Bioavailability of Tea Catechins and Its Improvement
Abstract
:1. Introduction
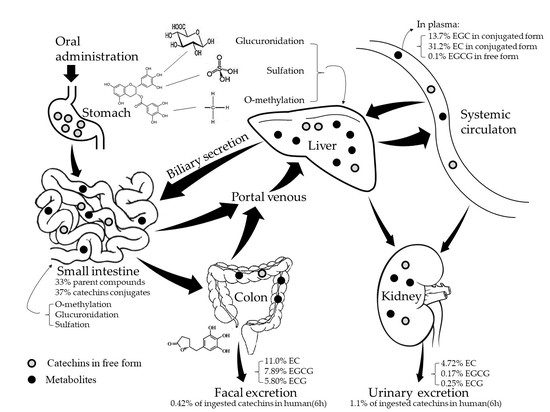
2. Absorption and Metabolism of Tea Catechins
3. Improving the Bioavailability of Catechins
3.1. Nanostructure-Based Drug Delivery System
3.1.1. Protein-Based Carriers
3.1.2. Carbohydrate-Based Carriers
3.1.3. Lipid-Based Carriers
3.1.4. Mechanism by Which Nano-Carriers Improving the Bioavailability of Catechins
3.2. Molecular Modification
3.3. Co-Administration of Catechins with Other Bioactive Components
4. Conclusions and Future Expectations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, W.; Kukula-Koch, W.; Komsta, Ł.; Marzec, Z.; Szwerc, W.; Głowniak, K. Green tea quality evaluation based on its catechins and metals composition in combination with chemometric analysis. Molecules 2018, 23, 1689. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T. Absorption, metabolism and antioxidative effects of tea catechin in humans. Biofactors 2000, 13, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.Y.; Li, Q.S.; Lin, X.M.; Qiao, R.Y.; Yang, R.; Li, X.M.; Dong, Z.B.; Xiang, L.P.; Zheng, X.Q.; Lu, J.L.; et al. Antidiabetic effects of tea. Molecules 2017, 22, 849. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.E.; Harrington, K.L.; Vollmer, T.R.; Ward, K.W.; Zhang, J.Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar] [PubMed]
- Oz, H.S.; Chen, T.S.; McClain, C.J.; de Villiers, W.J. Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem. 2005, 16, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.; de Villiers, W.J.S. Green tea polyphenols and sulfasalazine have parallel anti-inflammatory properties in colitis models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.P.; Wang, A.; Ye, J.H.; Zheng, X.Q.; Polito, C.A.; Lu, J.L.; Li, Q.S.; Liang, Y.R. Suppressive effects of tea catechins on breast cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Roberto, B.S.; Macedo, G.A.; Macedo, J.A.; Martins, I.M.; Nakajima, V.M.; Allwood, J.W.; Stewart, D.; McDougall, G.J. Immobilized tannase treatment alters polyphenolic composition in teas and their potential anti-obesity and hypoglycemic activities in vitro. Food Funct. 2016, 7, 3920–3932. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Dong, Z.; Yang, L.; Chen, X.; Gong, Z. Inhibition of proliferation of human lung cancer cells by green tea catechins is mediated by upregulation of let-7. Exp. Ther. Med. 2012, 4, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, C.A.; Bisson, W.H.; Dashwood, R.H. Tea catechins inhibit hepatocyte growth factor receptor (MET kinase) activity in human colon cancer cells: Kinetic and molecular docking studies. J. Med. Chem. 2009, 52, 6543–6545. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Oz, H.S.; Barve, S.; De Villiers, W.J.; McClain, C.J.; Varilek, G.W. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-κB activation by inhibiting IκB kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharm. 2001, 60, 528–533. [Google Scholar]
- Narumi, K.; Sonoda, J.I.; Shiotani, K.; Shigeru, M.; Shibata, M.; Kawachi, A.; Tomishige, E.; Sato, K.; Motoya, T. Simultaneous detection of green tea catechins and gallic acid in human serum after ingestion of green tea tablets using ion-pair high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B 2014, 945, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Lee, M.J.; Lu, H.; Meng, X.; Hong, J.J.J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Anavy, N.D.; Chow, H.H. Contribution of presystemic hepatic extraction to the low oral bioavailability of green tea catechins in rats. Drug Metab. Dispos. 2002, 30, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Krook, M.A.; Hagerman, A.E. Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res. Int. 2012, 49, 112–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zheng, Y.; Chow, M.S.S.; Zou, Z. Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int. J. Pharm. 2004, 287, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Ferguson, S.J.; Wuertzkozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic study of catechin stability: Effects of pH, concentration, and temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Lee, M.J.; Diamond, L.; Ju, J.; Hong, J.; Bose, M.; Newmark, H.L.; Yang, C.S. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 2006, 34, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar] [PubMed]
- Chow, H.H.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidem. Biomar. 2001, 10, 53–58. [Google Scholar]
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.C.; Wang, M.N.; Tseng, T.Y.; Sung, J.S.; Tsai, T.H. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Catterall, F.; King, L.J.; Clifford, M.N.; Ioannides, C. Bioavailability of dietary doses of 3H-labelled tea antioxidants (+)-catechin and (−)-epicatechin in rat. Xenobiotica 2003, 33, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Warden, B.A.; Smith, L.S.; Beecher, G.R.; Balentine, D.A.; Clevidence, B.A. Catechins are bioavailable in men and women drinking black tea throughout the day. J. Nutr. 2001, 131, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, M.J.; Li, H.E.; Yang, C.S. Absorption, distribution, and elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar] [PubMed]
- Yang, C.S.; Chen, L.; Lee, M.J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidem. Biomar. 1998, 7, 351–354. [Google Scholar]
- Lambert, J.D.; Sang, S.; Yang, C.S. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol. Pharm. 2007, 4, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.S.; Hakim, I.A. Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol. Res. 2011, 64, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Steiling, H.; Williamson, G.; Lean, M.E.; Crozier, A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010, 54, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2010, 53, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lambert, J.D.; Lee, S.H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, J.; Demeule, M.; Béliveau, R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. BBA Mol. Cell Res. 2002, 1542, 149–159. [Google Scholar] [CrossRef]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced uptake and transport of (+)-catechin and (−)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells. Int. J. Nanomed. 2014, 9, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, Y.; Li, R.C. Oral absorption and bioavailability of tea catechins. Planta Med. 2000, 66, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Dag, D.; Oztop, M.H. Formation and characterization of green tea extract loaded liposomes. J. Food Sci. 2017, 82, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.Q.; Peng, S.F.; Liu, W.; Gan, L.; Liu, W.L.; Liang, R.H.; Liu, C.M.; Niu, J.; Cao, Y.L.; Liu, Z.; et al. Improved in vitro, digestion stability of (−)-epigallocatechin gallate through nanoliposome encapsulation. Food Res. Int. 2014, 64, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Shi, Y.L.; Li, X.M.; Yang, R.; Cai, Z.Y.; Li, Q.S.; Ma, S.C.; Ye, J.H.; Lu, J.L.; Liang, Y.R.; et al. Food-grade encapsulation systems for (−)-epigallocatechin gallate. Molecules 2018, 23, 445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lim, L.T.; Kakuda, Y. Electrospun zein fibers as carriers to stabilize (-)-epigallocatechin gallate. J. Food Sci. 2009, 74, C233–C240. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.H.; Augustin, M.A. Nano- and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. Nutr. 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Hao, C.; Chen, Y.; Chen, L.; Fang, Z.; Liang, L. Formation of a multiligand complex of bovine serum albumin with retinol, resveratrol, and (−)-epigallocatechin-3-gallate for the protection of bioactive components. J. Agric. Food Chem. 2017, 65, 3019–3030. [Google Scholar]
- Li, Z.; Gu, L. Fabrication of self-assembled (−)-epigallocatechin gallate (EGCG) ovalbumin-dextran conjugate nanoparticles and their transport across monolayers of human intestinal epithelial Caco-2 cells. J. Agric. Food Chem. 2014, 62, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ha, J.; Zou, T.; Gu, L. Fabrication of coated bovine serum albumin (BSA)-epigallocatechin gallate (EGCG) nanoparticles and their transport across monolayers of human intestinal epithelial Caco-2 cells. Food Funct. 2014, 5, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Meena, R.; Paulraj, R. Fabrication of BSA-green tea polyphenols-chitosan nanoparticles and its role in radioprotection: A molecular and biochemical approach. J. Agric. Food Chem. 2016, 64, 6024–6034. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Mukherjee, P.K.; Kar, A.; Bahadur, S.; Al-Dhabi, N.A.; Duraipandiyan, V. Enhancement of photoprotection potential of catechin loaded nanoemulsion gel against UVA induced oxidative stress. J. Photochem. Photobiol. B 2016, 160, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Soler, C.; Lopez-Rubio, A. Stability and bioaccessibility of EGCG within edible micro-hydrogels. Chitosan vs. gelatin, a comparative study. Food Hydrocoll. 2016, 61, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Sabouri, S.; Geng, J.; Corredig, M. Tea polyphenols association to caseinate-stabilized oil–water interfaces. Food Hydrocoll. 2015, 51, 95–100. [Google Scholar] [CrossRef]
- Sabouri, S.; Wright, A.J.; Corredig, M. In vitro, digestion of sodium caseinate emulsions loaded with epigallocatechin gallate. Food Hydrocoll. 2017, 69, 350–358. [Google Scholar] [CrossRef]
- Haratifar, S.; Meckling, K.A.; Corredig, M. Antiproliferative activity of tea catechins associated with casein micelles, using HT29 colon cancer cells. J. Dairy Sci. 2014, 97, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, T.; Fernandez, M.L.; Luo, Y. Development of tannic acid cross-linked hollow zein nanoparticles as potential oral delivery vehicles for curcumin. Food Hydrocoll. 2016, 61, 821–831. [Google Scholar] [CrossRef]
- Bhushani, J.A.; Karthik, P.; Anandharamakrishnan, C. Nanoemulsion based delivery system for improved bioaccessibility and Caco-2cell monolayer permeability of green tea catechins. Food Hydrocoll. 2016, 56, 372–382. [Google Scholar] [CrossRef]
- Shi, M.; Huang, L.Y.; Nie, N.; Ye, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Binding of tea catechins to rice bran protein isolate: Interaction and protective effect during in vitro digestion. Food Res. Int. 2017, 93, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.A. Interaction of milk α- and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Zorilla, R.; Liang, L.; Remondetto, G.; Subirade, M. Interaction of epigallocatechin-3-gallate with β-lactoglobulin: Molecular characterization and biological implication. Dairy. Sci. Technol. 2011, 91, 629–644. [Google Scholar] [CrossRef]
- Son, Y.R.; Chung, J.H.; Ko, S.; Shim, S.M. Combinational enhancing effects of formulation and encapsulation on digestive stability and intestinal transport of green tea catechins. J. Microencapsul. 2016, 33, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Lee, S.J.; Chung, J.O.; Oh, Y.J.; Hwang, J.A.; Kim, Y.K.; Ko, S.; Shim, S.M. Effect of hydroxypropyl methyl cellulose phthalate coating on digestive stability and intestinal transport of green tea catechins. Integr. Med. Res. 2014, 3, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.Q.; Liu, W.; Liu, W.L.; Liang, R.H.; Li, T.; Liu, C.M.; Cao, Y.L.; Niu, J.; Liu, Z. Characterization and bioavailability of tea polyphenol nanoliposome prepared by combining an ethanol injection method with dynamic high-pressure microfluidization. J. Agric. Food Chem. 2014, 62, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Lee, W.R.; Shen, S.C.; Huang, Y.L. Effect of liposome encapsulation of tea catechins on their accumulation in basal cell carcinomas. J. Dermatol. Sci. 2006, 42, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.B.; Tsai, M.J.; Wu, P.C.; Tsai, Y.H.; Wu, Y.H.; Fang, J.Y. Elastic liposomes as carriers for oral delivery and the brain distribution of (+)-catechin. J. Drug Target. 2011, 19, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Tang, D.W.; Yu, S.H.; Ho, Y.C.; Huang, B.Q.; Tsai, G.J.; Hsieh, H.Y.; Sung, H.W.; Mi, F.L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Chen, L.; Yokoyama, W.; Williams, P.A.; Zhong, F. Niosomes consisting of tween-60 and cholesterol improve the chemical stability and antioxidant activity of (−)-epigallocatechin gallate under intestinal tract conditions. J. Agric. Food Chem. 2016, 64, 9180–9188. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Kajiya, K.; Naito, A.; Saitô, H.; Tuzi, S.; Tanio, M.; Suzuki, M.; Nanjo, F.; Suzuki, E.; Nakayama, T. Direct evidence of interaction of a green tea polyphenol, epigallocatechin gallate, with lipid bilayers by solid-state Nuclear Magnetic Resonance. Biosci. Biotechnol. Biochem. 2004, 68, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Peng, S.; Liu, W.; Chen, X.; Liu, C. A novel delivery system dextran sulfate coated amphiphilic chitosan derivatives-based nanoliposome: Capacity to improve in vitro, digestion stability of (−)-epigallocatechin gallate. Food Res. Int. 2015, 69, 114–120. [Google Scholar] [CrossRef]
- Hong, Z.; Xu, Y.; Yin, J.F.; Jin, J.; Jiang, Y.; Du, Q. Improving the effectiveness of (−)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Cellular uptake and cytotoxicity of chitosan–caseinophosphopeptides nanocomplexes loaded with epigallocatechin gallate. Carbohyd. Polym. 2012, 89, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jung, J.; Zhao, Y. Preparation, characterization and evaluation of antibacterial activity of catechins and catechins–Zn complex loaded β-chitosan nanoparticles of different particle sizes. Carbohyd. Polym. 2016, 137, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Hwang, T.L.; Huang, Y.L.; Fang, C.L. Enhancement of the transdermal delivery of catechins by liposomes incorporating anionic surfactants and ethanol. Int. J. Pharm. 2006, 310, 131–138. [Google Scholar] [CrossRef] [PubMed]
- De Pace, R.C.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.Y.; Wang, S. Anticancer activities of (−)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guan, R.; Xiao, C.; Lyu, F.; Cao, G.; Liu, M.; Gao, J. Optimization of catechin nanoliposomes and evaluation of their antioxidant activity and cytotoxicity. Sci. Adv. Mater. 2016, 9, 697–704. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its anti-atherogenic bioactivities in macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int. J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (−)-Epigallocatechin gallate (EGCG)-nanoethosomes as a transdermal delivery system for docetaxel to treat implanted human melanoma cell tumors in mice. Int. J. Pharm. 2016, 512, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Haratifar, S. Nanoencapsulation of Tea Catechins in Casein Micelles: Effects on Processing and Biological Functionalities. Environ. Sci. Pollut. R. 2012, 19, 2227–2237. [Google Scholar]
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Yu, Q.; Yang, L.; Sun, D.; Zhou, Y.; Liu, J. Epigallocatechin-3-gallate (EGCG)-stabilized selenium nanoparticles coated with Tet-1 peptide to reduce amyloid-β aggregation and cytotoxicity. ACS Appl. Mater. Interface 2014, 6, 8475–8487. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Bhatnagar, P.; Singh, M.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int. J. Nanomed. 2013, 8, 1451–1462. [Google Scholar]
- Lam, W.H.; Kazi, A.; Kuhn, D.J.; Chow, L.M.; Chan, A.S.; Dou, Q.P.; Chan, T.H. A potential prodrug for a green tea polyphenol proteasome inhibitor: Evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG]. Bioorgan. Med. Chem. 2004, 12, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Hong, J.; Kwon, S.J.; Lee, M.J.; Ho, C.T.; Yang, C.S. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab. Dispos. 2006, 34, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Ma, N.J.L.; Sang, S.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Peracetylated (-)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J. Agric. Food Chem. 2012, 60, 3441–3451. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.S.; Sang, S.; Cheng, K.H.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+ skin stem cells and skin tumors. Carcinogenesis 2013, 34, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Landis-Piwowar, K.R.; Huo, C.; Chen, D.I.; Milacic, V.; Shi, G.; Chan, T.H.; Dou, Q.P. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007, 67, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Chan, W.K.; Lee, T.W.; Lam, W.H.; Wang, X.; Chan, T.H.; Wong, Y.C. Effect of a prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate on the growth of androgen-independent prostate cancer in vivo. Nutr. Cancer 2008, 60, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Xu, H.; Man, G.C.W.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.Y.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent; anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A prodrug of green tea polyphenol (–)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Pamu, S.; Cui, Q.; Chan, T.H.; Dou, Q.P. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorgan. Med. Chem. 2012, 20, 3031–3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 51, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, S.M.; Yoo, S.H.; Ra, C.S.; Kim, Y.K.; Chung, J.O.; Lee, S.J. Digestive stability and absorption of green tea polyphenols: Influence of acid and xylitol addition. Food Res. Int. 2012, 45, 204–210. [Google Scholar] [CrossRef]
- Shin, S.C.; Choi, J.S. Effects of epigallocatechin gallate on the oral bioavailability and pharmacokinetics of tamoxifen and its main metabolite, 4-hydroxytamoxifen, in rats. Anti-Cancer Drug. 2009, 20, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Nabekura, T.; Kamiyama, S. Inhibition of P-glycoprotein function by tea catechins in KB-C2 cells. J. Pharm. Pharmacol. 2004, 56, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Malik, A.; Zaman, N.; Sarfaraz, S.; Siddiqui, I.A.; Syed, D.N.; Afaq, F.; Pasha, F.S.; Saleem, M.; Mukhtar, H. Combined inhibitory effects of green tea polyphenols and selective cyclooxygenase-2 inhibitors on the growth of human prostate cancer cells both in vitro and in vivo. Clin. Cancer Res. 2007, 13, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J. Cancer Res. Clin. 2015, 141, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Hong, J.; Kim, D.H.; Mishin, V.M.; Yang, C.S. Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J. Nutr. 2004, 134, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, G.; Zhang, M.; Zhou, Z.; Li, Q.; Strappe, P.; Blanchard, C. Epigallocatechin gallate (egcg) decorating soybean seed ferritin as a rutin nanocarrier with prolonged release property in the gastrointestinal tract. Plant Food Hum. Nutr. 2016, 71, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Scandlyn, M.J.; Stuart, E.C.; Somers-Edgar, T.J.; Menzies, A.R.; Rosengren, R.J. A new role for tamoxifen in oestrogen receptor-negative breast cancer when it is combined with epigallocatechin gallate. Br. J. Cancer 2008, 99, 1056–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, H.; Tighiouart, M.; Lee, J.E.; Shin, H.J.; Khuri, F.R.; Yang, C.S.; Chen, Z.; Shin, D.M. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int. J. Cancer 2010, 123, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.A.; Burke, P.; Coleman, D.T.; Bigelow, R.L.; Steffan, J.J.; Carroll, J.L.; Williams, B.J.; Cardelli, J.A. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin. Cancer Res. 2009, 15, 4885–4894. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Burm, J.P. Effects of oral epigallocatechin gallate on the pharmacokinetics of nicardipine in rats. Arch. Pharm. Res. 2009, 32, 1721–1725. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Rodrigues, M.; Marques, A.; Falcão, A.; Alves, G. Influence of the dual combination of silymarin and (−)-epigallocatechin gallate, natural dietary flavonoids, on the pharmacokinetics of oxcarbazepine in rats author names and affiliations. Food Chem. Toxicol. 2017, 106, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Choi, D.H.; Choi, J.S. Effects of oral epigallocatechin gallate on the oral pharmacokinetics of verapamil in rats. Biopharm. Drug Dispos. 2009, 30, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Nakayama, K.; Nakamura, M.; Sookwong, P.; Tsuduki, T.; Niino, H.; Kimura, F.; Miyazawa, T. Effects of co-administration of tea epigallocatechin-3-gallate (EGCG) and caffeine on absorption and metabolism of EGCG in humans. Biosci. Biotechnol. Biochem. 2009, 73, 2014–2017. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kwon, S.J.; Ju, J.; Bose, M.; Lee, M.J.; Hong, J.; Hao, X.; Yang, C.S. Effect of genistein on the bioavailability and intestinal cancer chemopreventive activity of (−)-epigallocatechin-3-gallate. Carcinogenesis 2008, 29, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.H.; Wang, X.; Yang, M.; Shi, X.; Huang, W.; Feng, Q. Combination of low concentration of (−)-epigallocatechin gallate (egcg) and curcumin strongly suppresses the growth of non-small cell lung cancer in vitro and in vivo through causing cell cycle arrest. Int. J. Mol. Sci. 2013, 14, 12023–12036. [Google Scholar] [CrossRef] [PubMed]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; Le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The combination of epigallocatechin gallate and curcumin suppresses ERα-breast cancer cell growth in vitro and in vivo. Int. J. Cancer 2010, 122, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Tang, A.; Lin, X.; Li, L.; Zhang, S.; Huang, Z.; Tang, H.; Li, Q.Q. Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. Int. J. Oncol. 2010, 37, 111–123. [Google Scholar] [PubMed]
- Oz, H.S.; Chen, T.S. Green-tea polyphenols downregulate cyclooxygenase and Bcl-2 activity in acetaminophen-induced hepatotoxicity. Digest. Dis. Sci. 2008, 53, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Materials of Carrier | Bioactive | Improvement of Effectiveness | Ref. |
|---|---|---|---|
| Chitosan and TPP | EGCG | Improved stability and increased plasma concentrations of EGCG | [42,66] |
| Chitosan | Catechin and EGCG | Enhanced the intestinal absorption of catechins | [69] |
| Carboxymethyl chitosan | EGCG | Improved stability and sustained release. | [73] |
| Chitosan and γ-glutamic acid | Catechins | Increased the paracellular transport of catechins with effective antioxidant activity. | [68] |
| Chitosan and polyaspartic acid | EGCG | Improved the effectiveness of EGCG against rabbit atherosclerosis. | [74] |
| Chitosan and caseinophosphopeptides | EGCG | Enhanced the intestinal permeability of catechins | [75] |
| Beta-chitosan | Catechins | Improved the antibacterial activity | [76] |
| Chitosan or poly-ε-lysine | EGCG | Improved the stability of EGCG and improved the permeability across intestine | [47] |
| Chitosan | TP | Improved the level of radioprotection of TP. | [48] |
| HPMCP or γ-CD | Catechins | Increased intestinal transport. | [59] |
| HPMCP | Catechins | Improved the stability of catechins and increased intestinal transport. | [60] |
| Lipsomes | Catechins | Enhanced the transdermal delivery of catechins. | [77] |
| Lipsomes | Green tea extract | Improved the stability of catechins. | [38] |
| Lipsomes | TP | Improved the stability of catechins. | [62] |
| Liposome | Catechins | Inducted greater basal cell carcinomas death at lower concentrations. | [63] |
| Nanolipsomes | EGCG | Induced apoptosis and inhibited proliferation of MCF7 breast cancer cells. | [78] |
| Nanolipsomes | Catechins | Improved the antioxidant activity | [79] |
| Nanostructured lipid | EGCG | Inhibitd atherosclerotic lesion development through decreasing macrophage cholesterol content and monocyte chemoattractant protein-1 expression. | [80] |
| Nanolipidic | EGCG | Improved α-secretase inducing ability of EGCG for the treatment of Alzheimer’s disease. | [81] |
| Nanoethosomes | EGCG | Enhancing the skin permeability. | [82] |
| Niosomes | EGCG | Improved the stability of catechins and exhibited stronger antioxidant ability. | [70] |
| Ovalbumin | EGCG | Enhanced the apparent permeability coefficient of EGCG on Caco-2 monolayers | [46] |
| Casein micelles | EGCG | Improved the stability of catechins, and decreased the proliferation of HT-29 cancer cells without affecting the bioefficacy of EGCG. | [83] |
| Casein micelles | Catechins | Improved the stability of catechins, and decreased the proliferation of HT-29 cancer cells in a manner similar to that of free EGCG. | [53] |
| Nanoemulsion gel | Catechins | Showed sustained release profile and enhanced photoprotection potential due to its improved skin permeability and bioavailability through transdermal route. | [49] |
| Zein | EGCG | Improved the stability of EGCG. | [41] |
| Rice bran protein isolate | Catechins | Improved the stability of catechins. | [56] |
| β-lactoglobulin | EGCG | Protected antioxidant activity of EGCG | [84] |
| Selenium nanoparticles and Tet-1 peptide | EGCG | Inhibited amyloid-β fibrillation and disaggregate preformed amyloid-β fibrils into nontoxic aggregates. | [85] |
| poly(lactide-co-glycolide) | EGCG | Showed a superior ability to prevent DMBA-induced DNA damage at much lower concentrations | [86] |
| Molecular Modification | Tested Cell Lines | Cancer Type | Major Effects | Ref. |
|---|---|---|---|---|
| Peracetylated EGCG | Jurkat T | Leukemic | Being more stable than free EGCG at neutral pH and showing greater efficacy in proteasome inhibition and cell death induction. | [87] |
| KYSE150, HCT116 | Esophageal and colon | Increasing the biological potency in vitro and the bioavailability of EGCG in esophageal or colon cancer cells. | [88] | |
| Colon | Showing stronger prevention potency to DSS-induced colitis than free EGCG. | [89] | ||
| CD34+ | Skin | Preventing skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+ skin stem cells and skin tumors | [90] | |
| MDA-MB-231 | Breast | Increasing the bioavailability, stability, and proteasome inhibition and anticancer activities of EGCG in human breast cancer cells and tumors. | [91] | |
| CWR22R | Prostate | Being more stable, increasing the therapeutic anticancer effects in androgen-independent prostate cancer | [92] | |
| Endometrium | Inhibiting the growth, development and angiogenesis of experimental endometriosis in mice, with improved efficacy, bioavailability, anti-oxidation and anti-angiogenesis capacities. | [93] | ||
| Inhibiting tumor angiogenesis through downregulation of VEGFA and HIF1α in tumor cell and chemokine(C-X-C motif) ligand 12 in host stroma. | [94] | |||
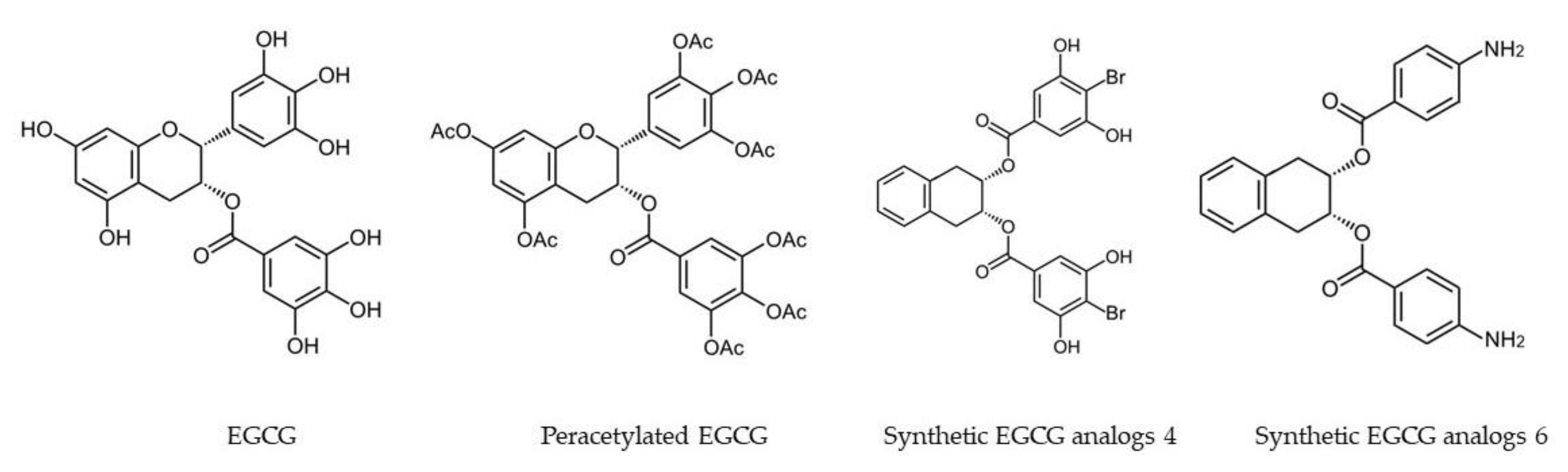
| Synthetic EGCG analogs 4 and 6 (Figure 2) | MDA-MB-231 | Breast | Activating AMPK, with inhibition of cell proliferation, up-regulation of the cyclin-dependent kinase inhibitor p21, down-regulation of mTOR pathway, and suppression of stem cell population in human breast cancer cells. | [95] |
| Tea Catechins | Complementary Bioactives | Effectiveness | Ref. |
|---|---|---|---|
| Catechins | Ascorbic acid (and sucrose or xylitol) | Increasing tea catechins recovery in a simulated in vitro digestion. | [96] |
| Improving catechins bioavailability by enhancing bioaccessibility and intestinal uptake. | [97] | ||
| Promoting intestinal transport of catechins in a dose-dependent manner. | [98] | ||
| Increasing bioavailability of green tea catechins. | [59] | ||
| EGCG | Piperine | Increasing EGCG bioavailability by inhibiting glucuronidation and gastrointestinal transit. | [103] |
| Rutin | Improving the stability and the prolonged release of rutin in simulated GI fluid, owing to the external attachment of EGCG to the ferritin cage, potentially reducing enzymolysis in GI fluid. | [104] | |
| Tamoxifen | Significantly improving the pharmacokinetics of orally administered tamoxifen. | [99] | |
| Promoting the suppressive effects on growth of ER-negative breast cancer, along with a decrease in expression of tumor proteins mTOR and the EGFR. | [105] | ||
| Erlotinib | Inhibiting pEGFR and pAKT, increasing activation of caspases 9, 3 and PARP, inhibiting cell proliferation and inducing apoptosis. | [106] | |
| Inhibiting cancer cell proliferation, increasingresponse to erlotinib. | [107] | ||
| Nicardipine | Increasing bioavailability of oral administered EGCG, resulting in inhibition both the hepatic CYP3A subfamily and intestinal P-gp. | [108] | |
| Oxcarbazepine | Enhancing the degree of systemic exposure tooxcarbazepine and licarbazepine in rats. | [109] | |
| Verapamil | Increasing significantly the bioavailability of verapamil. | [110] | |
| Caffeine | Enhancing the absorption of EGCG in humans. | [111] | |
| Genistein | Enhancing EGCG bioavailability and inhibiting tumorigenesis in mice. | [112] | |
| NS398 | Enhanced apoptosis induction in vitro and tumor growth inhibition in vivo. | [101] | |
| sulindac | Inducing apoptosis of cancer cells by promoting the expression of GADD153 and p21WAF1 genes. | [102] | |
| Curcumin | Enhancing cell cycle arrest at G1and S/G2 phases. | [113] | |
| Synergistic cytotoxicity to the cancer cells along with G2/M-phase cell cycle arrest. | [114] | ||
| ECG and EGCG | Doxorubicin | Enhancing sensitivity of cancer cells to doxorubicin and the accumulation of doxorubicin in cancer cells. | [115] |
| Green tea polyphenol | Acetaminophen | Green tea polyphenol supplementation attenuated hepatotoxicity by normalizing cyclooxygenase andB-cell lymphoma-2 activation, suggesting a potential use for in treating acetaminophen toxicity. | [116] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. https://doi.org/10.3390/molecules23092346
Cai Z-Y, Li X-M, Liang J-P, Xiang L-P, Wang K-R, Shi Y-L, Yang R, Shi M, Ye J-H, Lu J-L, et al. Bioavailability of Tea Catechins and Its Improvement. Molecules. 2018; 23(9):2346. https://doi.org/10.3390/molecules23092346
Chicago/Turabian StyleCai, Zhuo-Yu, Xu-Min Li, Jin-Pei Liang, Li-Ping Xiang, Kai-Rong Wang, Yun-Long Shi, Rui Yang, Meng Shi, Jian-Hui Ye, Jian-Liang Lu, and et al. 2018. "Bioavailability of Tea Catechins and Its Improvement" Molecules 23, no. 9: 2346. https://doi.org/10.3390/molecules23092346