Discovery of 4,5-Dihydro-1H-thieno[2′,3′:2,3]thiepino [4,5-c]pyrazole-3-carboxamide Derivatives as the Potential Epidermal Growth Factor Receptors for Tyrosine Kinase Inhibitors
Abstract
:1. Introduction
2. Results and Discussion
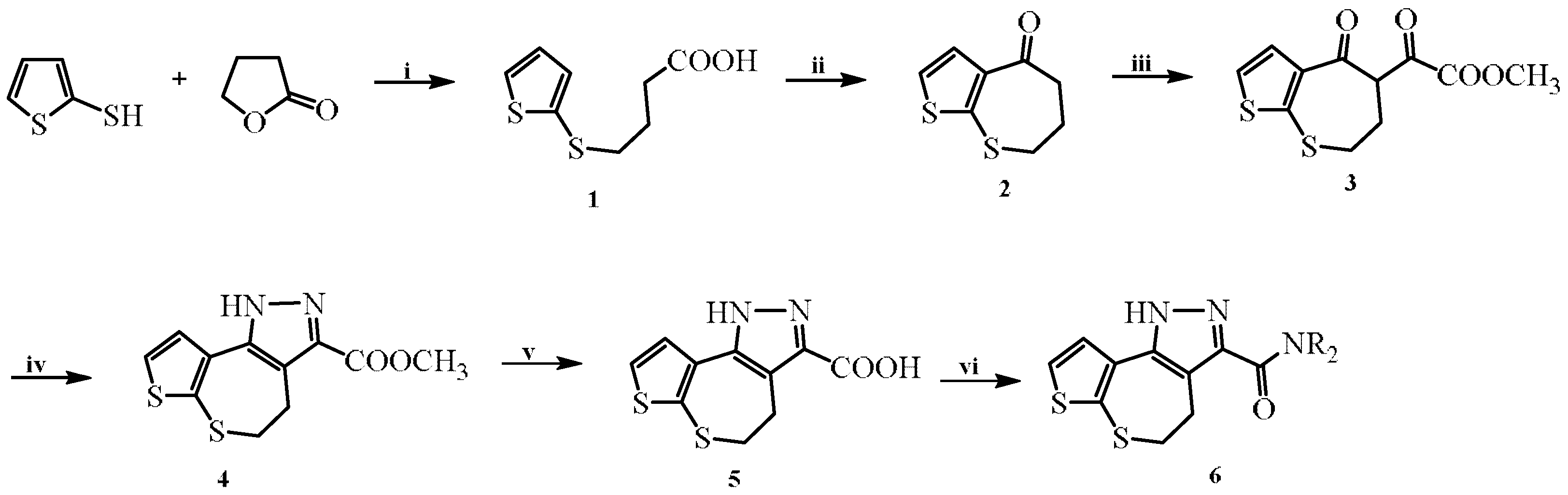
2.1. Synthesis
2.2. Biological Evaluation
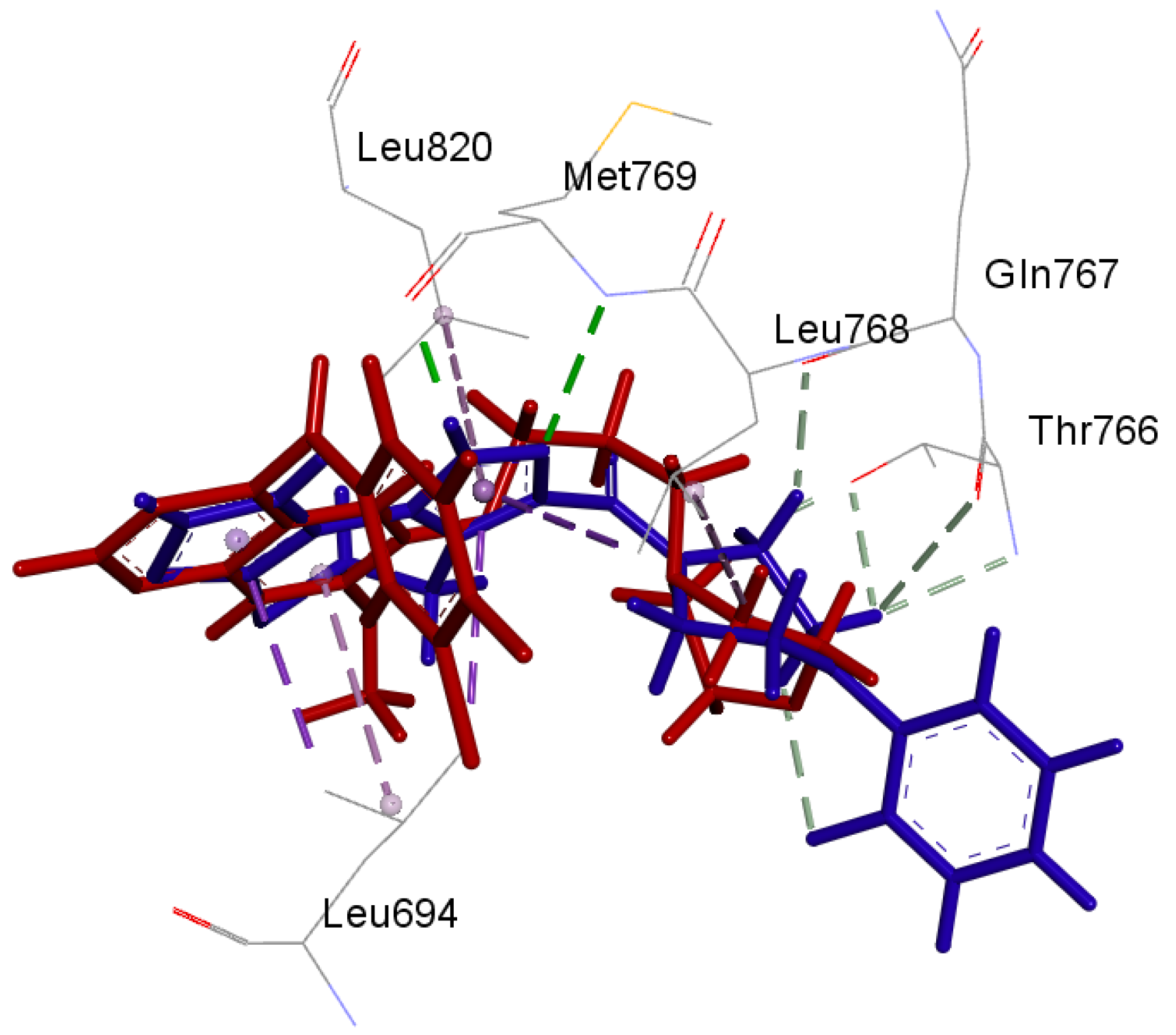
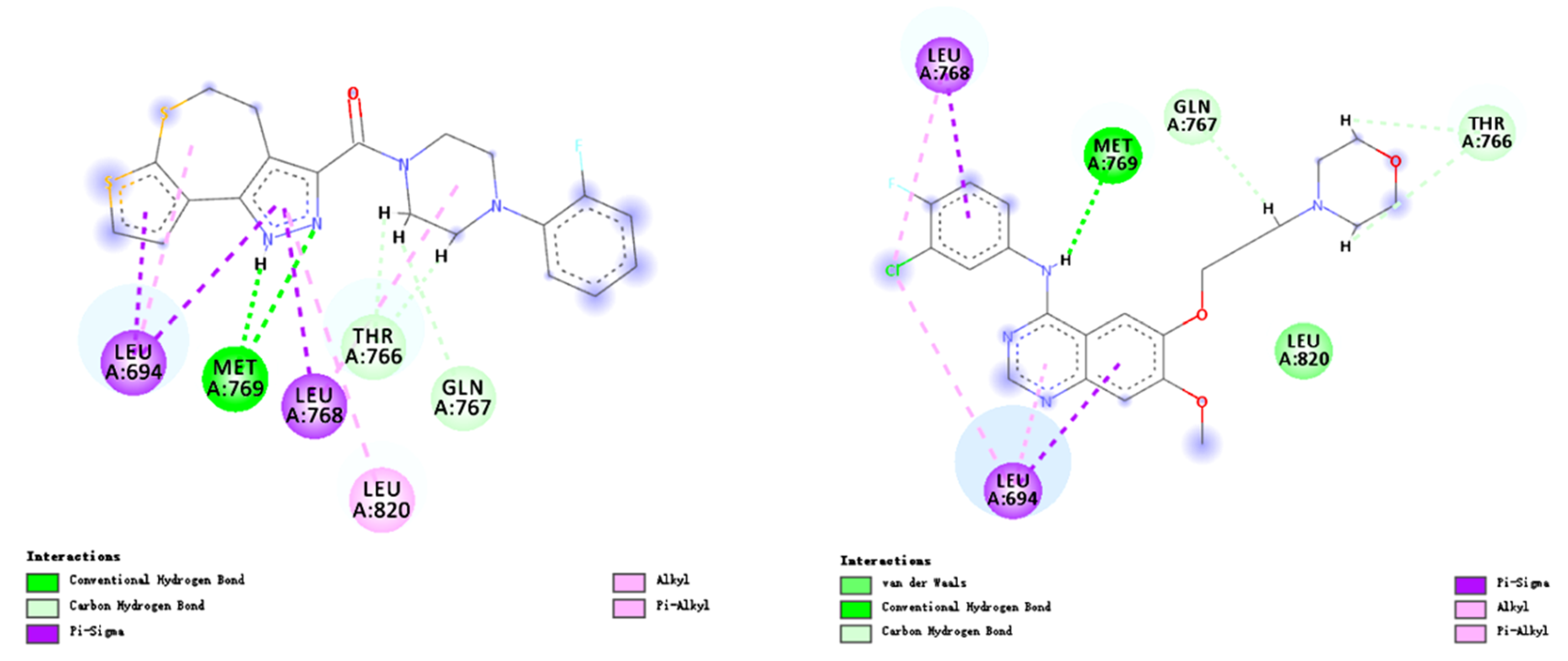
2.3. Docking Study
3. Experimental Section
3.1. Chemistry
3.1.1. Synthesis of 4-(Thiophen-2-ylthio)butanoic Acid (1)
3.1.2. Synthesis of 6,7-Dihydrothieno[2,3-b]thiepin-4(5H)-one (2)
3.1.3. Synthesis of Methyl Oxo(4-oxo-4,5,6,7-tetrahydrothieno[2,3-b]thiepin-5-yl)acetate (3)
3.1.4. Synthesis of Methyl 4,5-dihydro-1H-thieno[2′,3′:2,3]thiepino[4,5-c] pyrazole-3-carboxylate (4)
3.1.5. Synthesis of 4,5-Dihydro-1H-thieno[2′,3′:2,3]thiepin[4,5-c]pyrazole-3-carboxylic acid (5)
3.1.6. General Procedure for the Synthesis of 4,5-Dihydro-1H-thieno[2′,3′:2,3]thiepino[4,5-c]pyrazole-3-carboxamides (6a–6l)
3.2. Cell Proliferation Assay
3.3. Molecular Docking
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Antonello, A.; Tarozzi, A.; Morroni, F.; Cavalli, A.; Rosini, M.; Hrelia, P.; Bolognesi, M.L.; Melchiorre, C. Multitarget-directed drug design strategy: A novel molecule designed to block epidermal growth factor receptor (EGFR) and to exert proapoptotic effects. J. Med. Chem. 2006, 49, 6642–6645. [Google Scholar] [CrossRef] [PubMed]
- Moscatello, D.K.; Holgado-Madruga, M.; Godwin, A.K.; Ramirez, G.; Gunn, G.; Zoltick, P.W.; Biegel, J.A.; Hayes, R.L.; Wong, A.J. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995, 55, 5536–5539. [Google Scholar] [PubMed]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol. Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Zhang, H.; Berezov, A.; Wang, Q.; Zhang, G.; Drebin, J.; Murali, R.; Greene, M.I. ErbB receptors: From oncogenes to targeted cancer therapies. J. Clin. Investig. 2007, 117, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.J.; Kumar, R. Epidermal growth factor receptor family tyrosine kinases as signal integrators and therapeutic targets. Cancer Metastasis Rev. 2003, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Assefa, H.; Kamath, S.; Buolamwini, J.K. 3D-QSAR and docking studies on 4-anilinoquinazoline and 4-anilinoquinoline epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. J. Comput. Aided Mol. Des. 2003, 17, 475–493. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. Protein kinases—The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.W.; Bridges, A.J.; Denny, W.A.; Doherty, A.; Greis, K.D.; Hicks, J.L.; Hook, K.E.; Keller, P.R.; Leopold, W.R.; Loo, J.A.; et al. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc. Natl. Acad. Sci. USA 1998, 95, 12022–12027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyer, J.D.; Barbacci, E.G.; Iwata, K.K.; Arnold, L.; Boman, B.; Cunningham, A.; DiOrio, C.; Doty, J.; Morin, M.J.; Moyer, M.P.; et al. Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res. 1997, 57, 4838–4848. [Google Scholar] [PubMed]
- Park, J.H.; Liu, Y.; Lemmon, M.A.; Radhakrishnan, R. Erlotinib binds both inactive and active conformations of the EGFR tyrosine kinase domain. Biochem. J. 2012, 448, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponticello, G.S.; Freedman, M.B.; Habecker, C.N.; Holloway, M.K.; Amato, J.S.; Conn, R.S.; Baldwin, J.J. Utilization of α, β-unsaturated acids as Michael acceptors for the synthesis of thieno[2,3-b]thiopyrans. J. Org. Chem. 1988, 53, 9–13. [Google Scholar] [CrossRef]
- Wang, J.J.; Yin, B.Z.; Jiang, G.J.; Imafuku, K. Synthesis of isochroman-fused pyrazolone and pyrimidine derivatives. J. Heterocycl. Chem. 1990, 27, 1181–1184. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.R.; Yin, J.G.; Wang, J.J. Synthesis of isochromano[4,3-C]pyrazole substituted with biheterocycle at 3-position. J. Yantai Univ. Nat. Sci. Eng. Ed. 2002, 15, 113–117. [Google Scholar]
- Zhou, X.F.; Guo, J.L.; Ji, Y.M.; Pan, G.F.; Liu, T.; Zhu, H.; Zhao, J.P. Reciprocal negative regulation between EGFR and DEPTOR plays an important role in the progression of lung adenocarcinoma. Mol. Cancer Res. 2016, 14, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Kao, L.T. Cytotoxic enhancement of hexapeptide-conjugated micelles in EGFR high-expressed cancer cells. Expert Opin. Drug Deliv. 2014, 11, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Luan, T.; Kong, J.; Zhang, Y.; Wang, H.F. Synthesis and anti-tumor activities of 4-anilinoquinoline derivatives. Molecules 2016, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the all target compounds are available from the authors. |
| No. | Substituents | IC50 (μM) | |
|---|---|---|---|
| A549 | HepG2 | ||
| 6a | 4-methylpiperazin-1-yl | 23.44 ± 3.32 | >200 |
| 6b | 4-phenylpiperazin-1-yl | 21.38 ± 14.39 | >200 |
| 6c | 4-(4-methylphenyl)piperazin-1-yl | 11.03 ± 1.96 | >200 |
| 6d | 4-(4-methoxyphenyl)piperazin-1-yl | 13.13 ± 1.21 | >200 |
| 6e | 4-(4-fluorophenyl)piperazin-1-yl | 92.71 ± 23.90 | >200 |
| 6f | 4-(2-methylphenyl)piperazin-1-yl | 3.79 ± 13.39 | >200 |
| 6g | 4-(2-fluorophenyl)piperazin-1-yl | 9.68 ± 1.95 | >200 |
| 6h | 4-(2-chlorophenyl)piperazin-1-yl | 28.68 ± 11.71 | >200 |
| 6i | 4-(diphenylmethyl)piperazin-1-yl | 104.98 ± 44.66 | >200 |
| 6j | pyrrolidin-1-yl | 25.98 ± 8.00 | >200 |
| 6k | piperidin-1-yl | 27.94 ± 13.79 | >200 |
| 6l | morpholin-4-yl | 12.62 ± 14.76 | >200 |
| Gifitinib | 8.58 ± 1.65 | >200 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, J.; Lu, Q.; Wang, X.; Sun, R.; Jin, Z.; Zhan, X.; Hu, J.; Wan, D.C.-c.; Hu, C. Discovery of 4,5-Dihydro-1H-thieno[2′,3′:2,3]thiepino [4,5-c]pyrazole-3-carboxamide Derivatives as the Potential Epidermal Growth Factor Receptors for Tyrosine Kinase Inhibitors. Molecules 2018, 23, 1980. https://doi.org/10.3390/molecules23081980
Ke J, Lu Q, Wang X, Sun R, Jin Z, Zhan X, Hu J, Wan DC-c, Hu C. Discovery of 4,5-Dihydro-1H-thieno[2′,3′:2,3]thiepino [4,5-c]pyrazole-3-carboxamide Derivatives as the Potential Epidermal Growth Factor Receptors for Tyrosine Kinase Inhibitors. Molecules. 2018; 23(8):1980. https://doi.org/10.3390/molecules23081980
Chicago/Turabian StyleKe, Jia, Qi Lu, Xin Wang, Rui Sun, Zhe Jin, Xiaoyi Zhan, Jianshu Hu, David Chi-cheong Wan, and Chun Hu. 2018. "Discovery of 4,5-Dihydro-1H-thieno[2′,3′:2,3]thiepino [4,5-c]pyrazole-3-carboxamide Derivatives as the Potential Epidermal Growth Factor Receptors for Tyrosine Kinase Inhibitors" Molecules 23, no. 8: 1980. https://doi.org/10.3390/molecules23081980







