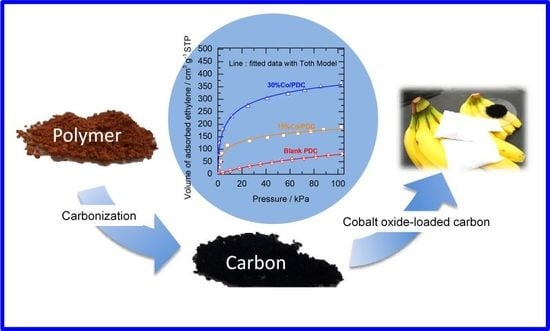
Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit
Abstract
:1. Introduction
2. Results and Discussion
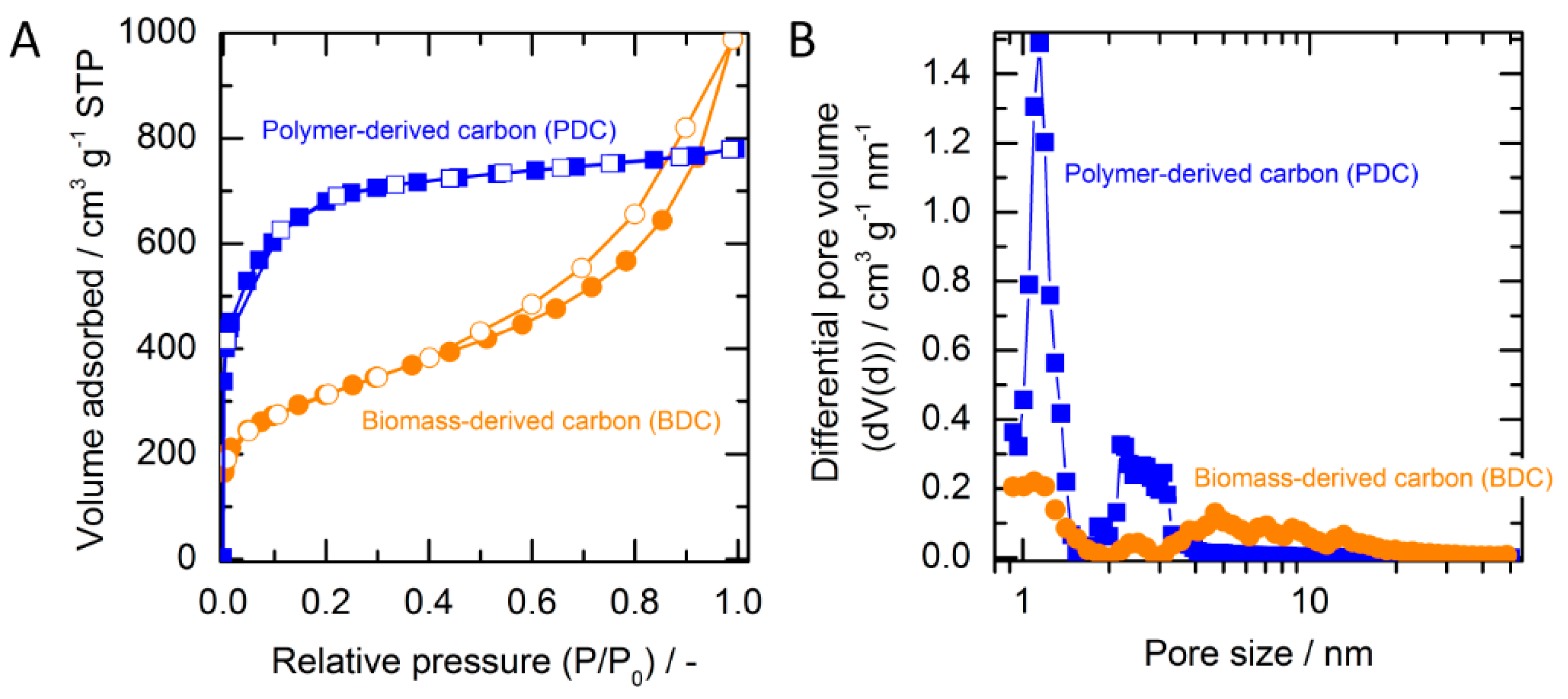
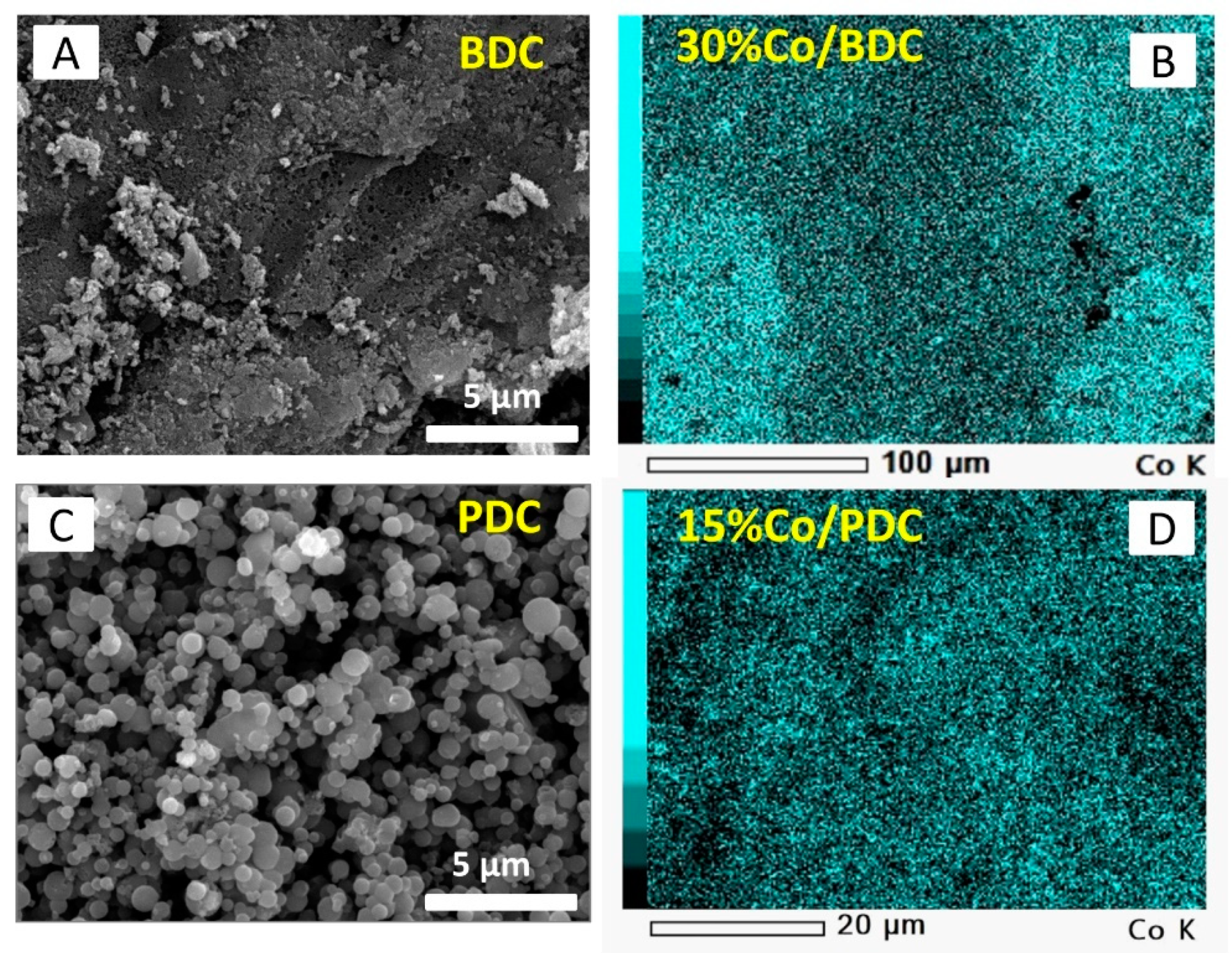
2.1. Adsorbent Characterization
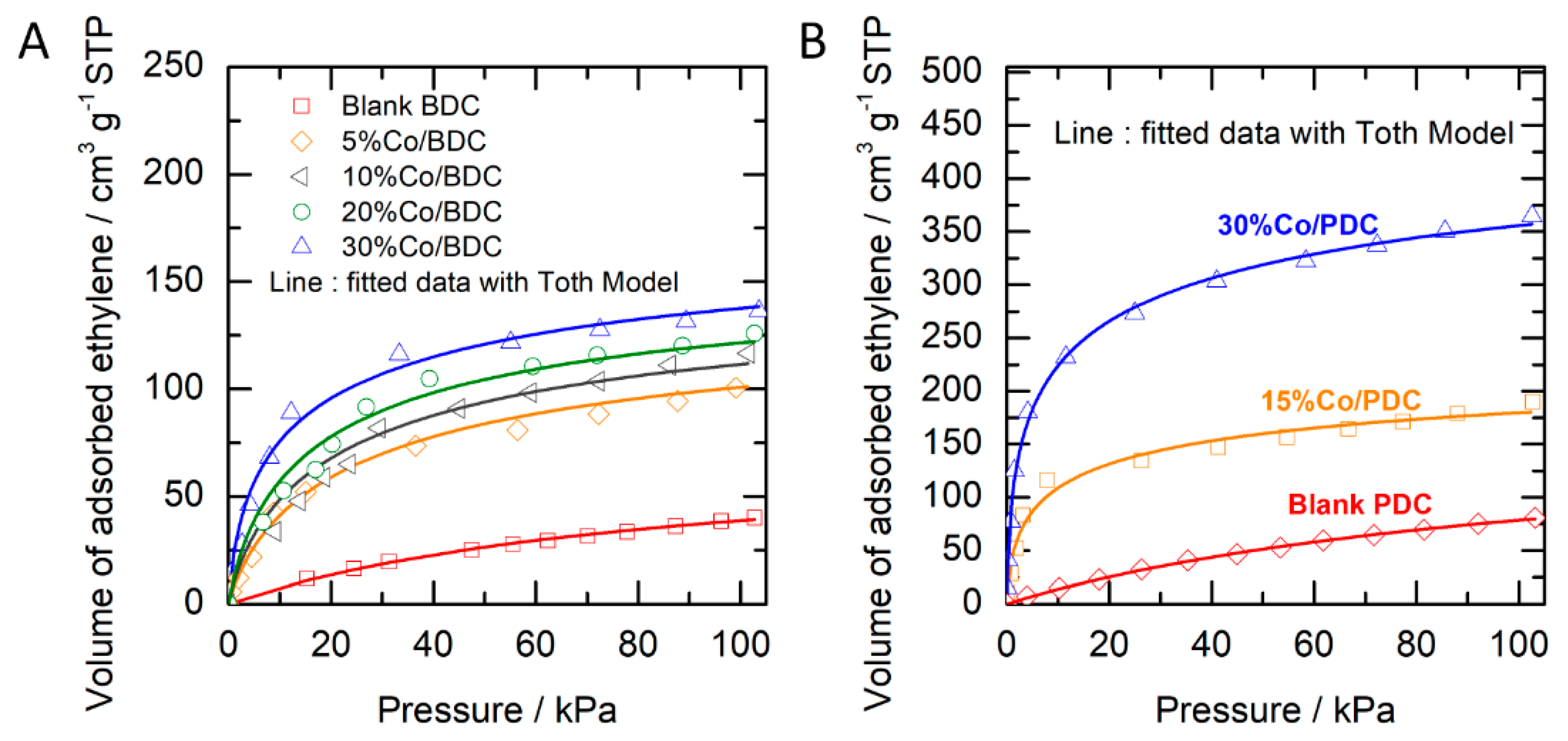
2.2. Ethylene Adsorption
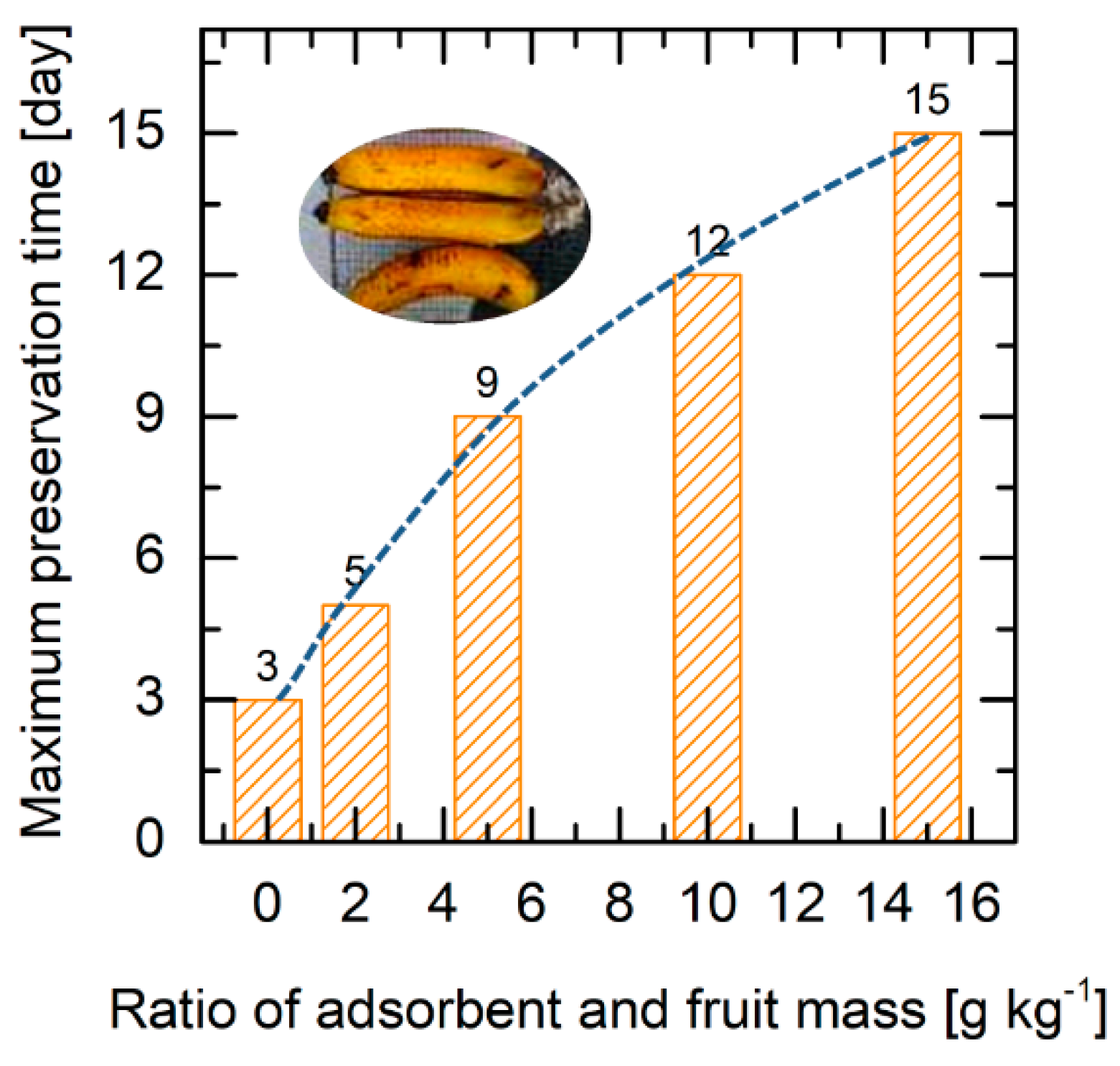
2.3. Fruit Preservation Test
3. Materials and Methods
3.1. Nanoporous Carbon Preparation
3.2. Cobalt Oxide-Impregnated Nanoporous Carbon Preparation
3.3. Characterization Methods
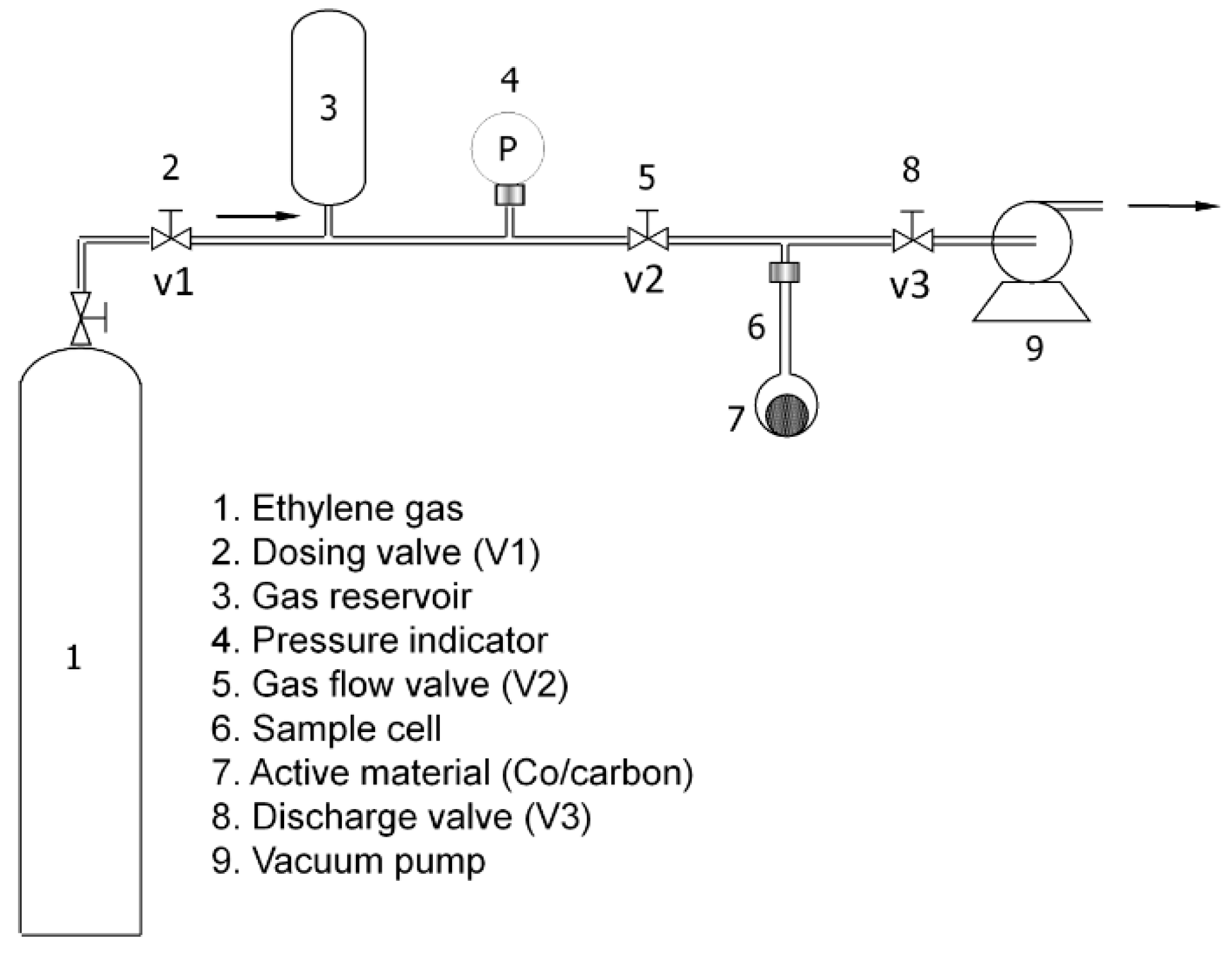
3.4. Ethylene Adsorption Measurement
3.5. Preservation Test
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dermesonluoglu, E.; Katsaros, G.; Tsevdou, M.; Giannakourou, M.; Taoukis, P. Kinetic study of quality indices and shelf life modelling of frozen spinach under dynamic conditions of the cold chain. J. Food Eng. 2015, 148, 13–23. [Google Scholar] [CrossRef]
- Paull, R.E. Effect of temperature and relative humidity on fresh commodity quality. Postharvest Biol. Technol. 1999, 15, 263–277. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Bailén, G.; Serrano, M.; Guillén, F.; Valverde, J.M.; Zapata, P.; Castillo, S.; Valero, D. Tools to maintain postharvest fruit and vegetable quality through the inhibition of ethylene action: A review. Crit. Rev. Food Sci. Nutr. 2007, 47, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; Warton, M.A. Efficacy of potassium permanganate impregnated into alumina beads to reduce atmospheric ethylene. J. Am. Soc. Hortic. Sci. 2004, 129, 433–438. [Google Scholar] [CrossRef]
- Jayaraman, K.S.; Raju, P.S. Development and evaluation of a permanganate-based ethylene scrubber for extending the shelf life of fresh fruits and vegetables. J. Food Sci. Technol. India 1992, 29, 77–83. [Google Scholar]
- Singh, R.; Giri, S. Shelf-life study of Guava under active packaging: An experiment with potassium permanganate salt as ethylene absorbent. J. Food Saf. Food Qual. 2014, 65, 32–39. [Google Scholar]
- Cao, J.; Li, X.; Wu, K.; Jiang, W.; Qu, G. Preparation of a novel PdCl2–CuSO4–based ethylene scavenger supported by acidified activated carbon powder and its effects on quality and ethylene metabolism of broccoli during shelf-life. Postharvest Biol. Technol. 2015, 99, 50–57. [Google Scholar] [CrossRef]
- Sue-aok, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Study of ethylene adsorption on zeolite NaY modified with group I metal ions. Appl. Surf. Sci. 2010, 256, 3997–4002. [Google Scholar] [CrossRef]
- Mukti, N.I.F.; Prasetyo, I.; Mindaryani, A. Preparasi karbon teremban oksida cobalt dari limbah kulit manggis sebagai adsorben penjerap etilen untuk pengawetan buah. Reaktor 2015, 15, 165–174. [Google Scholar] [CrossRef]
- Mukti, N.I.F. Pemanfaatan Limbah Sisa Ekstraksi Kulit Manggis Sebagai Bahan Pembuat Adsorben Penjerap Etilen Untuk Pengawetan Buah. Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2015. [Google Scholar]
- Zhao, X.S.; Bao, X.Y.; Guo, W.; Lee, F.Y. Immobilizing catalysts on porous materials. Mater. Today 2006, 9, 32–39. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Tan, W.; Han, W.; Jiang, Y.; Guo, G. Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal. 2014, 309, 408–418. [Google Scholar] [CrossRef]
- Zuo, S.; Liu, F.; Tong, J.; Qi, C. Complete oxidation of benzene with cobalt oxide and ceria using the mesoporous support SBA-16. Appl. Catal. A Gen. 2013, 467, 1–6. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Wang, J.; Qiao, W.; Long, D.; Ling, L. Low temperature catalytic combustion of ethylene over cobalt oxide supported mesoporous carbon spheres. Chem. Eng. J. 2016, 293, 243–251. [Google Scholar] [CrossRef]
- Prasetyo, I.; Mukti, N.I.F.; Fahrurrozi, M.; Ariyanto, T. Removing Ethylene by Adsorption using Cobalt Oxide-Loaded Nanoporous Carbon. Asean J. Chem. Eng. 2018, 18, 9–16. [Google Scholar]
- Sharma, S.; Pollet, B.G. Support materials for PEMFC and DMFC electrocatalysts—A review. J. Power Sources 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Sigot, L.; Fontseré Obis, M.; Benbelkacem, H.; Germain, P.; Ducom, G. Comparing the performance of a 13X zeolite and an impregnated activated carbon for H2S removal from biogas to fuel an SOFC: Influence of water. Int. J. Hydrogen Energy 2016, 41, 18533–18541. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yang, G.; Zhang, J.; Wang, Y.; Yao, M. Activated Carbon Preparation from Lignin by H3PO4 Activation and Its Application to Gas Separation. Chem. Eng. Technol. 2011, 35, 309–316. [Google Scholar] [CrossRef]
- Yi, H.; Li, F.; Ning, P.; Tang, X.; Peng, J.; Li, Y.; Deng, H. Adsorption separation of CO2, CH4, and N2 on microwave activated carbon. Chem. Eng. J. 2013, 215, 635–642. [Google Scholar] [CrossRef]
- Lenghaus, K.; GuangHua Qiao, G.; Solomon, D.H.; Gomez, C.; Rodriguez-Reinoso, F.; Sepulveda-Escribano, A. Controlling carbon microporosity: The structure of carbons obtained from different phenolic resin precursors. Carbon 2002, 40, 743–749. [Google Scholar] [CrossRef]
- Glasel, J.; Diao, J.Y.; Feng, Z.B.; Hilgart, M.; Wolker, T.; Su, D.S.; Etzold, B.J.M. Mesoporous and graphitic carbide-derived carbons as selective and stable catalysts for the dehydrogenation reaction. Chem. Mater. 2015, 27, 5719–5725. [Google Scholar] [CrossRef]
- Moreira, R.F.P.M.; José, H.J.; Rodrigues, A.E. Modification of pore size in activated carbon by polymer deposition and its effects on molecular sieve selectivity. Carbon 2001, 39, 2269–2276. [Google Scholar] [CrossRef]
- Prasetyo, I.; Rochmadi, R.; Wahyono, E.; Ariyanto, T. Controlling synthesis of polymer-derived carbon molecular sieve and its performance for CO2/CH4 separation. Eng. J. 2017, 21, 83–94. [Google Scholar] [CrossRef]
- Choi, B.-U.; Choi, D.-K.; Lee, Y.-W.; Lee, B.-K.; Kim, S.-H. Adsorption equilibria of methane, ethane, ethylene, nitrogen, and hydrogen onto activated carbon. J. Chem. Eng. Data 2003, 48, 603–607. [Google Scholar] [CrossRef]
- Ariyanto, T.; Kern, A.; Etzold, B.J.M.; Zhang, G.-R. Carbide-derived carbon with hollow core structure and its performance as catalyst support for methanol electro-oxidation. Electrochem. Commun. 2017, 82, 12–15. [Google Scholar] [CrossRef]
- Fraga, M.A.; Jordao, E.; Mendes, M.J.; Freitas, M.M.A.; Faria, J.L.; Figueiredo, J.L. Properties of carbon-supported platinum catalysts: Role of carbon surface sites. J. Catal. 2002, 209, 355–364. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics, 2nd ed.; Imperial College Press: London, UK, 1998. [Google Scholar]
- Fogler, H.S. Essentials of Chemical Reaction Engineering, 5th ed.; Pearson Education: London, UK, 2010. [Google Scholar]
- Prasetyo, I.; Rochmadi; Ariyanto, T.; Yunanto, R. Simple method to produce nanoporous carbon for various applications by pyrolysis of specially synthesized phenolic resin. Indones. J. Chem. 2013, 13, 95–100. [Google Scholar] [CrossRef]
- Becker, H.; Turek, T.; Güttel, R. Study of temperature-programmed calcination of cobalt-based catalysts under NO-containing atmosphere. Catal. Today 2013, 215, 8–12. [Google Scholar] [CrossRef]
- Wang, C.-B.; Lin, H.-K.; Tang, C.-W. Thermal Characterization and Microstructure Change of Cobalt Oxides. Catal. Lett. 2004, 94, 69–74. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A. V Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N2-sorption data by DR-, BET- and t-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Porous Textural Parameter | SSAa, m2 g−1 | %SSAMic b, % | VTc, cm3 g−1 | %Vmic d, % | dve, nm |
|---|---|---|---|---|---|
| Commercial activated carbon | 1050 | 55 | 0.46 | 82 | 1.75 |
| Biomass-derived carbon (BDC) | 1080 | 32 | 1.53 | 10 | 5.66 |
| Polymer-derived carbon (PDC) | 2390 | 84 | 1.21 | 60 | 2.03 |
| 15% Co/PDC | 1469 | 92 | 0.70 | 70 | 1.92 |
| 30% Co/PDC | 928 | 87 | 0.49 | 63 | 2.10 |
| Parameter | Cobalt Oxide/Biomass-Derived Carbon | Cobalt oxide/Polymer-Derived Carbon | ||||||
|---|---|---|---|---|---|---|---|---|
| Blank BDC | 5%Co/BDC | 10%Co/BDC | 20%Co/BDC | 30%Co/BDC | Blank PDC | 15%Co/PDC | 30%Co/PDC | |
| Cµs, cm3 g−1 | 76.57 | 147.08 | 159.35 | 164.38 | 195.51 | 174.24 | 273.10 | 553.70 |
| b, kPa−1 | 0.01 | 0.06 | 0.08 | 0.10 | 0.27 | 0.01 | 0.92 | 1.76 |
| t, - | 0.94 | 0.67 | 0.65 | 0.66 | 0.51 | 0.95 | 0.39 | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasetyo, I.; Mukti, N.I.F.; Ariyanto, T. Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit. Molecules 2019, 24, 1507. https://doi.org/10.3390/molecules24081507
Prasetyo I, Mukti NIF, Ariyanto T. Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit. Molecules. 2019; 24(8):1507. https://doi.org/10.3390/molecules24081507
Chicago/Turabian StylePrasetyo, Imam, Nur Indah Fajar Mukti, and Teguh Ariyanto. 2019. "Ethylene Adsorption Using Cobalt Oxide-Loaded Polymer-Derived Nanoporous Carbon and Its Application to Extend Shelf Life of Fruit" Molecules 24, no. 8: 1507. https://doi.org/10.3390/molecules24081507