Bulnesia sarmientoi Supercritical Fluid Extract Exhibits Necroptotic Effects and Anti-Metastatic Activity on Lung Cancer Cells
Abstract
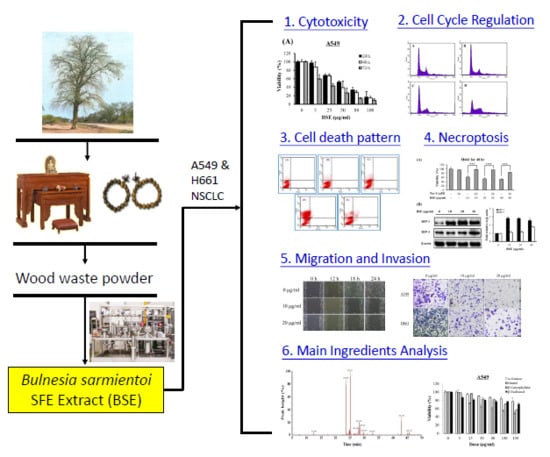
:1. Introduction
2. Results and Discussion
2.1. Effects of BSE on Anti-Proliferation of Human Lung Cancer Cells
2.2. Effects of BSE on Cell Cycle Regulation of Lung Cancer Cells
2.3. BSE Treatment Caused A549 and H661 Necrosis but not Apoptosis
2.4. Effect of BSE on Protein Expression Related to Necroptosis
2.5. BSE Inhibits the Migration and Invasion of A549 and H661 Cells
2.6. Cytotoxicity of Main Ingredients of BSE on Lung Cancer Cells
3. Materials and Methods
3.1. Reagents
3.2. Preparation of BSE
3.3. Cell Culture
3.4. Cytotoxicity Assay
3.5. Flow Cytometric Analysis on Cell Cycle
3.6. Measurement of Apoptotic Ratio of A549 Cells
3.7. Cell Migration by Wound Healing Assay
3.8. Cell Migration and Invasion Assays by Boyden Chamber
3.9. Western Blot Analysis
3.10. Gas Chromatography-Mass Spectrometry
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cho, J.H. Immunotherapy for non-small-cell lung cancer: Current status and future obstacles. Immune Netw. 2017, 17, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited the role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Jabbour, S.K.; Aisner, J. Current state of immunotherapy for non–small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Christofferson, D.E.; Yuan, J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell. Biol. 2010, 22, 263–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galluzzi, L.; Kepp, O.; Kroemer, G. RIP kinases initiate programmed necrosis. J. Mol. Cell. Biol. 2009, 1, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Huang, Z.; Boyce, M.; Li, Y.; Jagtap, P.; Mizushima, N.; Cuny, G.D.; Mitchison, T.J.; Moskowitz, M.A.; Yuan, J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005, 1, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.M.; Degterev, A.; David, J.; Rosenbaum, P.S.; Roth, S.; Grotta, J.C.; Cuny, G.D.; Yuan, J.; Savitz, S.I. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J. Neurosci. Res. 2010, 88, 1569–1576. [Google Scholar] [CrossRef]
- Smith, C.C.; Davidson, S.M.; Lim, S.Y.; Simpkin, J.C.; Hothersall, J.S.; Yellon, D.M. Necrostatin: A potentially novel cardioprotective agent? Cardiovasc. Drugs Ther. 2007, 21, 227–233. [Google Scholar] [CrossRef]
- Huang, C.; Luo, Y.; Zhao, J.; Yang, F.; Zhao, H.; Fan, W.; Ge, P. Shikonin kills glioma cells through necroptosis mediated by RIP-1. PLoS ONE 2013, 8, e66326. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, Z.; Duan, H.; Sun, L.; Zhang, S.; Chen, M.; Wang, Y.; Gao, Q.; Song, Y.; Zhu, X.; et al. Deoxypodophyllotoxin triggers necroptosis in human non-small cell lung cancer NCI-H460 cells. Biomed. Pharmacother. 2013, 67, 701–706. [Google Scholar] [CrossRef]
- Yan, C.; Oh, J.S.; Yoo, S.H.; Lee, J.S.; Yoon, Y.G.; Oh, Y.J.; Jang, M.S.; Lee, S.Y.; Yang, J.; Lee, S.H.; et al. The targeted inhibition of mitochondrial Hsp90 overcomes the apoptosis resistance conferred by Bcl-2 in Hep3B cells via necroptosis. Toxicol. Appl. Pharmacol. 2013, 266, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Aaes, T.L.; Kagan, V.E.; D’Herde, K.; Bachert, C.; Leybaert, L.; Vandenabeele, P.; Krysko, D.V. Necroptotic cell death in anti–cancer therapy. Immunol. Rev. 2017, 280, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.; Miller, G. Molecular pathways: The necrosome—A target for cancer therapy. Clin. Cancer Res. 2017, 23, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The organ microenvironment and cancer metastasis. Differentiation 2005, 70, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Arvelo, F.; Cotte, C. Metalloproteinases in tumor progression. Review. Investig. Clin. 2006, 47, 185–205. [Google Scholar] [PubMed]
- Imanaka, H.H.; Koide, K.; Shimizu, T.; Asai, N.; Kinouchi Shimizu, A.; Ishikado, T.; Makino, N.O. Chemoprevention of tumor metastasis by liposomal β-sitosterol intake. Biol. Pharm. Bull. 2008, 31, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M.P.A.; Garcia, J.M.; del Corral, M.A.; Castro Gomez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Tian, J.; Qian, K.; Zhao, X.B.; Morris-Natschke, S.L.; Yang, L.; Nan, X.; Tian, X.; Lee, K.H. Recent progress on C-4-modified podophyllotoxin analogs as potent antitumor agents. Med. Res. Rev. 2015, 35, 1–62. [Google Scholar] [CrossRef]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical pharmacokinetics of paclitaxel monotherapy: An updated literature review. Clin. Pharmacokinet. 2018, 57, 7–19. [Google Scholar] [CrossRef]
- Cazzaniga, M.E.; Camerini, A.; Addeo, R.; Nolè, F.; Munzone, E.; Collovà, E.; Del Conte, A.; Mencoboni, M.; Papaldo, P.; Pasini, F.; et al. Metronomic oral vinorelbine in advanced breast cancer and non-small-cell lung cancer: Current status and future development. Future Oncol. 2016, 12, 373–387. [Google Scholar] [CrossRef]
- Venditto, V.J.; Simanek, E.E. Cancer therapies utilizing the camptothecins: A review of in vivo literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, Y.; Wang, L.; Mo, C.; Zhang, J.; Zhang, W. d-Galactose induces necroptotic cell death in neuroblastoma cell lines. J. Cell. Biochem. 2011, 112, 3834–3844. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, N.; Leon, L.; Carraway, K.L.; Gorin, F. 5-Benzylglycinyl-amiloride kills proliferating and nonproliferating malignant glioma cells through caspase-independent necroptosis mediated by apoptosis-inducing factor. J. Pharmacol. Exp. Ther. 2013, 344, 600–615. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Cristofanon, S.; Fulda, S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell. Death Differ. 2013, 20, 1161–1173. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Bao, J.; Lin, W.; Gao, H.; Zhao, W.; Zhang, Q.; Leung, C.H.; Ma, D.L.; Lu, J.; Chen, X. 2-Methoxy-6-acetyl-7-methyljuglone (MAM), a natural naphthoquinone, induces NO-dependent apoptosis and necroptosis by H2O2-dependent JNK activation in cancer cells. Free Radic. Biol. Med. 2016, 92, 61–77. [Google Scholar] [CrossRef]
- Jing, L.; Song, F.; Liu, Z.; Li, J.; Wu, B.; Fu, Z.; Jiang, J.; Chen, Z. MLKL-PITPα signaling-mediated necroptosis contributes to cisplatin-triggered cell death in lung cancer A549 cells. Cancer Lett. 2018, 414, 136–146. [Google Scholar] [CrossRef]
- Han, W.; Li, L.; Qiu, S.; Lu, Q.; Pan, Q.; Gu, Y. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol. Cancer Ther. 2007, 6, 1641–1649. [Google Scholar] [CrossRef] [Green Version]
- Xuan, Y.; Hu, X. Naturally-occurring shikonin analogues—A class of necroptotic inducers that circumvent cancer drug resistance. Cancer Lett. 2009, 274, 233–242. [Google Scholar] [CrossRef]
- Fu, Z.; Deng, B.; Liao, Y.; Shan, L.; Yin, F.; Wang, Z. The anti-tumor effect of shikonin on osteosarcoma by inducing RIP1 and RIP3 dependent necroptosis. BMC Cancer 2013, 13, 580. [Google Scholar] [CrossRef]
- Wada, N.; Kawano, Y.; Fujiwara, S.; Kikukawa, Y.; Okuno, Y.; Tasaki, M.; Ueda, M.; Ando, Y.; Yoshinaga, K.; Ri, M.; et al. Shikonin dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells. Int. J. Oncol. 2015, 46, 963–972. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Xu, W.; Ding, J.; Yin, F.; Xu, J.; Sun, W.; Wang, H.; Sun, M.; Cai, Z.; et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis. Theranostics 2018, 8, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Uquiche, E.; Cirano, N.; Millao, S. Supercritical fluid extraction of essential oil from Leptocarpha rivularis using CO2. Ind. Crops Prod. 2015, 77, 307–314. [Google Scholar] [CrossRef]
- Rai, A.; Bhargava, R.; Mohanty, B. Simulation of supercritical fluid extraction of essential oil from natural products. J. Appl. Res. Med. Aromat. Plants 2017, 5, 1–9. [Google Scholar] [CrossRef]
- Priyanka, S.K. Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J. Clean. Prod. 2018, 188, 816–824. [Google Scholar] [CrossRef]
- Rodilla, J.M.; Silva, L.A.; Martinez, N.; Lorenzo, D.; Davyt, D.; Castillo, L.; Giménez, C.; Cabrera, R.; González-Coloma, A.; Zrostlíková, J.; et al. Advances in the identification and agrochemical importance of sesquiterpenoids from Bulnesia sarmientoi essential oil. Ind. Crops Prod. 2011, 33, 497–503. [Google Scholar] [CrossRef]
- Hiebert, M.R.; Flores-Giubi, M.E.; Barua, J.E.; Molina-Salinas, G.M.; Ferro, E.A.; Alvarenga, N.L. Antimycobacterial activity of the ethanolic extract of the wood of Bulnesia Sarmientoi Lorentz Ex. Griseb. Rev. Latinoamer. Quím. 2012, 40, 7–12. [Google Scholar]
- Kamruzzaman, S.M.; Endale, M.; Oh, W.J.; Park, S.C.; Kim, K.S.; Hong, J.H.; Kwak, Y.S.; Yun, B.S.; Rhee, M.H. Inhibitory effects of Bulnesia sarmienti aqueous extract on agonist-induced platelet activation and thrombus formation involves mitogen-activated protein kinases. J. Ethnopharmacol. 2010, 130, 614–620. [Google Scholar] [CrossRef]
- Mollah, M.L.; Kim, J.O.; Lee, G.D.; Park, C.H.; Hong, J.H.; Kim, H.Y.; Kim, K.S. Growth–inhibitory effects of a Bulnesia sarmienti aqueous extract on A549 cells in vitro and S180 cells in vivo. Immunopharmacol. Immunotoxicol. 2009, 31, 492–498. [Google Scholar] [CrossRef]
- Mollah, M.L.; Song, J.C.; Park, C.H.; Lee, G.D.; Hong, J.H.; Ryoo, Z.Y.; Lee, S.; Chang, K.T.; Kim, K.S. Anticancer activity and apoptotic effects of Bulnesia sarmienti against human lung cancer H460 cells. Oncol. Res. 2009, 18, 259–267. [Google Scholar] [CrossRef]
- Kim, D.; Mollah, M.L.; Kim, K. Induction of apoptosis of SW480 human colon cancer cells by (−)-epicatechin isolated from Bulnesia sarmienti. Anticancer Res. 2012, 32, 5353–5361. [Google Scholar] [PubMed]
- Wu, W.; Liu, P.; Li, J. Necroptosis: An emerging form of programmed cell death. Crit. Rev. Oncol. Hematol. 2012, 82, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Jouan-Lanhouet, S.; Arshad, M.; Piquet-Pellorce, C.; Martin-Chouly, C.; Le Moigne-Muller, G.; Van Herreweghe, F. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell. Death Differ. 2012, 19, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Vanlangenakker, N.; Vanden Berghe, T.; Vandenabeele, P. Many stimuli pull the necrotic trigger, an overview. Cell. Death Differ. 2012, 19, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Sefton, M.A.; Sumby, C.J.; Tiekink, E.R.; Taylor, D.K. Mechanistic studies on the autoxidation of α-guaiene: Structural diversity of the sesquiterpenoid downstream products. J. Nat. Prod. 2015, 78, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, S.E.; Wiirzler, L.A.M.; Cavalcante, H.A.O.; Uchida, N.S.; de Souza Silva-Comar, F.M.; Cardia, G.F.E.; da Silva, E.L.; Aguiar, R.P.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of patchouli (Pogostemon cablin) essential oil on in vitro and in vivo leukocytes behavior in acute inflammatory response. Biomed. Pharmacother. 2016, 84, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Gao, Y.; Lai, P.X. Chemical composition, antioxidant, antimicrobial and cytotoxic activities of essential oil from Premna microphylla Turczaninow. Molecules 2017, 22, 381. [Google Scholar] [CrossRef] [PubMed]
- De Alencar, D.C.; Pinheiro, M.L.; Pereira, J.L.; de Carvalho, J.E.; Campos, F.R.; Serain, A.F.; Tirico, R.B.; Hernández-Tasco, A.J.; Costa, E.V.; Salvador, M. Chemical composition of the essential oil from the leaves of Anaxagorea brevipes (Annonaceae) and evaluation of its bioactivity. Nat. Prod. Res. 2016, 30, 1088–1092. [Google Scholar] [CrossRef]
- Shu, Z.; Pu, J.; Chen, L.; Zhang, Y.; Rahman, K.; Qin, L.; Zheng, C. Alisma orientale: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese mdicine. Am. J. Chin. Med. 2016, 44, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, J.; Luo, Y.; Huang, N.; Zhen, N.; Zhou, Y.; Sun, F.; Li, Z.; Pan, Q.; Li, Y. (−)-Guaiol regulates RAD51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget 2016, 7, 62585–62597. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, J.; Wu, J.; Huang, N.; Cui, Z.; Luo, Y.; Sun, F.; Pan, Q.; Li, Y.; Yang, Q. (−)-Guaiol regulates autophagic cell death depending on mTOR signaling in NSCLC. Cancer Biol. Ther. 2018, 19, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β–caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Selestino Neta, M.C.; Vittorazzi, C.; Guimarães, A.C.; Martins, J.D.; Fronza, M.; Endringer, D.C.; Scherer, R. Effects of β-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24-h time-kill curve studies. Pharm. Biol. 2017, 55, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Hsu, H.F.; Huang, K.H.; Wu, J.M.; Kuo, S.M.; Ling, X.H.; Houng, J.Y. Anti-proliferative effects of Siegesbeckia orientalis ethanol extract on human endometrial RL-95 cancer cells. Molecules 2014, 19, 19980–19994. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |










| Cell Line | BSE (μg/mL) | Cisplatin (μg/mL) | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| A549 | 59.0 | 43.7 | 18.1 | 58.7 | 44.3 | 19.2 |
| H661 | 46.6 | 44.6 | 24.7 | 79.6 | 16.9 | 16.1 |
| MRC-5 | 120.8 | 89.7 | 61.1 | 34.1 | 10.7 | 7.8 |
| BSE Conc. (μg/mL) | A549 Cells | H661 Cells | ||||||
|---|---|---|---|---|---|---|---|---|
| SubG1 (%) | G0/G1 (%) * | S (%) * | G2/M (%) * | SubG1 (%) | G0/G1 (%) * | S (%) * | G2/M (%) * | |
| 0 | 0.37 ± 0.08 | 65.89 ± 1.33 | 10.61 ± 0.55 | 23.50 ± 1.83 | 2.96 ± 0.40 | 76.75 ± 0.51 | 4.72 ± 0.29 | 18.53 ± 0.23 |
| 22 | 0.56 ± 0.07 | 65.87 ± 3.50 | 9.99 ± 0.58 | 24.15 ± 3.30 | 4.48 ± 0.50 | 77.64 ± 0.36 | 4.80 ± 0.33 | 17.56 ± 0.42 |
| 44 | 1.54 ± 0.10 | 65.06 ± 1.64 | 11.78 ± 0.49. | 23.16 ± 2.12 | 5.97 ± 0.47 | 72.28 ± 0.99 | 7.33 ± 0.24 | 20.39 ± 1.04 |
| 88 | 4.59 ± 0.16 | 42.25 ± 2.10 | 19.86 ± 3.83 | 37.89 ± 5.93 | 14.17 ± 0.31 | 52.85 ± 2.33 | 11.43 ± 1.49 | 35.72 ± 2.88 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-L.; Chang, J.-C.; Fang, L.-W.; Hsu, H.-F.; Lee, L.-C.; Yang, J.-F.; Liang, M.-T.; Hsiao, P.-C.; Wang, C.-P.; Wang, S.-W.; et al. Bulnesia sarmientoi Supercritical Fluid Extract Exhibits Necroptotic Effects and Anti-Metastatic Activity on Lung Cancer Cells. Molecules 2018, 23, 3304. https://doi.org/10.3390/molecules23123304
Wang H-L, Chang J-C, Fang L-W, Hsu H-F, Lee L-C, Yang J-F, Liang M-T, Hsiao P-C, Wang C-P, Wang S-W, et al. Bulnesia sarmientoi Supercritical Fluid Extract Exhibits Necroptotic Effects and Anti-Metastatic Activity on Lung Cancer Cells. Molecules. 2018; 23(12):3304. https://doi.org/10.3390/molecules23123304
Chicago/Turabian StyleWang, Heng-Long, Jung-Che Chang, Li-Wen Fang, Hsia-Fen Hsu, Li-Chiun Lee, Jyh-Ferng Yang, Ming-Tsai Liang, Pei-Chi Hsiao, Chao-Ping Wang, Shih-Wei Wang, and et al. 2018. "Bulnesia sarmientoi Supercritical Fluid Extract Exhibits Necroptotic Effects and Anti-Metastatic Activity on Lung Cancer Cells" Molecules 23, no. 12: 3304. https://doi.org/10.3390/molecules23123304