Catalytic Activity of Polynuclear vs. Dinuclear Aroylhydrazone Cu(II) Complexes in Microwave-Assisted Oxidation of Neat Aliphatic and Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
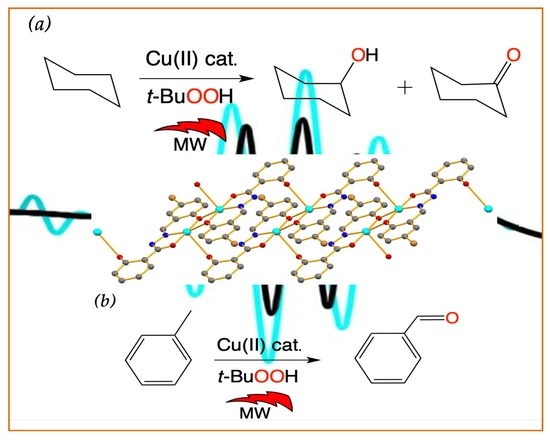
2.2. Microwave-Assisted Peroxidative Oxidation of Cyclohexane and Toluene
3. Materials and Methods
3.1. General Materials and Equipment
3.2. Syntheses of the Pro-Ligand H2L
3.3. Synthesis of [Cu(L)]n (1)
3.4. X-Ray Measurements
3.5. Catalytic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estes, L.L.; Schweizer, M. Fibers, 4 Polyamide Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 2011; Volume 11, pp. 41–49. [Google Scholar]
- Ahmed, J.; Ahuja, S.; Ali, I.; Anderson, C.; Anderson, M.J.; Arai, Y.; Braglia, M.; Babirad, S.; Bag, D.; Bagchi, A.; et al. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Clerici, M.G.; Ricci, M.; Strukul, G. Metal-Catalysis in Industrial Organic Processes; Chiusoli, G.P., Maitlis, P.M., Eds.; Royal Society of Chemistry: Cambridge, UK, 2006. [Google Scholar]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes: Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 15–16, 2236–2252. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Vanadium complexes: Recent progress in oxidation catalysis. Coord. Chem. Rev. 2015, 301–302, 200–239. [Google Scholar] [CrossRef]
- Shul’pin, G.B. New trends in oxidative functionalization of carbon–hydrogen bonds: A review. Catalysts 2016, 6, 50. [Google Scholar] [CrossRef]
- Albrecht, M.; Lindner, M.M. Cleavage of unreactive bonds with pincer metal complexes. Dalton Trans. 2011, 40, 8733–8744. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Musa, S. Coordination versatility of sp3-Hybridized pincer ligands toward ligand—Metal cooperative catalysis. ACS Catal. 2012, 2, 2456–2466. [Google Scholar] [CrossRef]
- Biniuri, Y.; Albada, B.; Wolff, M.; Golub, E.; Gelman, D.; Willner, I. Cu2+ or Fe3+ terpyridine/aptamer conjugates: Nucleoapzymes catalyzing the oxidation of dopamine to aminochrome. ACS Catal. 2018, 8, 1802–1809. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Korlyukov, A.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Lamaty, F.; Bantreil, X.; Villemejeanne, B.; et al. Si10Cu6N4 cage hexacoppersilsesquioxanes containing N ligands: Synthesis, structure, and high catalytic activity in peroxide oxidations. Inorg. Chem. 2017, 56, 15026–15040. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Fernández-Baeza, J.; Lara-Sánchez, A.; Sánchez-Barba, L.F. Metal complexes with heteroscorpionate ligands based on the bis(pyrazol-1-yl)methane moiety: Catalytic chemistry. Coord. Chem. Rev. 2013, 257, 1806–1868. [Google Scholar] [CrossRef]
- Potter, M.E.; Paterson, A.J.; Raja, R. Transition metal versus heavy metal synergy in selective catalytic oxidations. ACS Catal. 2012, 2, 2446–2451. [Google Scholar] [CrossRef]
- Gramage-Doria, R.; Armspach, D.; Matt, D. Metallated cavitands (calixarenes, resorcinarenes, cyclodextrins) with internal coordination sites. Coord. Chem. Rev. 2013, 257, 776–816. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Catalysis by metal nanoparticles embedded on metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 5262–5284. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.; Martins, L.M.D.R.S.; Mahmudov, K.T.; Kopylovich, M.N.; Drew, M.G.B.; Pettinari, C.; Pombeiro, A.J.L. Microwave-assisted and solvent-free peroxidative oxidation of 1-phenylethanol to acetophenone with a Cu(II)-TEMPO catalytic system. Catal. Commun. 2014, 48, 4048–4058. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Scorpionate vanadium, iron and copper complexes as selective catalysts for the peroxidative oxidation of cyclohexane under mild conditions. Adv. Synth. Catal. 2008, 350, 706–716. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Hazra, S.; Pombeiro, A.J.L. Catalytic oxidation of cyclohexane with hydrogen peroxide and a tetracopper(II) complex in an ionic liquid. Rendus Rendu Chim. 2015, 18, 758–765. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Nasani, R.; Saha, M.; Mobin, S.M.; Mukhopadhyay, S.; Pombeiro, A.J.L. Greener selective cycloalkane oxidation with hydrogen peroxide catalyzed by copper-5-(4-pyridyl)tetrazolate metal-organic frameworks. Molecules 2015, 20, 19203–19220. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear Cu(II) structural isomers: Coordination, magnetism, electrochemistry and catalytic activity toward oxidation of alkanes. Eur. J. Inorg. Chem. 2015, 3959–3969. [Google Scholar] [CrossRef]
- Timokhin, I.; Pettinari, C.; Marchetti, F.; Pettinari, R.; Condello, F.; Galli, S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Novel coordination polymers with (pyrazolato)-based tectons: Catalytic activity in the peroxidative oxidation of alcohols and cyclohexane. Cryst. Growth Des. 2015, 15, 2303–2317. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Sabbatini, A.; Drew, M.G.B.; Martins, L.M.D.R.S.; Pettinari, C.; Pombeiro, A.J.L. Cooperative Metal-Ligand Assisted E/Z Isomerisation and Cyano-groups Activation at CuII and CoII Complexes of Arylhydrazones of Active Methylene Nitriles. Inorg. Chem. 2014, 53, 9946–9958. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Aroylhydrazone Cu(II) complexes in keto form: Structural characterization and catalytic activity towards cyclohexane oxidation. Molecules 2016, 21, 425. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Levitsky, M.M.; Khrustalev, V.N.; Zubavichus, Y.V.; Shul’pina, L.S.; Shubina, E.S.; Shul’pin, G.B. Mild and regioselective hydroxylation of methyl group in neocuproine: Approach to an N,O-ligated Cu6 cage phenylsilsesquioxane. Organometallics 2018, 37, 168–171. [Google Scholar] [CrossRef]
- Allen, S.E.; Walvoord, R.R.; Salinas, R.P.; Kozlowski, M.C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, J.; Zhao, M.; Lv, Y.; Li, G.; Hu, C. Partial oxidation of toluene in CH3COOH by H2O2 in the presence of VO(acac)2 catalyst. J. Phys. Chem. 2009, 113, 14270–14278. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Catalytic oxidation of alcohols: Recent advances. Adv. Organomet. Chem. 2015, 63, 91–174. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Microwaves in Catalysis: Methodology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Oxido vanadium complexes with tridentate aroylhydrazone as catalyst precursors for solvent-free microwave-assisted oxidation of alcohol. Appl. Catal. A Gen. 2015, 493, 50–57. [Google Scholar] [CrossRef]
- Alexandru, M.; Cazacu, M.; Arvinte, A.; Shova, S.; Turta, C.; Simionescu, B.C.; Dobrov, A.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; et al. m-Chlorido-bridged dimanganese(II) complexes of the Schiff base derived from [2+2]condensation of 2,6-diformyl-4-methylphenol and 1,3-bis(3-aminopropyl)tetramethyldisiloxane: Structure, magnetism, electrochemical behaviour and catalytic oxidation of secondary alcohols. Eur. J. Inorg. Chem. 2014, 120–131. [Google Scholar] [CrossRef]
- Karmakar, A.; Martins, L.M.D.R.S.; Hazra, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Metal–organic frameworks with pyridyl-based isophthalic acid and their catalytic applications in microwave assisted peroxidative oxidation of alcohols and Henry reaction. Cryst. Growth Des. 2016, 16, 1837–1849. [Google Scholar] [CrossRef]
- Sutradhar, M.; Rajeshwari; Barman, T.R.; Fernandes, A.R.; Paradinha, F.; Roma-Rodrigues, C.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Mixed ligand aroylhydrazone and N-donor heterocyclic Lewis base Cu(II) complexes as potential antiproliferative agents. J. Inorg. Biochem. 2017, 175, 267–275. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Barman, T.R.; Scorcelletti, F.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Microwave-assisted peroxidative oxidation of toluene and 1-phenylethanol with monomeric keto and polymeric enol aroylhydrazone Cu(II) complexes. Mol. Catal. 2017, 439, 224–232. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mahmudov, K.T.; Silva, M.F.C.G.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Silva, T.F.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Trends in properties of para-substituted 3-(phenylhydrazo)pentane-2,4-diones. J. Phys. Org. Chem. 2011, 24, 764–773. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation: A quantitative measure of distortion in coordination polyhedral. Science 1971, 172, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Maharramov, A.M.; Mahmudov, K.T.; Kopylovich, M.N.; Pombeiro, A.J.L. Non-Covalent Interactions in the Synthesis and Design of New Compounds; Maharramov, A.M., Mahmudov, K.T., Kopylovich, M.N., Pombeiro, A.J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Shul’pin, G.B.; Nizova, G.V. Formation of alkyl peroxides in oxidation of alkanes by H2O2 catalyzed by transition metal complexes. React. Kinet. Catal. Lett. 1992, 48, 333–338. [Google Scholar] [CrossRef]
- Bruker, S. APEX2 & SAINT; AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





| 1 | |
|---|---|
| Empirical formula | C14H9BrCuN2O3 |
| Formula weight | 396.68 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| Temperature/K | 150 (2) |
| a/Å | 31.799 (2) |
| b/Å | 6.3515 (4) |
| c/Å | 14.2176 (10) |
| α/° | 90 |
| β/° | 114.420 (4) |
| γ/° | 90 |
| V (Å3) | 2614.6 (3) |
| Z | 8 |
| Dcalc (g cm−3) | 2.015 |
| μ(Mo Kα) (mm−1) | 4.74 |
| Rfls. collected/unique/observed | 9952/2668/1906 |
| Rint | 0.059 |
| Final R1 a, wR2 b (I ≥ 2σ) | 0.039, 0.074 |
| Goodness-of-fit on F2 | 1.01 |
| N1—Cu1 | 1.914 (3) | O1—Cu1—O2 | 174.99 (11) |
| O1—Cu1 | 1.920 (2) | N1—Cu1—O2 i | 164.20 (13) |
| O2—Cu1 | 1.936 (2) | O1—Cu1—O2 i | 104.25 (11) |
| O2—Cu1 i | 1.977 (3) | O2—Cu1—O2 i | 80.16 (12) |
| Cu1—O2 i | 1.976 (3) | N1—Cu1—Cu1 i | 131.93 (10) |
| Cu1—Cu1 i | 2.9945 (9) | O1—Cu1—Cu1 i | 143.78 (8) |
| N1—Cu1—O1 | 82.22 (12) | O2—Cu1—Cu1 i | 40.58 (8) |
| N1—Cu1—O2 | 92.92 (12) | O2i—Cu1—Cu1 i | 39.58 (7) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutradhar, M.; Barman, T.R.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Catalytic Activity of Polynuclear vs. Dinuclear Aroylhydrazone Cu(II) Complexes in Microwave-Assisted Oxidation of Neat Aliphatic and Aromatic Hydrocarbons. Molecules 2019, 24, 47. https://doi.org/10.3390/molecules24010047
Sutradhar M, Barman TR, Pombeiro AJL, Martins LMDRS. Catalytic Activity of Polynuclear vs. Dinuclear Aroylhydrazone Cu(II) Complexes in Microwave-Assisted Oxidation of Neat Aliphatic and Aromatic Hydrocarbons. Molecules. 2019; 24(1):47. https://doi.org/10.3390/molecules24010047
Chicago/Turabian StyleSutradhar, Manas, Tannistha Roy Barman, Armando J. L. Pombeiro, and Luísa M.D.R.S. Martins. 2019. "Catalytic Activity of Polynuclear vs. Dinuclear Aroylhydrazone Cu(II) Complexes in Microwave-Assisted Oxidation of Neat Aliphatic and Aromatic Hydrocarbons" Molecules 24, no. 1: 47. https://doi.org/10.3390/molecules24010047
APA StyleSutradhar, M., Barman, T. R., Pombeiro, A. J. L., & Martins, L. M. D. R. S. (2019). Catalytic Activity of Polynuclear vs. Dinuclear Aroylhydrazone Cu(II) Complexes in Microwave-Assisted Oxidation of Neat Aliphatic and Aromatic Hydrocarbons. Molecules, 24(1), 47. https://doi.org/10.3390/molecules24010047