Molecular Dynamics Simulation of a Jet in a Binary System at Supercritical Environment
Abstract
:1. Introduction
2. Methodology
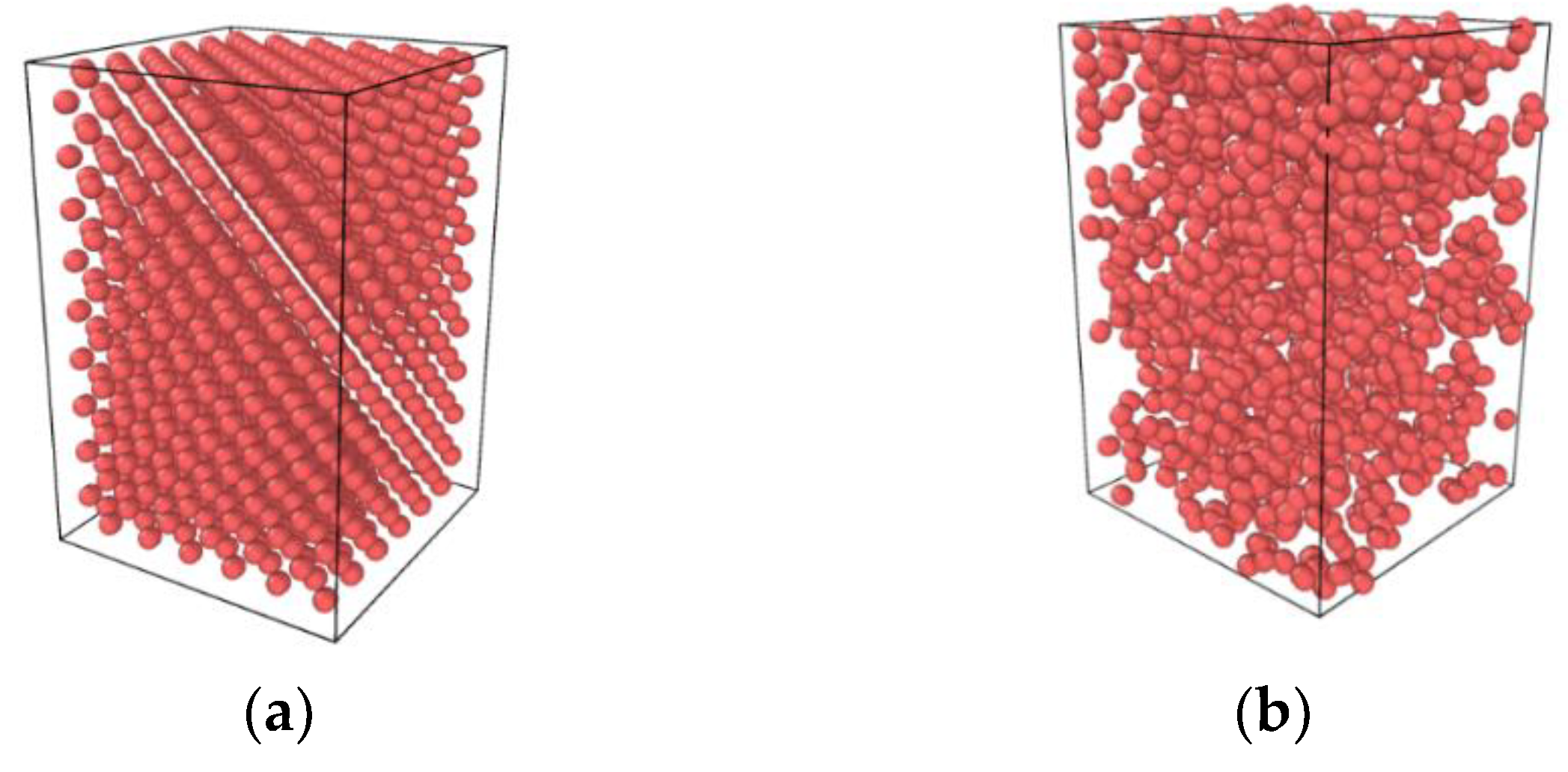
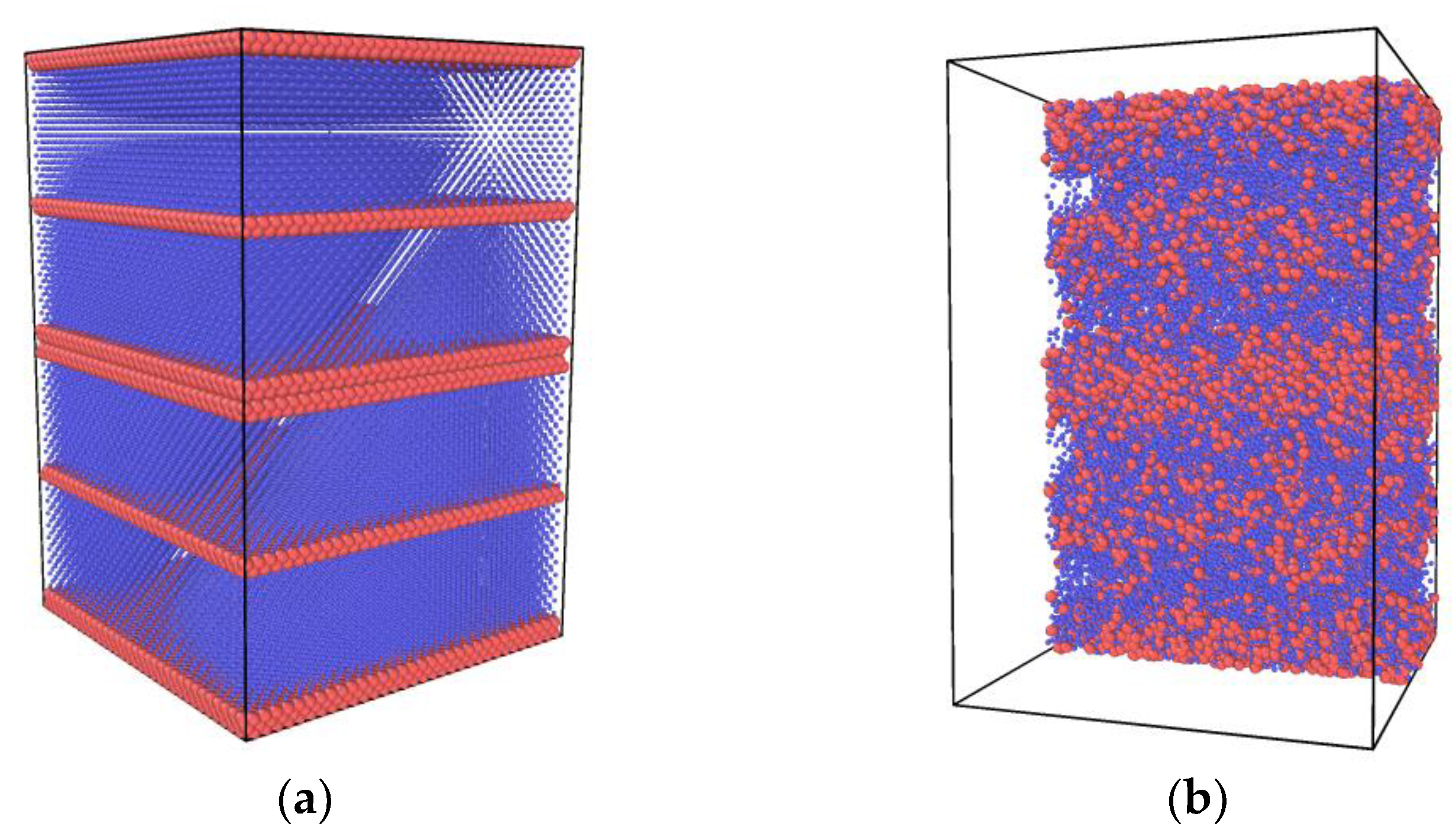
2.1. Molecular Dynamics Model
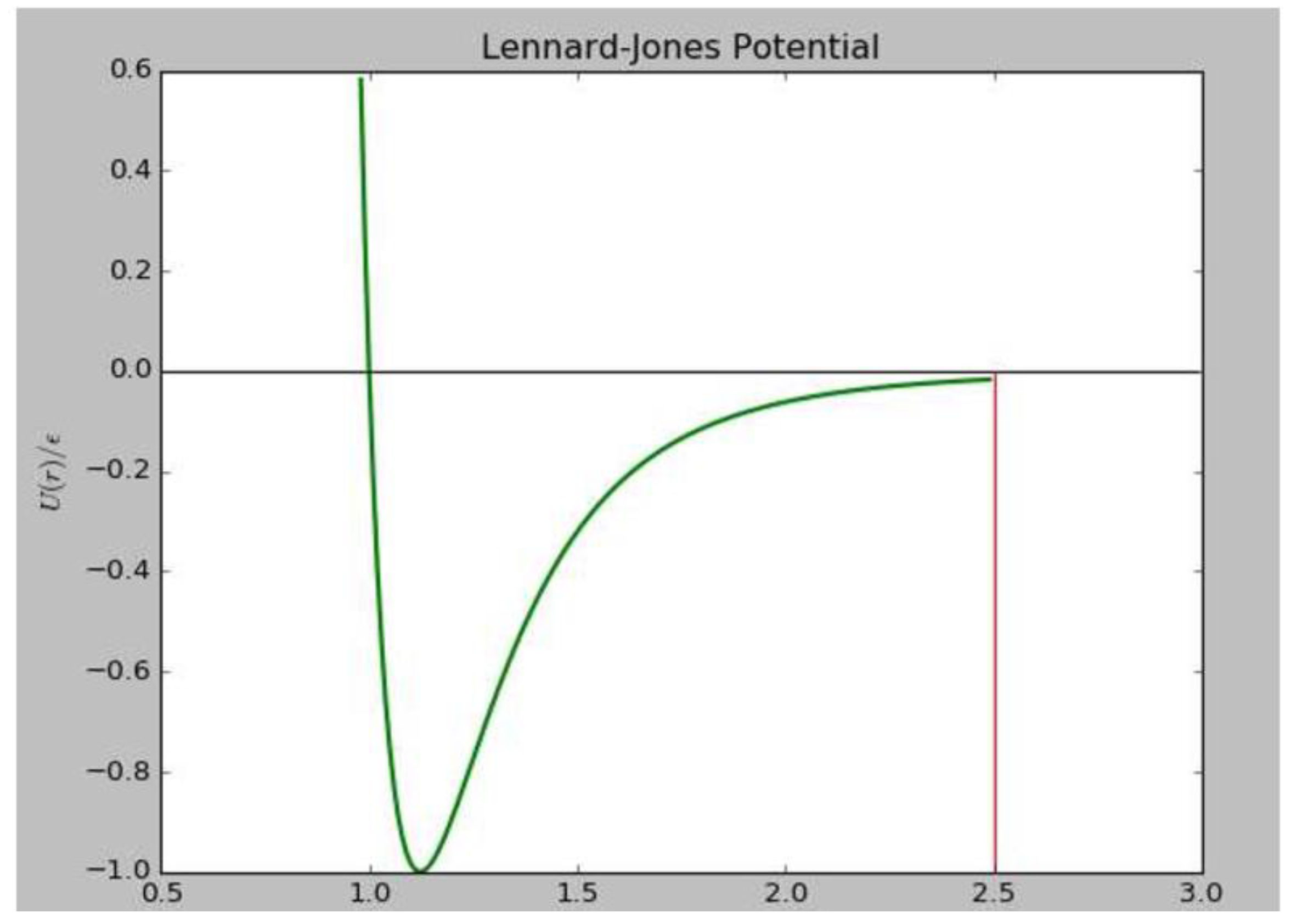
2.2. Force Routine
2.3. Equation of State
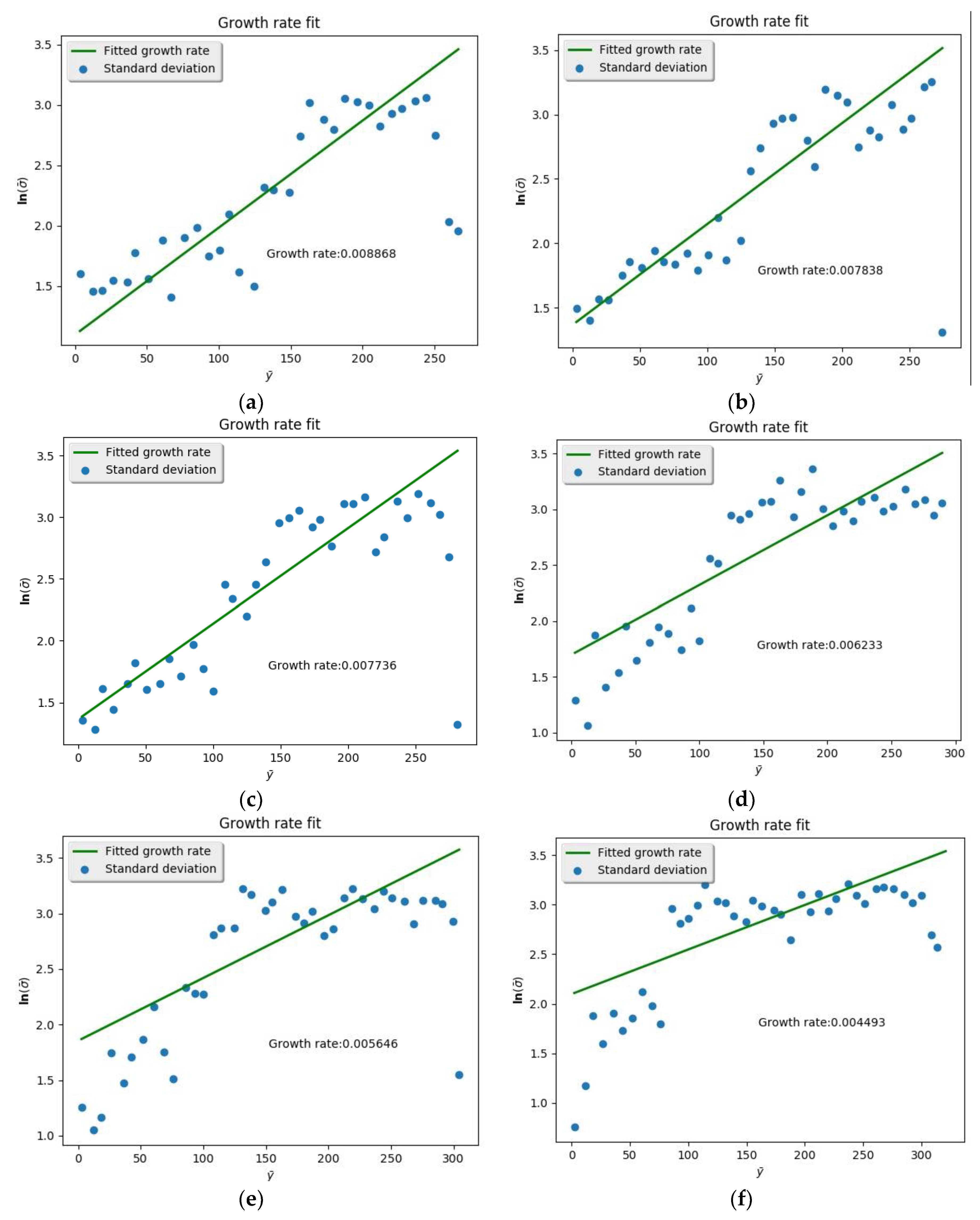
2.4. Spatial Growth Rate
3. Results and Discussion
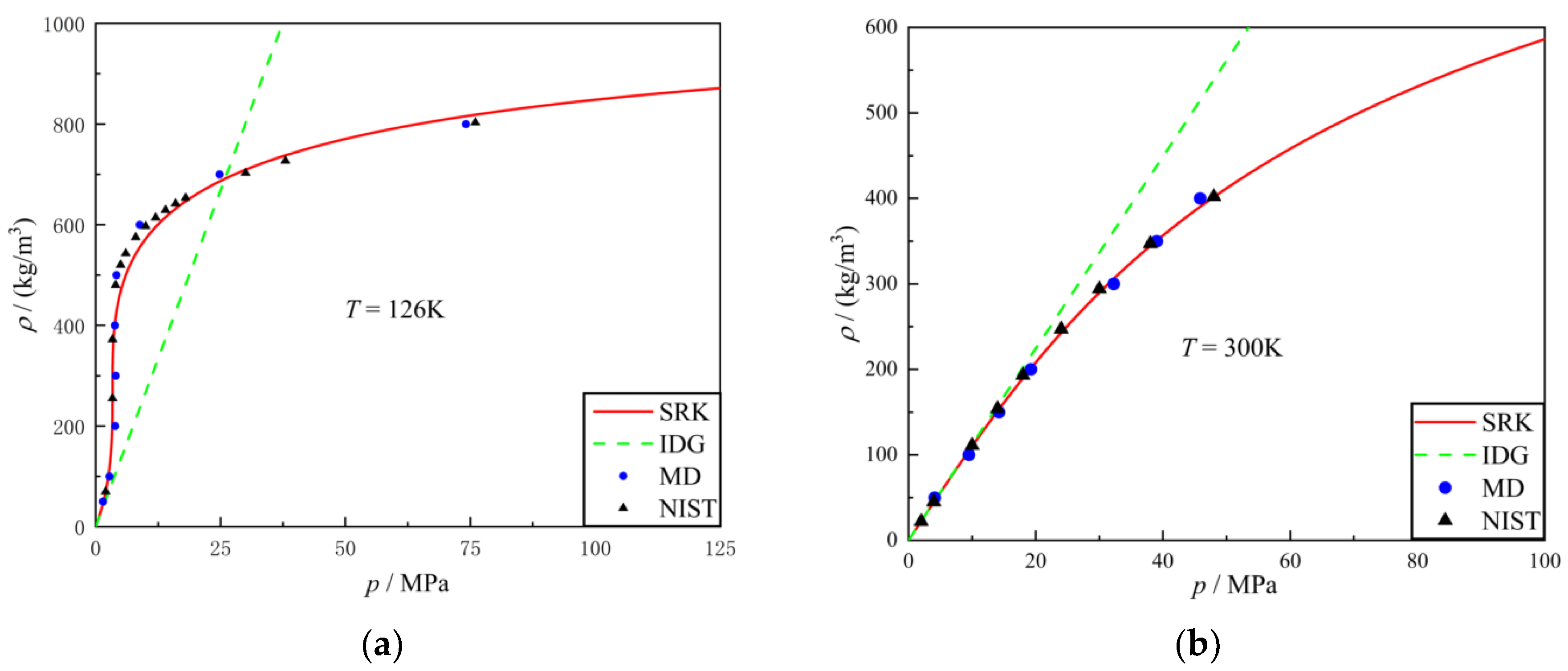
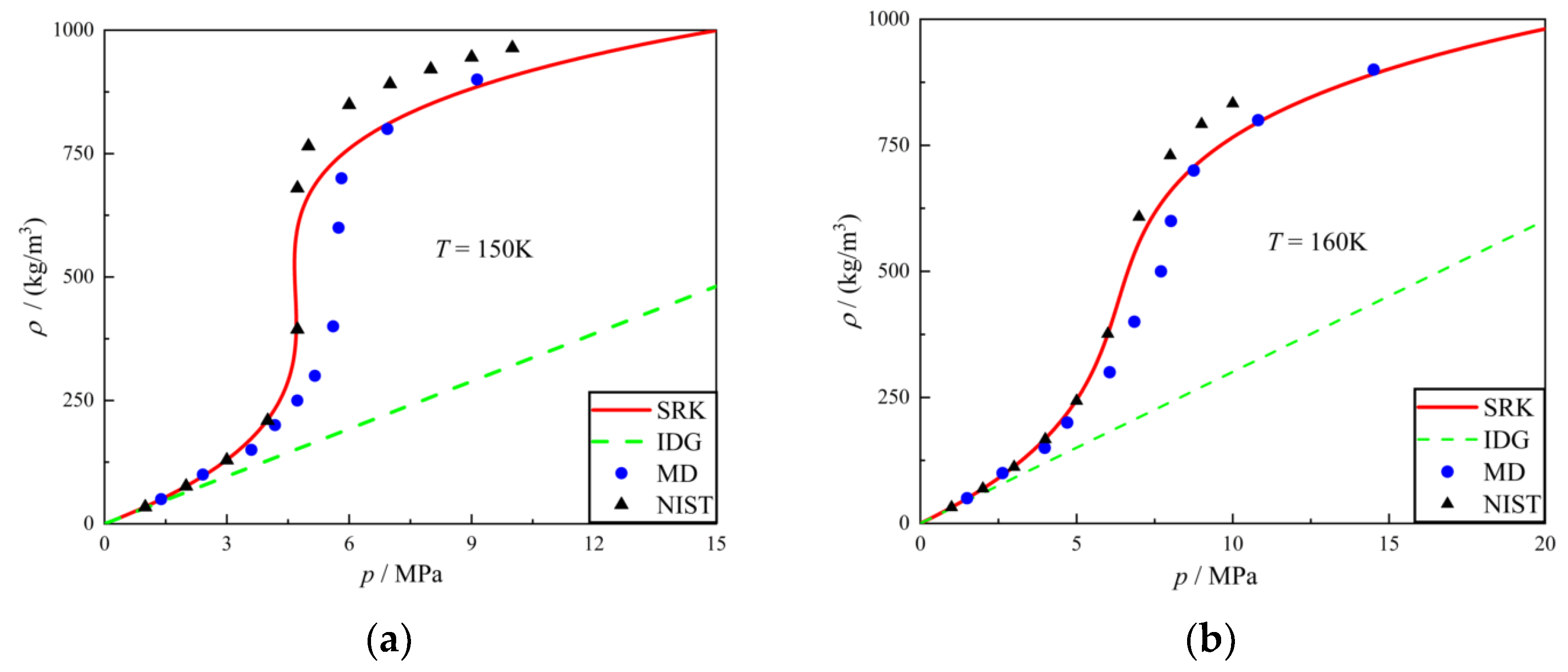
3.1. Equation of State
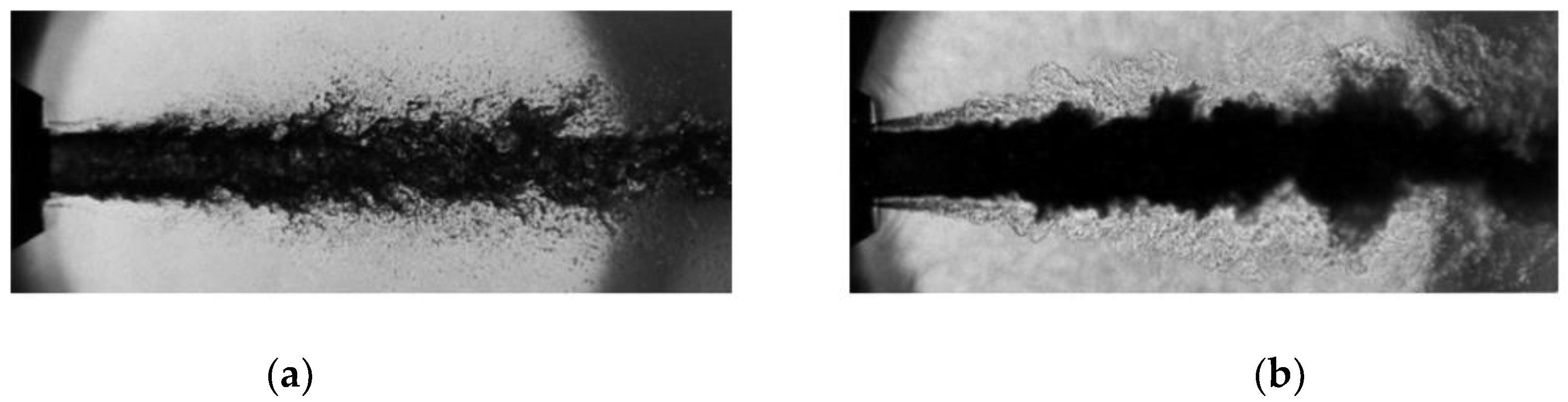
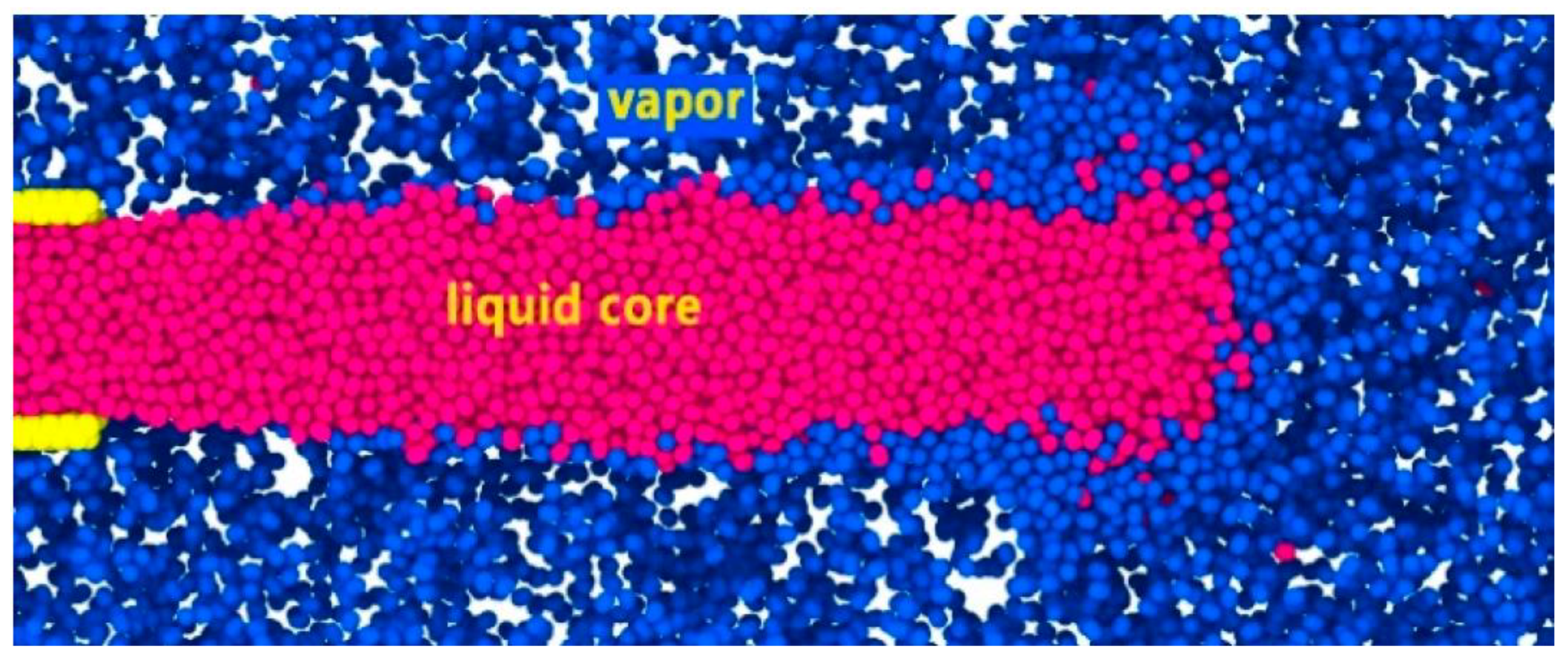
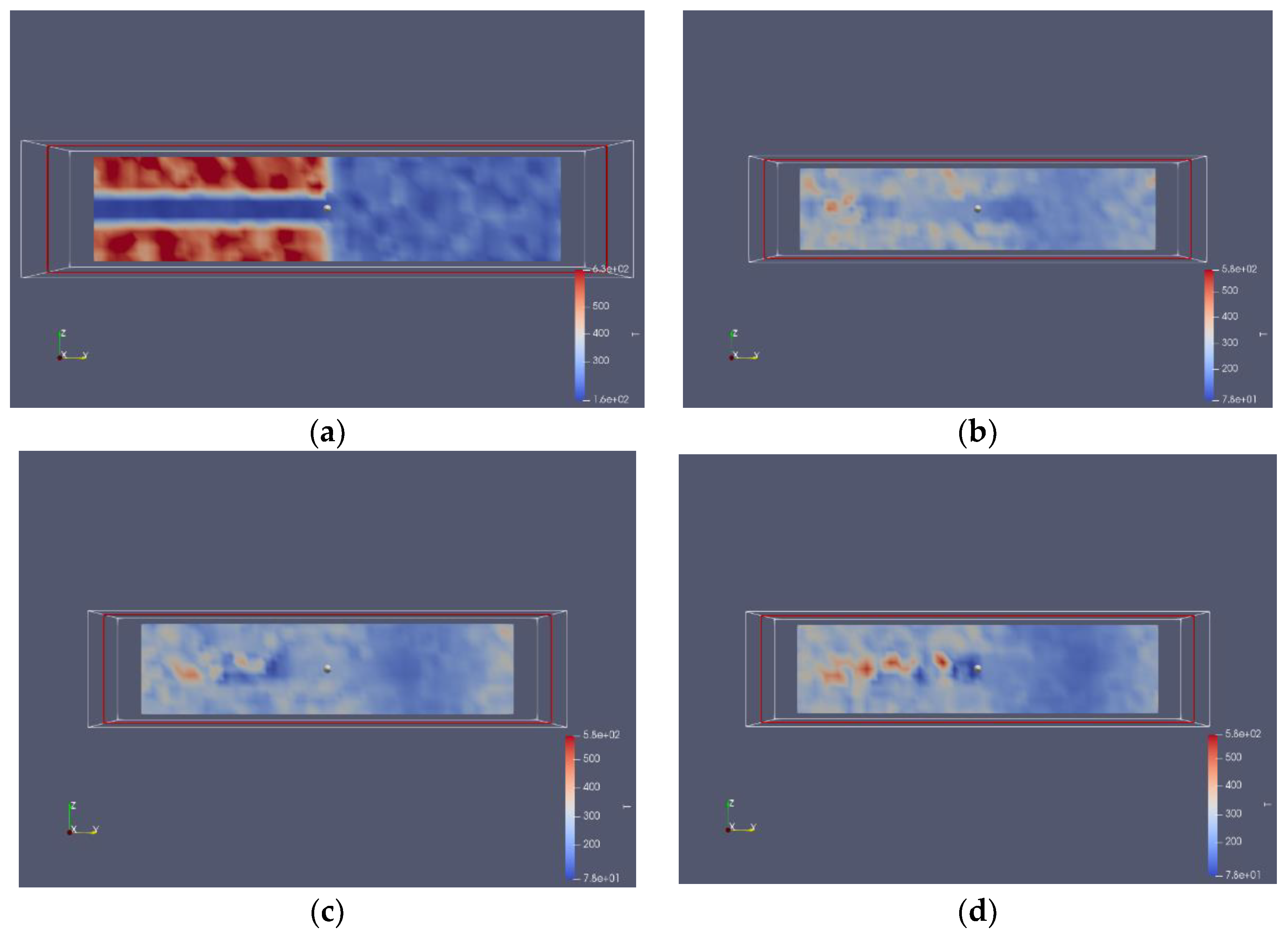
3.2. Nitrogen Jet Profile
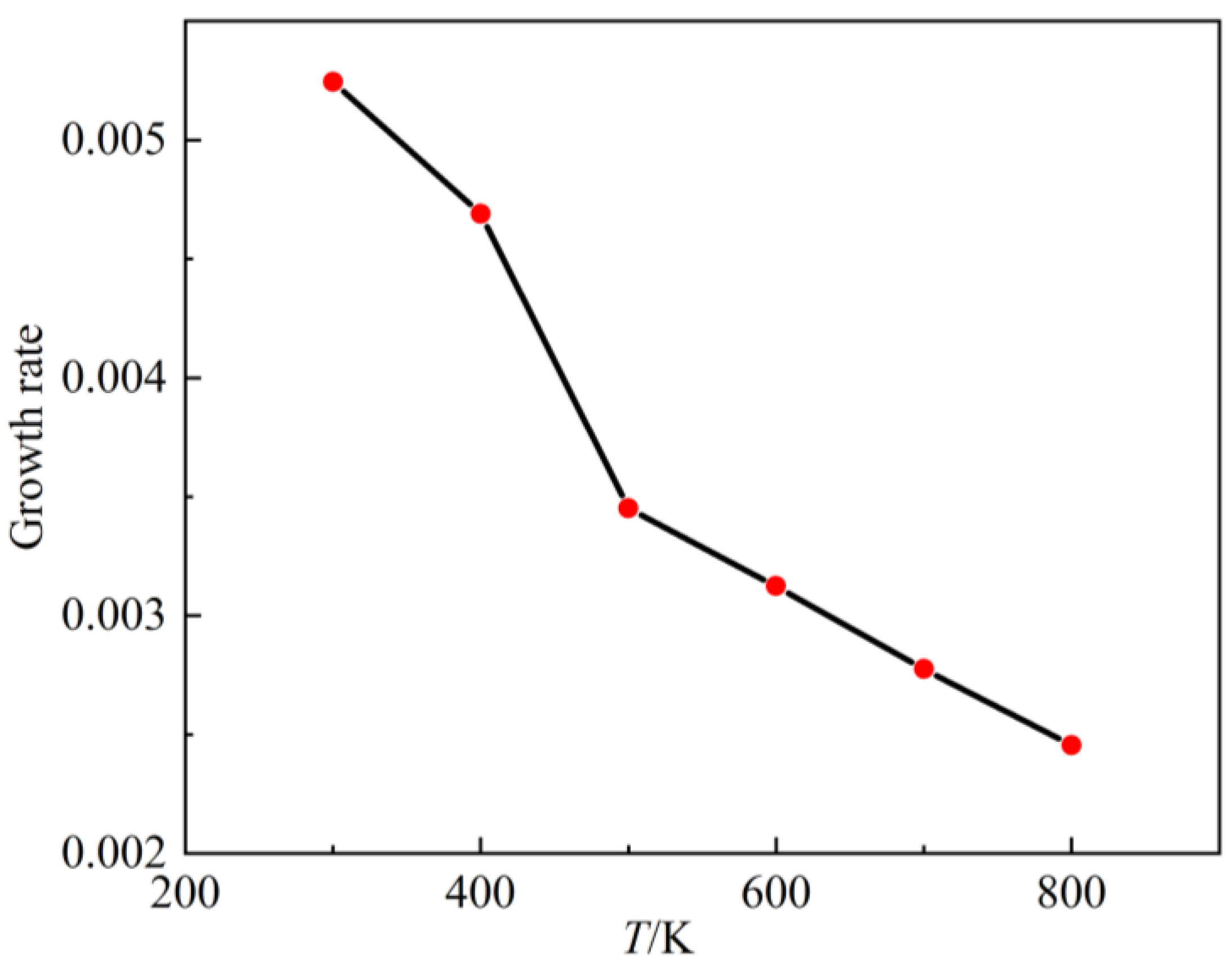
3.3. Spatial Growth Rate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xie, M. Mechanism and Modeling of Transcritical/Supercritical Fuel Spray and Mixture Formation in Internal Combustion Engines. J. Combust. Sci. Technol. 2014, 1, 20–21. [Google Scholar]
- Mayer, W.O.H.; Schik, A.H.A.; Vielle, B.; Chauveau, C.; G-ogravekalp, I.; Talley, D.G.; Woodward, R.D. Atomization and Breakup of Cryogenic Propellants Under High-Pressure Subcritical and Supercritical Conditions. J. Propuls. Power 1998, 14, 835–842. [Google Scholar] [CrossRef]
- Dahms, R.N.; Oefelein, J.C. On the transition between two-phase and single-phase interface dynamics in multicomponent fluids at supercritical pressures. Phys. Fluids 2013, 25, 092103. [Google Scholar] [CrossRef]
- Lekner, J.; Henderson, J.R. Theoretical determination of the thickness of a liquid-vapour interface. Phys. A 1978, 94, 545–558. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, Y.; Yang, V. Near-field flame dynamics of liquid oxygen/kerosene bi-swirl injectors at supercritical conditions. Combust. Flame 2018, 190, 1–11. [Google Scholar] [CrossRef]
- Hiroumi, T.; Susumu, T.; Nobuhiro, Y.; Koji, O. A numerical study on a temporal mixing layer under transcritical conditions. Comput. Fluids 2013, 85, 93–104. [Google Scholar]
- Ribert, P.G.; Lartigue, G.; Domingo, P. Large-eddy simulation of supercritical fluid injection. J. Supercrit. Fluids 2013, 84, 61–73. [Google Scholar]
- Lapenna, P.E.; Creta, F. Mixing under transcritical conditions: An a-priori study using direct numerical simulation. J. Supercrit. Fluids 2017, 128, 263–278. [Google Scholar] [CrossRef]
- Ludwig, K.F.; Micci, M. Molecular dynamics simulations of rayleigh and first wind-induced breakup. At. Sprays 2011, 21, 275–281. [Google Scholar] [CrossRef]
- Branam, R.D.; Micci, M.M. Comparison of wall models for the molecular dynamics simulation of microflows. Nanoscale Microscale Thermophys. Eng. 2009, 13, 1–12. [Google Scholar] [CrossRef]
- Chehroudi, B.; Cohn, R.; Talley, D. Cryogenic shear layers: Experiments and phenomenological modeling of the initial growth rate under subcritical and supercritical conditions. Int. J. Heat Fluid Flow 2002, 23, 554–563. [Google Scholar] [CrossRef]
- Chehroudi, B. Recent experimental efforts on high-pressure supercritical injection for liquid rockets and their implications. Int. J. Aerosp. Eng. 2012, 2012, 1–31. [Google Scholar] [CrossRef]
- Tani, H.; Teramoto, S.; Okamoto, K. High-speed observations of cryogenic single and coaxial jets under subcritical and transcritical conditions. Exp. Fluids 2015, 56. [Google Scholar] [CrossRef]
- Chapela, G.A.; Saville, G.; Rowlinson, J.S. Computer simulation of the gas/liquid surface. Faraday Discuss. Chem. Soc. 1975, 59, 22–28. [Google Scholar] [CrossRef]
- Nijmeijer, M.; Barker, A.; Bruin, C. A molecular dynamics simulation of the Leonard-Jones liquid-vapor interface. J. Chem. Phys. 1988, 89, 3789–3792. [Google Scholar] [CrossRef]
- Chen, L. Area dependence of the surface tension of a lennard-jones fluid from molecular dynamics simulations. J. Chem. Phys. 1995, 103, 10214–10216. [Google Scholar] [CrossRef]
- Moseler, M.; Landman, U. Formation, stability, and breakup of nanojets. Science 2000, 289, 1165–1169. [Google Scholar] [CrossRef]
- Shin, H.; Suh, D.; Yoon, W. Non-equilibrium molecular dynamics of nanojet injection in a high pressure environment. Microfluid. Nanofluidics 2008, 5, 561–570. [Google Scholar] [CrossRef]
- Zhang, Y. Molecular Dynamics Study on the Mechanism of Nanoscale Jet Instability Reaching Supercritical Conditions. Appl. Sci. 2018. accepted. [Google Scholar]
- Sandia National Laboratories. LAMMPS Documentation [DB/OL]. Available online: https://lammps.sandia.gov/doc/Manual.html (accessed on 27 November 2018).
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Liu, T. Shear-Coaxial Injection and Mixing of Cryogenic Fluids Under Supercritical Conditions. Ph.D. Thesis, The Pennsylvania State University, State College, PA, USA, 2007. [Google Scholar]
- Soave, G. Equilibrium constants from a modified redlich-kwong equation of state. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Bian, X.; Du, Z.; Tang, Y.; Du, J. Measurement and correlation of compressibility factor of high co2-content natural gas. J. Pet. Sci. Eng. 2012, 82–83, 38–43. [Google Scholar] [CrossRef]
- Charpentier, J.B.; Renoult, M.C.; Crumeyrolle, O. Growth rate measurement in free jet experiments. Exp. Fluids 2017, 58. [Google Scholar] [CrossRef]
- Rayleigh, J.W.S. On the instability of jets. Proc. Lord. Math. Soc. 1878, 10, 4–13. [Google Scholar] [CrossRef]
- Zong, N.; Meng, H.; Hsieh, S.-Y.; Yang, V. A numerical study of cryogenic fluid injection and mixing under supercritical conditions. Phys. Fluids 2004, 16, 4248–4261. [Google Scholar] [CrossRef]
- Bellan, J. Supercritical (and subcritical) fluid behavior and modeling: Drops, streams, shear and mixing layers, jets and sprays. Prog. Energy Combust. Sci. 2000, 26, 329–366. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Fang, Z.; Zhang, Y.; Yang, L. Molecular Dynamics Simulation of a Jet in a Binary System at Supercritical Environment. Molecules 2019, 24, 31. https://doi.org/10.3390/molecules24010031
Fu Q, Fang Z, Zhang Y, Yang L. Molecular Dynamics Simulation of a Jet in a Binary System at Supercritical Environment. Molecules. 2019; 24(1):31. https://doi.org/10.3390/molecules24010031
Chicago/Turabian StyleFu, Qingfei, Zixuan Fang, Yunxiao Zhang, and Lijun Yang. 2019. "Molecular Dynamics Simulation of a Jet in a Binary System at Supercritical Environment" Molecules 24, no. 1: 31. https://doi.org/10.3390/molecules24010031



