Polysaccharide-Enriched Fraction from Amillariella Mellea Fruiting Body Improves Insulin Resistance
Abstract
:1. Introduction
2. Results
2.1. Preparation of the Polysaccharide-Enriched Fraction from Amillariella Mellea Fruiting Body
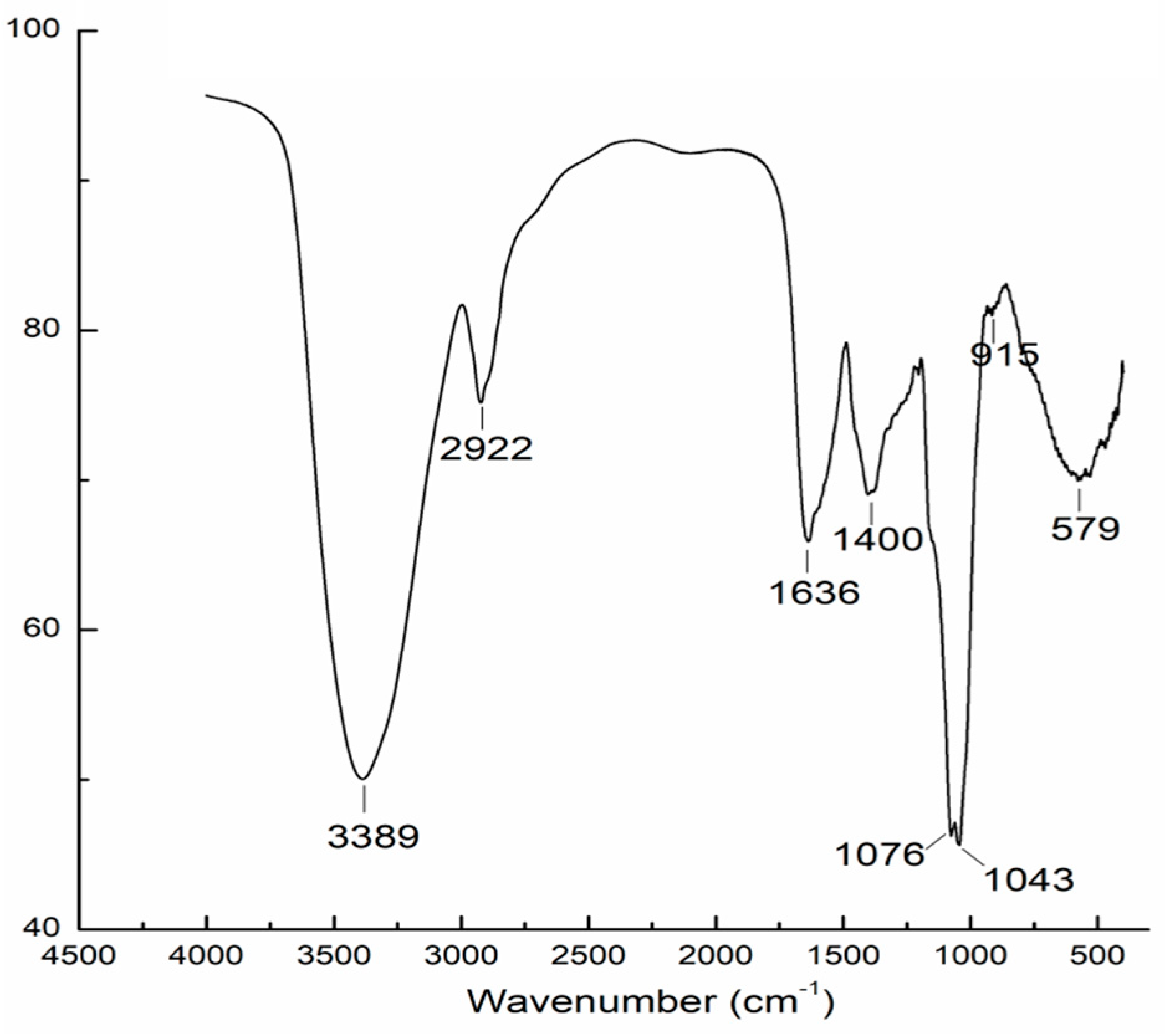
2.2. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.3. AAMP Reduced Fasting Blood Glucose in HFD/DEX-Treated Rat
2.4. AAMP Improved Glucose Intolerance in HFD/DEX-Treated Rat
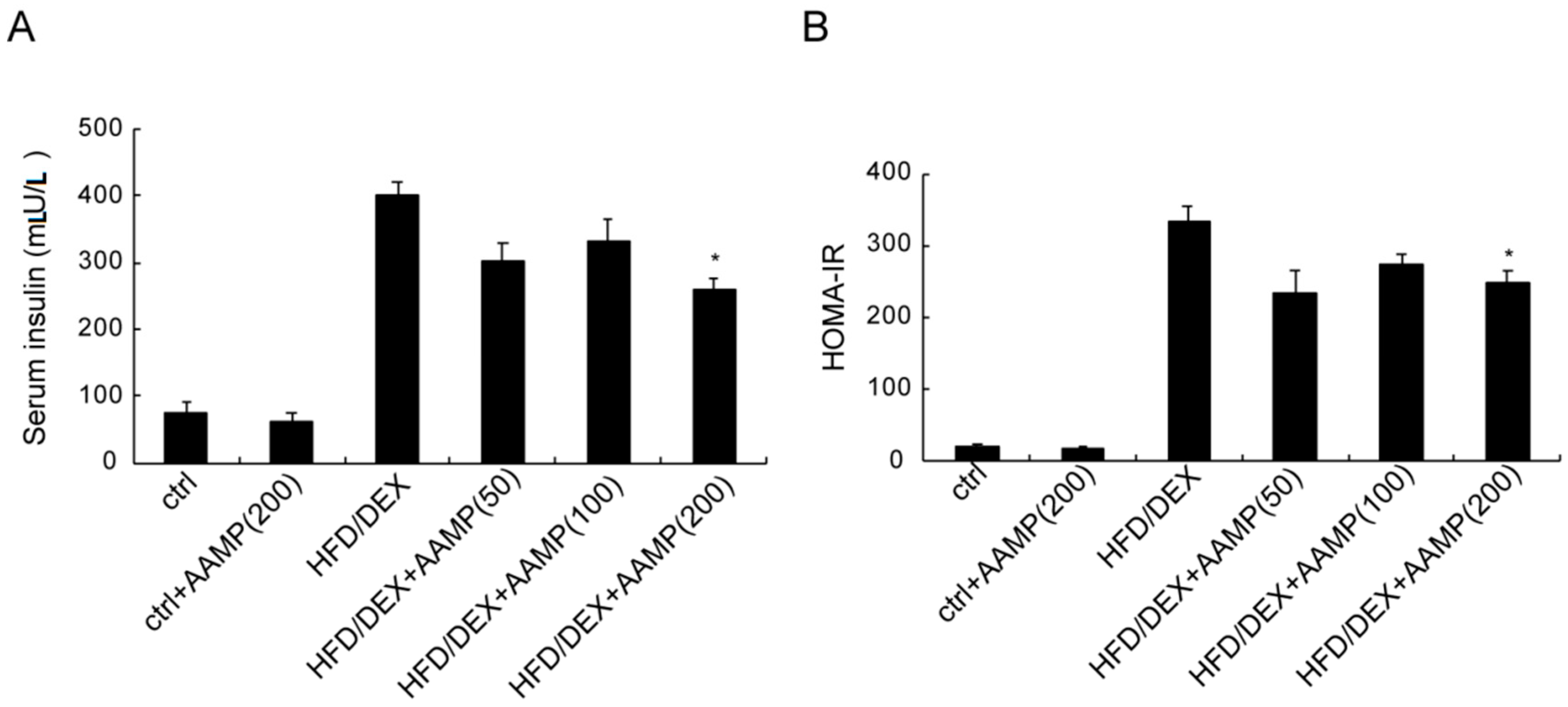
2.5. AAMP Ameliorated Insulin Resistance in HFD/DEX-Treated Rat
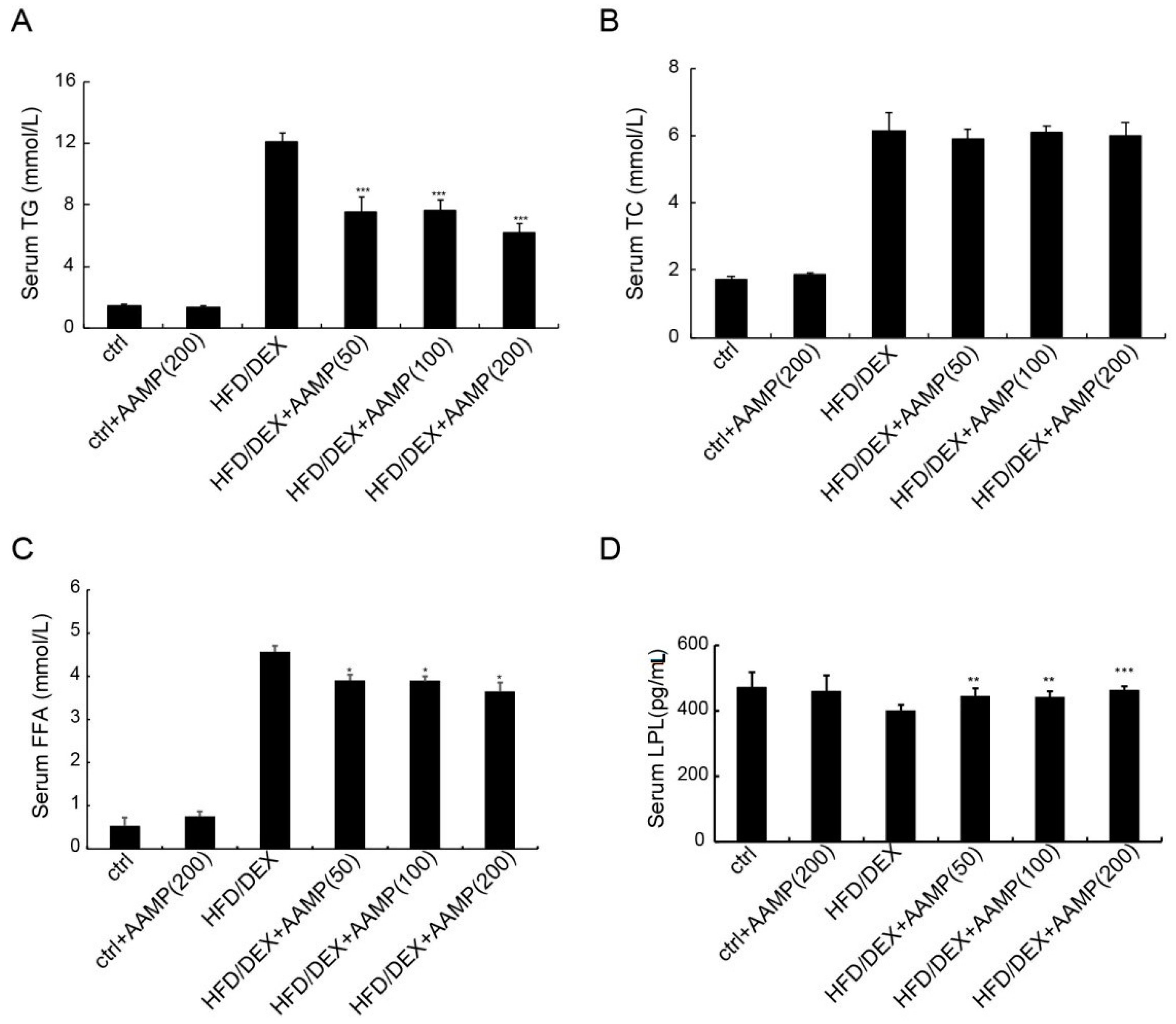
2.6. AAMP Lowered Serum Triglyceride and Free Fatty Acids in HFD/DEX-Treated Rat
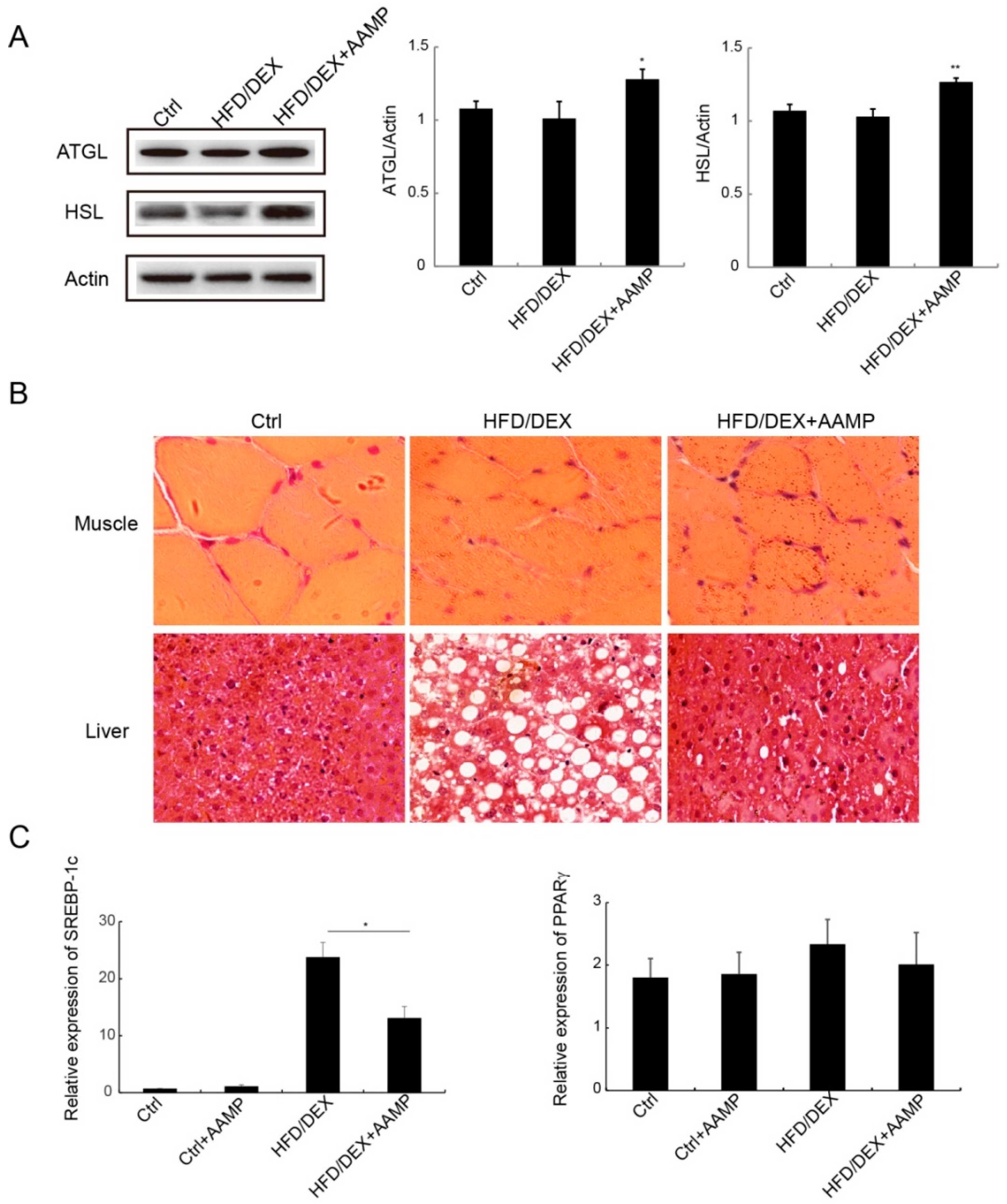
2.7. AAMP Enhanced Lipolysis and Suppressed Lipogenesis
3. Discussion
4. Materials and Methods
4.1. Preparation of the Polysaccharide-Enriched Fraction
4.2. Fourier Transform Infrared Spectroscopy Analysis
4.3. Experimental Protocols
4.4. Fasting Blood Glucose Test
4.5. Glucose Tolerance Test (GTT)
4.6. Biochemical Indicators Detection
4.7. H and E Staining
4.8. Western Blots
4.9. Quantitative Real-Time PCR (RT-qPCR)
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Farese, R.V., Jr.; Zechner, R.; Newgard, C.B.; Walther, T.C. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 2012, 15, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Effects of free fatty acids (FFA) on glucose metabolism: Significance for insulin resistance and type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2003, 111, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Bio. 2008, 9, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yamamoto, J.; Iwasaki, S.; Asaba, H.; Hamura, H.; Ikeda, Y.; Watanabe, M.; Magoori, K.; Ioka, R.X.; Tachibana, K.; et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc. Natl. Acad. Sci. USA 2003, 100, 15924–15929. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Wang, Y.; Cline, G.W.; Rabin-Court, A.; Song, J.D.; Dufour, S.; Zhang, X.M.; Petersen, K.F.; Shulman, G.I. Leptin Mediates a Glucose-Fatty Acid Cycle to Maintain Glucose Homeostasis in Starvation. Cell 2018, 172, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Anuradha, C.V. Glucocorticoid Antagonism Reduces Insulin Resistance and Associated Lipid Abnormalities in High-Fructose-Fed Mice. Can. J. Diabetes 2017, 41, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ooi, V.E.C.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Park, J.Y.; Seok, S.; Jang, T.S.; Park, H.B.; Shim, S.H.; Kang, K.S.; Kim, K.H. Antigastritis effects of Armillariella tabescens (Scop.) Sing. and the identification of its anti-inflammatory metabolites. J. Pharm. Pharmacol. 2018, 70, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.T.; Wu, S.J.; Tsai, J.Y.; Lai, M.N. Antioxidant activities of cultured Armillariella mellea. Prikl. Biokhim. Mikrobiol. 2007, 43, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G.L. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cao, Y.P.; Yu, M.; Shen, Y.S. Polysaccharide from the rhizomorph of Armillaria mella (AMP-1) protects INS-1 cells from alloxan injury. Chin. Pharmacol. Bull. 2008, 24, 1160–1165. [Google Scholar]
- Kozarski, M.; Klaus, A.; Niksic, M.; Vrvic, M.M.; Todorovic, N.; Jakovljevic, D.; Griensven, L.J. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Casu, B.; Scovenna, G.; Cifonelli, A.J.; Perlin, A.S. Infra-red spectra of glycosaminoglycans in deuterium oxide and deuterium chloride solution: Quantitative evaluation of uronic acid and acetamidodeoxyhexose moieties. Carbohydr. Res. 1978, 63, 13–27. [Google Scholar] [CrossRef]
- Barker, S.A.; Bourne, E.J.; Stacey, M.; Whiffen, D.H. Infra-red spectra of carbohydrates. Part I. Some derivatives of D-glucopyranose. J. Chem. Soc. 1954, 1, 171–176. [Google Scholar] [CrossRef]
- Zabielski, P.; Hady, H.R.; Chacinska, M.; Roszczyc, K.; Gorski, J.; Blachnio-Zabielska, A.U. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018, 8, 7249. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Johnson, J.H.; Ohneda, M.; McAllister, C.T.; Inman, L.; Alam, T.; Unger, R.H. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J. Clin. Invest. 1992, 90, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Lund, S.; Hougaard, K.S. Chronic high-fat diet increases acute neuroendocrine stress response independently of prenatal dexamethasone treatment in male rats. Acta Neuropsychiatr. 2014, 26, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Inamdar, M.N.; Viswanatha, G.L. Protective effect of lemongrass oil against dexamethasone induced hyperlipidemia in rats: Possible role of decreased lecithin cholesterol acetyl transferase activity. Asian Pac. J. Trop. Med. 2011, 4, 658–660. [Google Scholar] [CrossRef]
- Kim, J.Y.; Garcia-Carbonell, R.; Yamachika, S.; Zhao, P.; Dhar, D.; Loomba, R.; Kaufman, R.J.; Saltiel, A.R.; Karin, M. ER Stress Drives Lipogenesis and Steatohepatitis via Caspase-2 Activation of S1P. Cell 2018, 175, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yaauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPARγ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Souza-Mello, V. Peroxisome proliferator-activated receptors as targets to treat non-alcoholic fatty liver disease. World J. hepatol. 2015, 7, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.Q.; Li, X.Z.; Yang, S.W.; Yang, L.L.; Fan, Y.Y.; Zhou, Y.F. Pectic Bee Pollen Polysaccharide from Rosa rugosa Alleviates Diet-Induced Hepatic Steatosis and Insulin Resistance via Induction of AMPK/mTOR-Mediated Autophagy. Molecules 2017, 22, 699. [Google Scholar] [CrossRef]
- Sedmak, J.J.; Grossberg, S.E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 1997, 79, 544–552. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Bi, H.T.; Li, X.H.; Ni, W.H.; Han, H.; Li, N.; Wang, B.Q.; Zhou, Y.F.; Tai, G.H. Total fractionation and characterization of the water-soluble polysaccharides isolated from Panax ginseng CA Meyer. Carbohydr. Polym. 2009, 77, 544–552. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Gene Symbol | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| SREBP-1c | GGAGCCATGGATTGCACATT | GCTTCCAGAGAGGAGGCCAG |
| PPARγ | CCCTTTACCACGGTTGATTTCTC | GCAGGCTCTACTTTGATCGCACT |
| GAPDH | ATGATTCTACCCACGGCAAG | CTGGAAGATGGTGATGGGTT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Meng, Y.; Yan, J.; Wang, N.; Xue, Z.; Zhang, H.; Fan, Y. Polysaccharide-Enriched Fraction from Amillariella Mellea Fruiting Body Improves Insulin Resistance. Molecules 2019, 24, 46. https://doi.org/10.3390/molecules24010046
Yang S, Meng Y, Yan J, Wang N, Xue Z, Zhang H, Fan Y. Polysaccharide-Enriched Fraction from Amillariella Mellea Fruiting Body Improves Insulin Resistance. Molecules. 2019; 24(1):46. https://doi.org/10.3390/molecules24010046
Chicago/Turabian StyleYang, Siwen, Yuhan Meng, Jingmin Yan, Na Wang, Zhujun Xue, Hang Zhang, and Yuying Fan. 2019. "Polysaccharide-Enriched Fraction from Amillariella Mellea Fruiting Body Improves Insulin Resistance" Molecules 24, no. 1: 46. https://doi.org/10.3390/molecules24010046






