A H2O2-Responsive Boron Dipyrromethene-Based Photosensitizer for Imaging-Guided Photodynamic Therapy
Abstract
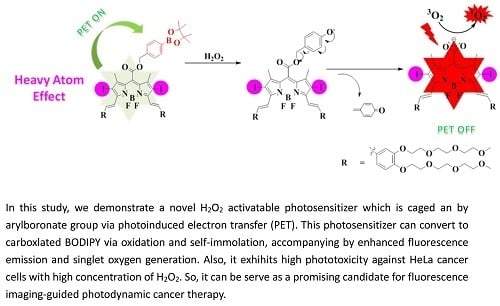
:1. Introduction
2. Results and Discussion
2.1. Molecular Design and Chemical Synthesis
2.2. Photochemical and Photophysical Properties
2.3. In Vitro Photodynamic Activity Study
2.3.1. Cellular Fluorescence Imaging
2.3.2. Measurements of Intracellular ROS
2.3.3. Cytotoxicity Studies
2.3.4. Subcellular Localization Studies
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. Synthesis of Compound 4
3.2.2. Synthesis of Compound 5
3.2.3. Synthesis of Compound 6
3.2.4. Synthesis of Compound 7
3.3. Photo-Physical and Photo-Chemical Studies
3.3.1. Absorption and Fluorescence Studies
3.3.2. Detection of Singlet Oxygen (1O2) Generation Efficiency
3.4. In Vitro Studies
3.4.1. Cell Culture and Conditions
3.4.2. Photocytotoxicity Studies
3.4.3. Measurements of Intracellular ROS
3.4.4. Subcellular Localization Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ban, Q.; Bai, T.; Duan, X.; Kong, J. Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers. Biomater. Sci. 2017, 5, 190–210. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wetzel, I.; Dréau, D.; Cho, H. 3D miniaturization of human organs for drug discovery. Adv. Healthc. Mater. 2017, 7, 1700551. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Lo, P.C.; Jiang, X.J.; Fong, W.P.; Ng, D.K.P. Synthesis and in vitro photodynamic activities of di-alpha-substituted zinc(ii) phthalocyanine derivatives. Dalton Trans. 2009, 21, 4129–4135. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Sloman, L.; He, Z.; Aksimentiev, A. Nanopore sequencing: Graphene nanopores for protein sequencing. Adv. Funct. Mater. 2016, 26, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.P.S.; Lo, P.C.; Chan, E.Y.M.; Fong, W.P.; Ko, W.H.; Ng, D.K.P. Synthesis and in vitro photodynamic activity of novel galactose-containing phthalocyanines. Tetrahedron Lett. 2005, 46, 1551–1554. [Google Scholar] [CrossRef]
- Huang, Z. A Review of progress in clinical photodynamic therapy. Thecnol. Cancer Res. Treat 2005, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.Y.; Ke, M.R.; Zhang, Y.; Huang, J.D.; Yoon, J. A tumor-pH-responsive supramolecular photosensitizer for activatable photodynamic therapy with minimal in vivo skin phototoxicity. Theranostics 2017, 7, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolemen, S.; Yoon, J.; Akkaya, E.U. Activatable photosensitizers: Agents for selective photodynamic therapy. Adv. Funct. Mater. 2017, 27, 1604053. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- Wu, W.; Shao, X.; Zhao, J.; Wu, M. Photodynamic therapy: Controllable photodynamic therapy implemented by regulating singlet oxygen efficiency. Adv. Sci. 2017, 4, 1700113. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D. Photodynamic therapy of cancer: An update. Ca. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Alshememry, A.K.; El-Tokhy, S.S.; Unsworth, L.D. Using properties of tumor microenvironments for controlling local, on-demand delivery from biopolymer-based nanocarriers. Curr. Pharm. Des. 2017, 23, 5358–5391. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Tang, Q.; Xiao, W.; Li, J.; Chen, D.; Zhang, Y.; Shao, J. A fullerene-rhodamine b photosensitizer with pH-activated visible-light absorbance/fluorescence/photodynamic therapy. J. Mater. Chem. 2018, 6, 2778–2784. [Google Scholar]
- Yan, S.; Chen, J.; Cai, L.; Xu, P.; Zhang, Y.; Li, S.; Hu, P.; Chen, X.; Huang, M.; Chen, Z. Phthalocyanine-based photosensitizer with tumor-pH-responsive properties for cancer theranostics. J. Mater. Chem. 2018, 6, 6080–6088. [Google Scholar] [CrossRef]
- Wong, R.C.H.; Ng, D.K.P.; Fong, W.P.; Lo, P.C. Encapsulating pH-responsive doxorubicin-phthalocyanine conjugates in mesoporous silica nanoparticles for combined photodynamic therapy and controlled chemotherapy. Chem. Eur. J. 2017, 23, 16505–16515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, A.; Li, J.; Wu, M.; Cai, Z.; Wu, L.; Wei, Z.; Yang, H.; Liu, X.; Liu, J. Tumor microenvironment activable self-assembled DNA hybrids for pH and redox dual-responsive chemotherapy/PDT treatment of hepatocellular carcinoma. Adv. Sci. 2017, 4, 1600460. [Google Scholar] [CrossRef] [PubMed]
- Yuki, I.; Mako, K.; Fumiaki, O.; Masayuki, M.; Takuya, T.; Toru, K.; Tasuku, U.; Kenjiro, H.; Tetsuo, N.; Yasuteru, U. Selective ablation of β-galactosidase-expressing cells with a rationally designed activatable photosensitizer. Angew. Chem. Int. Ed. Engl. 2014, 53, 6772–6775. [Google Scholar]
- Yoon, H.Y.; Koo, H.; Choi, K.Y.; Lee, S.J.; Kim, K.; Kwon, I.C.; Leary, J.F.; Park, K.; Yuk, S.H.; Park, J.H. Tumor-targeting hyaluronic acid nanoparticles for photodynamic imaging and therapy. Biomaterials 2012, 33, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Chan, M.W.; Qi, Q.; Chen, J.; Zheng, G. Porphyrin FRET acceptors for apoptosis induction and monitoring. J. Am. Chem. Soc. 2011, 133, 18580–18582. [Google Scholar] [CrossRef] [PubMed]
- Turan, I.S.; Cakmak, F.P.; Yildirim, D.C.; Cetin-Atalay, C.; Akkaya, E.U. Near-IR absorbing BODIPY derivatives as glutathione-activated photosensitizers for selective photodynamic action. Chem. Eur. J. 2015, 20, 16088–16092. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Kuang, S.; Li, G.; Jin, C.; Ji, L.; Chao, H. A GSH-activatable ruthenium(ii)-azo photosensitizer for two-photon photodynamic therapy. Chem. Commun. 2017, 53, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.M.; Jin, Z.; Lv, Y.; Yang, C.; Zhang, X.B.; Tan, W.; Yu, R.Q. An activatable two-photon fluorescence nanoprobe for bioimaging of glutathione in living cells and tissues. Anal. Chem. 2014, 86, 12321–12326. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Hu, X.X.; Li, K.; Liu, Y.; Rong, Q.; Zhu, L.; Yuan, L.; Qu, F.L.; Zhang, X.B.; Tan, W. A mitochondrial-targeted prodrug for NIR imaging guided and synergetic NIR photodynamic-chemo cancer therapy. Chem. Sci. 2017, 8, 7689–7695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhao, M.; Yang, Y.; Liang, Y.; Sun, C.; Gao, W.; Li, S.; He, B.; Pu, Y. A reactive oxygen species (ROS)-responsive low molecular weight gel co-loaded with doxorubicin and Zn(II) phthalocyanine tetrasulfonic acid for combined chemo-photodynamic therapy. J. Mater. Chem. 2017, 5, 9157–9164. [Google Scholar] [CrossRef]
- Durantini, A.M.; Greene, L.E.; Lincoln, R.; Martínez, S.R.; Cosa, G. Reactive oxygen species mediated activation of a dormant singlet oxygen photosensitizer: From autocatalytic singlet oxygen amplification to chemicontrolled photodynamic therapy. J. Am. Chem. Soc. 2016, 138, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, L.; Zhu, M.; Wan, G.; Li, C.; Zhang, J.; Wang, Y.; Wang, Y. Reactive oxygen species-responsive nanoparticles based on peglated prodrug for targeted treatment of oral tongue squamous cell carcinoma by combining photodynamic therapy and chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 29260–29272. [Google Scholar] [CrossRef] [PubMed]
- Pak, Y.; Swamy, K.; Yoon, J. Recent progress in fluorescent imaging probes. Sensors 2015, 15, 24374–24396. [Google Scholar] [CrossRef]
- Liang, X.; Xu, X.; Qiao, D.; Yin, Z.; Shang, L. The dual mechanism ICT-FRET-based fluorescent probe for the selective detection of hydrogen peroxide. Chem. Asian J. 2017, 12, 3187–3194. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, X.; Lu, Z.; Wang, X.; Wang, Z. A fluorescent probe for hydrogen peroxide in vivo based on the modulation of intramolecular charge transfer. Anal. Chem. 2017, 89, 5278–5310. [Google Scholar] [CrossRef]
- Xiao, H.; Li, P.; Zhang, S.; Zhang, W.; Zhang, W.; Tang, B. Simultaneous fluorescence visualization of mitochondrial hydrogen peroxide and zinc ions in live cells and in vivo. Chem. Commun. 2016, 52, 12741–12744. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, M.; Chen, P.; Fan, Q.; Wang, W.; Huang, W. Enhancing hydrophilicity of photoacoustic probes for effective ratiometric imaging of hydrogen peroxide. J. Mater. Chem. 2018, 6, 4531–4538. [Google Scholar] [CrossRef]
- Shang, X.; Song, X.; Faller, C.; Lai, R.; Li, H.; Cerny, R.; Niu, W.; Guo, J. Fluorogenic protein labeling using a genetically encoded unstrained alkene. Chem. Sci. 2017, 8, 1141–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.Y.; Ha, J.H.; Park, S.N. Selective release system for antioxidative and anti-inflammatory activities using H2O2-responsive therapeutic nanoparticles. Biomacromolecules 2017, 18, 3197–3206. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tian, J.; He, W.; Guo, Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.R.; Bittner, G.C.V.D.; Chang, C.J. Boronate Oxidation as a Bioorthogonal Reaction Approach for Studying the Chemistry of Hydrogen Peroxide in Living Systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; He, X.; Su, M.; Zhai, W.; Zhang, H.; Li, C. A general strategy toward highly fluorogenic bioprobes emitting across the visible spectrum. J. Am. Chem. Soc. 2017, 139, 10157–10163. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zhou, P.-Z.; Ma, J.-L.; Jia, X. Trifluoromethyl boron dipyrromethene derivatives as potential photosensitizers for photodynamic therapy. Molecules 2018, 23, 458. [Google Scholar] [CrossRef]
- Zhang, F.L.; Huang, Q.; Zheng, K.; Li, J.; Liu, J.Y.; Xue, J.P. A novel strategy for targeting photodynamic therapy. Molecular combo of photodynamic agent zinc(II) phthalocyanine and small molecule target-based anticancer drug erlotinib. Chem. Commun. 2013, 49, 9570–9572. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, Y.; Xie, M.; Zou, L.; Wang, Z.; Liu, S.; Zhao, Q. Halogenated Aza-BODIPY for imaging-guided synergistic photodynamic and photothermal tumor therapy. Adv. Healthc. Mater. 2018, 7, e1800606. [Google Scholar] [CrossRef]
- Eun-Joong, K.; Sankarprasad, B.; Hyunseung, L.; Hyun Min, K.; Chaejoon, C.; Sukhendu, M.; Kwan Soo, H.; Jong Seung, K. An activatable prodrug for the treatment of metastatic tumors. J. Am. Chem. Soc. 2014, 136, 13888–13894. [Google Scholar]
- Jiang, X.J.; Lo, P.C.; Tsang, Y.M.; Yeung, S.L.; Fong, W.P.; Ng, D.K.P. Phthalocyanine-polyamine conjugates as pH-controlled photosensitizers for photodynamic therapy. Chem. Eur. J. 2010, 16, 4777–4783. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |













| Solvent | Compounds | (nm) | (nm) | ΦF b | Stokes Shifts (nm) |
|---|---|---|---|---|---|
| DMSO | 6 | 632 | 664 | 0.17 | 32 |
| 7 | 707 | 755 | 0.067 | 48 | |
| PBS (0.05% Tween) | 6 | 647 | 678 | 0.13 | 31 |
| 7 | 705 | 739 | 0.042 | 34 | |
| DMSO:PBS = 1:4 | 6 | 652 | 696 | 0.089 | 44 |
| 7 | 723 | 753 | 0.011 | 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-W.; Su, D.; Li, X.-Q.; Cao, J.-J.; Yang, D.-C.; Liu, J.-Y. A H2O2-Responsive Boron Dipyrromethene-Based Photosensitizer for Imaging-Guided Photodynamic Therapy. Molecules 2019, 24, 32. https://doi.org/10.3390/molecules24010032
Wang Z-W, Su D, Li X-Q, Cao J-J, Yang D-C, Liu J-Y. A H2O2-Responsive Boron Dipyrromethene-Based Photosensitizer for Imaging-Guided Photodynamic Therapy. Molecules. 2019; 24(1):32. https://doi.org/10.3390/molecules24010032
Chicago/Turabian StyleWang, Zhi-Wei, Dan Su, Xiao-Qiang Li, Jing-Jing Cao, De-Chao Yang, and Jian-Yong Liu. 2019. "A H2O2-Responsive Boron Dipyrromethene-Based Photosensitizer for Imaging-Guided Photodynamic Therapy" Molecules 24, no. 1: 32. https://doi.org/10.3390/molecules24010032
APA StyleWang, Z.-W., Su, D., Li, X.-Q., Cao, J.-J., Yang, D.-C., & Liu, J.-Y. (2019). A H2O2-Responsive Boron Dipyrromethene-Based Photosensitizer for Imaging-Guided Photodynamic Therapy. Molecules, 24(1), 32. https://doi.org/10.3390/molecules24010032