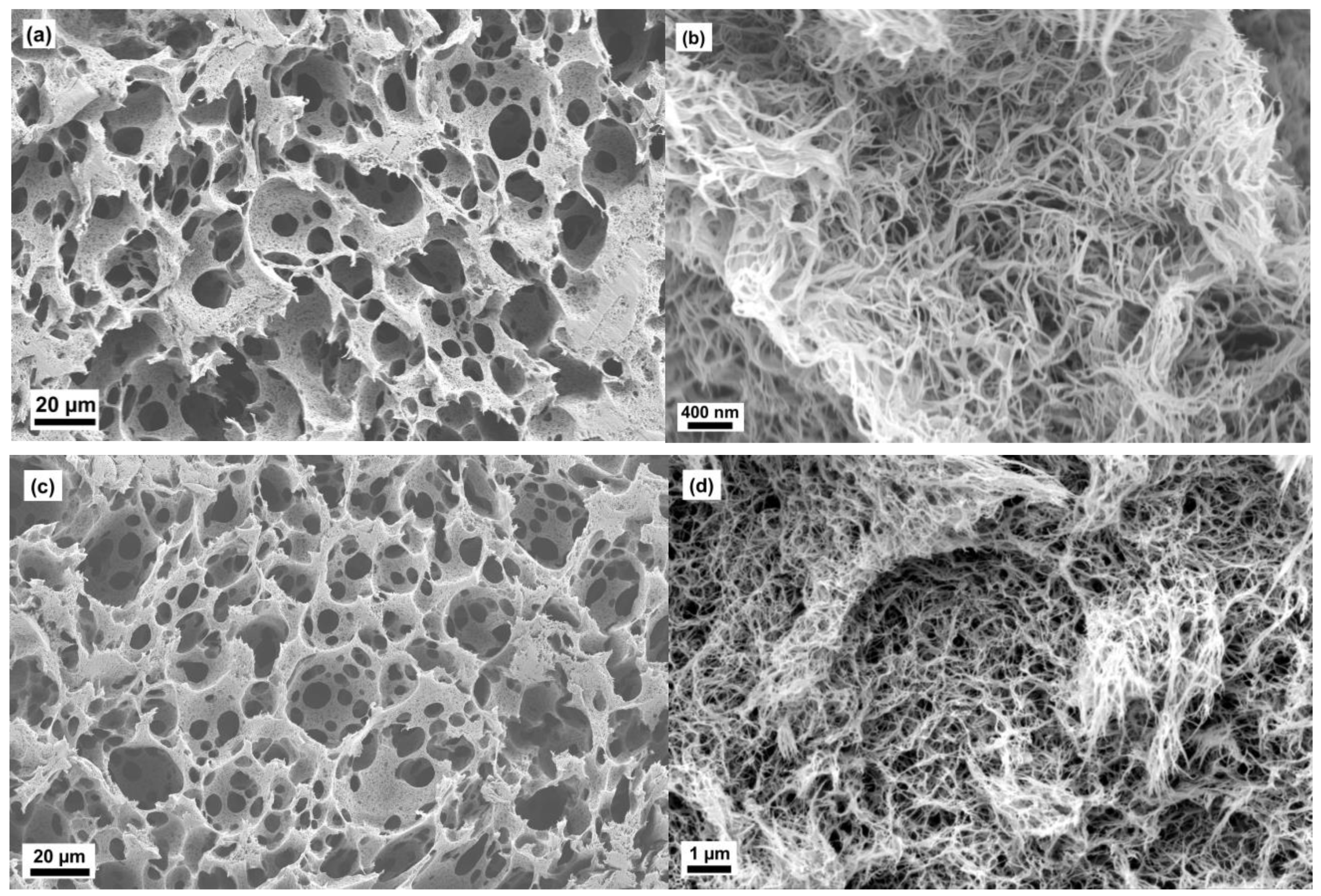
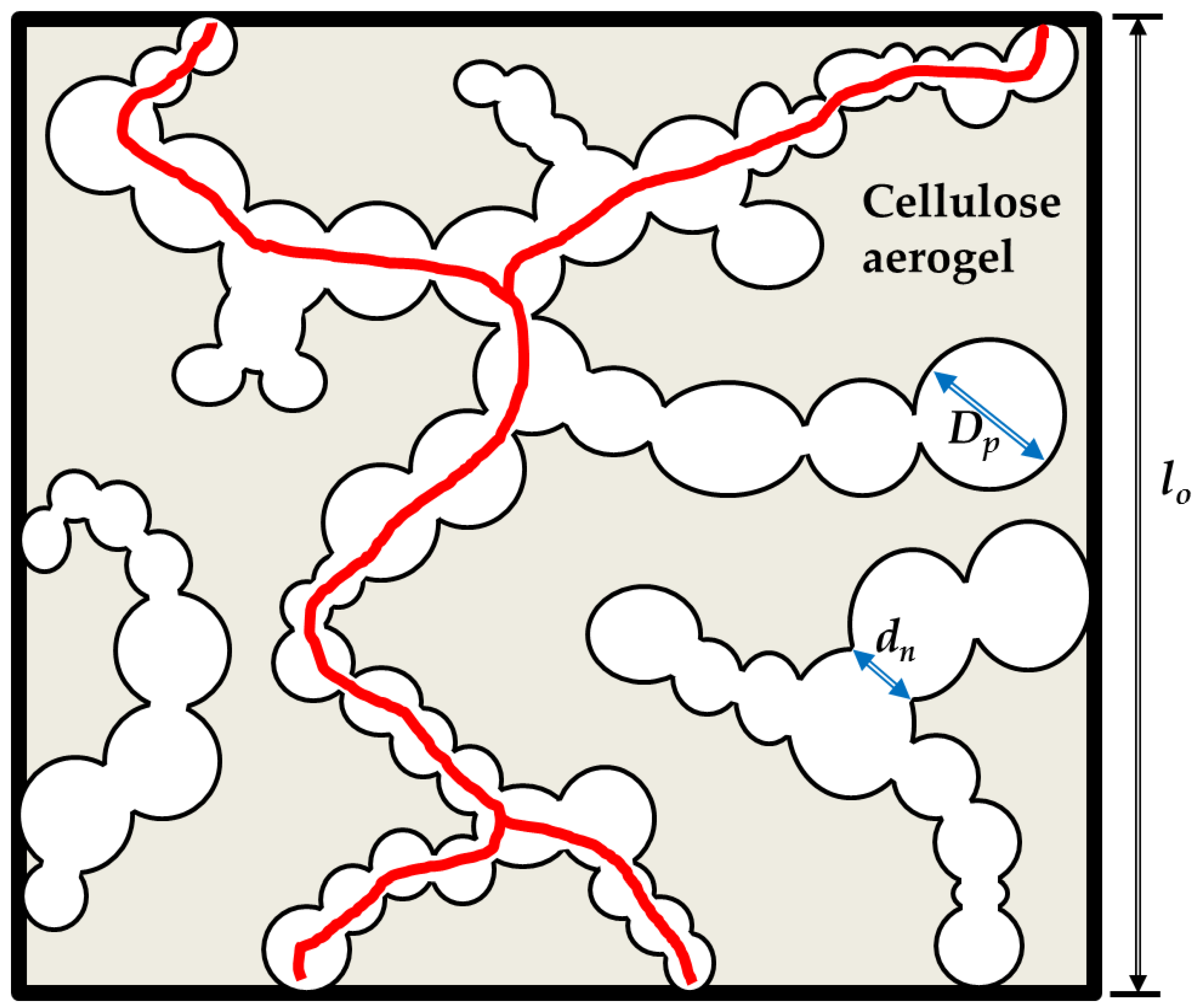
2.2. Microstructure of Aerogels of Cellulose
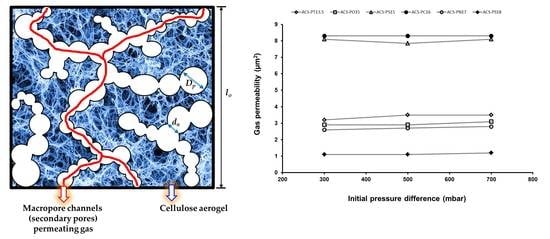
2.3. Gas Permeability Data Analysis
- the pressure–time curves followed the theoretical model made for pure Darcy flow used to fit the curves and extract the permeability constant,
- the permeability was independent of the pressure difference and
- the permeability varied with the surfactant used.
3. Materials and Methods
3.1. Synthesis of Cellulose Aerogels
3.2. Characterisation Methods
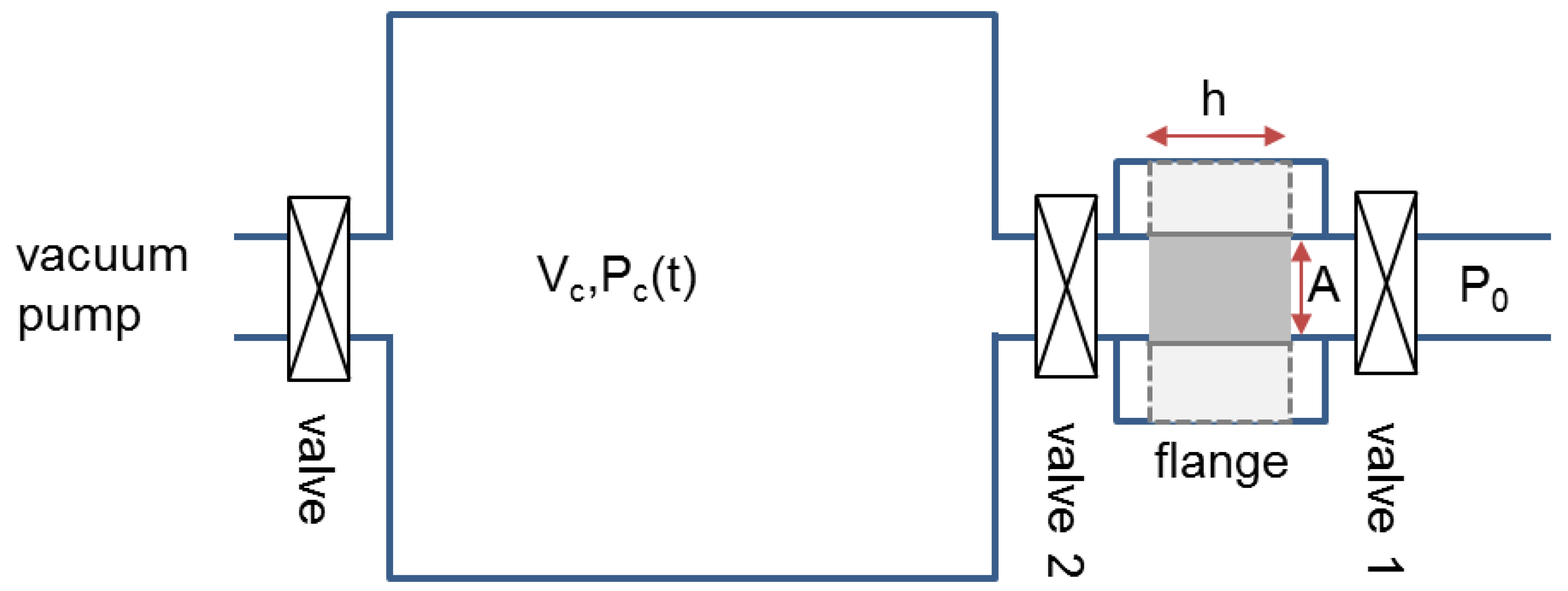
3.3. Experimental Set Up for Gas Permeability Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ratke, L. Monoliths and fibrous cellulose aerogels. In Aerogels Handbook; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; p. 173. [Google Scholar]
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019. [Google Scholar] [CrossRef]
- Boldizar, A.; Klason, C.; Kubat, J. Prehydrolysed cellulose as reinforcing filler for thermoplastics. Int. J. Polym. Mater. 1987, 11, 229–262. [Google Scholar] [CrossRef]
- Berglund, L.A. Cellulose-based nanocomposites. In Natural Fibers, Biopolymers, and Biocomposites; Mohanty, A.K., Misra, M., Drzal, L.T., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 807–832. [Google Scholar]
- Olsson, R.T.; Samir, M.A.S.A.; Salazar-Alvarez, G.; Belova, L.; Ström, V.; Berglund, L.A.; Ikkala, O.; Nogues, J.; Gedde, U.W. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat. Nanotechnol. 2010, 5, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Cervin, N.T.; Aulin, C.; Larsson, P.T.; Wågberg, L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 2012, 19, 401–410. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Le, N.T.; Le, A.T.T.; Hoang, N.; Tan, V.B.C.; Duong, H.M. Cellulose Aerogel from Paper Waste for Crude Oil Spill Cleaning. Ind. Eng. Chem. Res. 2013, 52, 18386–18391. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically cross-linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 297–298. [Google Scholar] [CrossRef]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Vuoti, S.; Laatikainen, E.; Heikkinen, H.; Johansson, L.-S.; Saharinen, E.; Retulainen, E. Chemical modification of cellulosic fibers for better convertibility in packaging applications. Carbohydr. Polym. 2013, 96, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.D. New chemically modified cellulosic fibers. Ind. Eng. Chem. 1958, 50, 73. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Licciardello, A. Surface chemical modification of natural cellulose fibers. J. Appl. Polym. Sci. 2002, 83, 38–45. [Google Scholar] [CrossRef]
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated Cellulose Surface Modification: A Review. Materials 2013, 6, 1745–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Ganesan, K.; Barowski, A.; Ratke, L.; Milow, B. Influence of hierarchical porous structures on the mechanical properties of cellulose aerogels. J. Sol-Gel Sci. Technol. 2019, 89, 156–165. [Google Scholar] [CrossRef]
- Deng, M.; Zhou, Q.; Du, A.; Kasteren, J.; Wang, Y. Preparation of nanoporous cellulose faoms from cellulose-ionic liquid solutions. Mater. Lett. 2009, 63, 1851–1854. [Google Scholar] [CrossRef]
- Pircher, N.; Fischhuber, D.; Carbajal, L.; Strauß, C.; Nedelec, J.-M.; Kasper, C.; Rosenau, T.; Liebner, F. Preparation and Reinforcement of Dual-Porous Biocompatible Cellulose Scaffolds for Tissue Engineering. Macromol. Mater. Eng. 2015, 300, 911–924. [Google Scholar] [CrossRef]
- Ganesan, K.; Ratke, L. Facile preparation of monolithic -carrageenan aerogels. Soft Matter 2014, 10, 3218–3224. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef]
- Kaviany, M. Principles of Heat Transfer in Porous Media, 2 ed.; Springer-Verlag New York: Berlin, Germany, 1995. [Google Scholar]
- Tanikawa, W.; Shimamoto, T. Klinkenberg effect for gas permeability and its comparison to water permeability for porous sedimentary rocks. Hydrol. Earth Syst. Sci. Discuss. 2006, 3, 1315–1338. [Google Scholar] [CrossRef]
- Scheidegger, A.E. The Physics of Flow through Porous Media, 3 ed.; University of Toronto Press: Toronto, ON, Canada, 1974; p. 353. [Google Scholar]
- Hoepfner, S.; Ratke, L.; Milow, B. Synthesis and characterisation of nanofibrillar cellulose aerogels. Cellulose 2008, 15, 121–129. [Google Scholar] [CrossRef]
- Karadagli, I.; Schulz, B.; Schestakow, M.; Milow, B.; Gries, T.; Ratke, L. Production of porous cellulose aerogel fibers by an extrusion process. J. Supercrit. Fluids 2015, 106, 105–114. [Google Scholar] [CrossRef]
- Reuß, M.; Ratke, L. Characterisation of Carbon-AeroSands. Giessereiforschung 2009, 61, 24–33. [Google Scholar]
- Torrent, R.J. A two-chamber vacuum cell for measuring the coefficient of permeability to air of the concrete cover on site. Mater. Struct. 1992, 25, 358–365. [Google Scholar] [CrossRef]
- Churchill, S.W. Viscous Flows: The Practical Use of Theory; Butterworth publishers: Oxford, UK, 1988; p. 602. [Google Scholar]
- Vazquez, J.L. The Porous Medium Equation: Mathematical Theory; Oxford University Press: Oxford, UK, 2006; p. 648. [Google Scholar]
- Jannot, Y.; Lasseux, D. A new quasi-steady method to measure gas permeability of weakly permeable porous media. Rev. Sci. Instrum. 2012, 83, 015113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, R.E.; Williams, R.J.J. Diffusion in Gases and Porous Media; Springer Science+Business Media: New York, NY, USA, 1980. [Google Scholar]


| Sample | Envelope Density, ρe (kg/m3) | Total Porosity (%) | BET Specific Surface Area (m2/g) |
|---|---|---|---|
| AC-2 | 63 ± 0.15 | 95.9 | 287 |
| AC-4 | 108.8 ± 12.4 | 92.9 | 314 |
| AC-6 | 137.9 ± 1 | 91.0 | 282 |
| ACS-PT13.5 | 62.5 ± 2.5 | 95.9 | 304 |
| ACS-PO15 | 53.3 ± 0.9 | 96.4 | 301 |
| ACS-PS15 | 63.9 ± 0.2 | 95.8 | 327 |
| ACS-PC16 | 67.3 ± 1 | 95.6 | 296 |
| ACS-PN17 | 60.1 ± 0.9 | 96.1 | 262 |
| ACS-PS18 | 55.8 ± 2.3 | 96.3 | 245 |
| Sample | Volume Fraction of Macropores | Secondary Pore Diameter, µm | Neck Diameter, µm | Cell Wall Thickness, µm |
|---|---|---|---|---|
| ACS-PT13.5 | 58 ± 4 | 50–600 | 10–20 | 0.75–100 |
| ACS-PO15 | 55 ± 4 | 100–400 | 50–100 | 35–175 |
| ACS-PS15 | 60 ± 4 | 75–150 | 20–75 | 10–50 |
| ACS-PC16 | 54 ± 4 | 50–110 | 10–40 | 10–20 |
| ACS-PN17 | 71 ± 4 | 30–40 | 4–20 | 1–3 |
| ACS-PS18 | 72 ± 4 | 20–30 | 2–10 | 0.25–2 |
| Sample | Permeability Constant, K, µm2 |
|---|---|
| AC-2 | 0.0465 a |
| AC-4 | 0.005 a |
| AC-6 | 0.0036 a |
| ACS-PT13.5 | 3.40 b |
| ACS-PO15 | 4.61 b |
| ACS-PS15 | 8.02 b |
| ACS-PC16 | 8.30 b |
| ACS-PN17 | 2.70 b |
| ACS-PS18 | 1.13 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesan, K.; Barowski, A.; Ratke, L. Gas Permeability of Cellulose Aerogels with a Designed Dual Pore Space System. Molecules 2019, 24, 2688. https://doi.org/10.3390/molecules24152688
Ganesan K, Barowski A, Ratke L. Gas Permeability of Cellulose Aerogels with a Designed Dual Pore Space System. Molecules. 2019; 24(15):2688. https://doi.org/10.3390/molecules24152688
Chicago/Turabian StyleGanesan, Kathirvel, Adam Barowski, and Lorenz Ratke. 2019. "Gas Permeability of Cellulose Aerogels with a Designed Dual Pore Space System" Molecules 24, no. 15: 2688. https://doi.org/10.3390/molecules24152688