Recent Advances in the Microwave-Assisted Production of Hydroxymethylfurfural by Hydrolysis of Cellulose Derivatives—A Review
Abstract
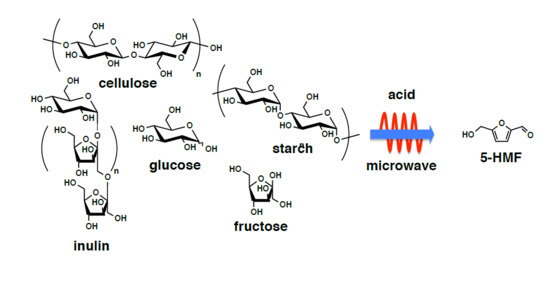
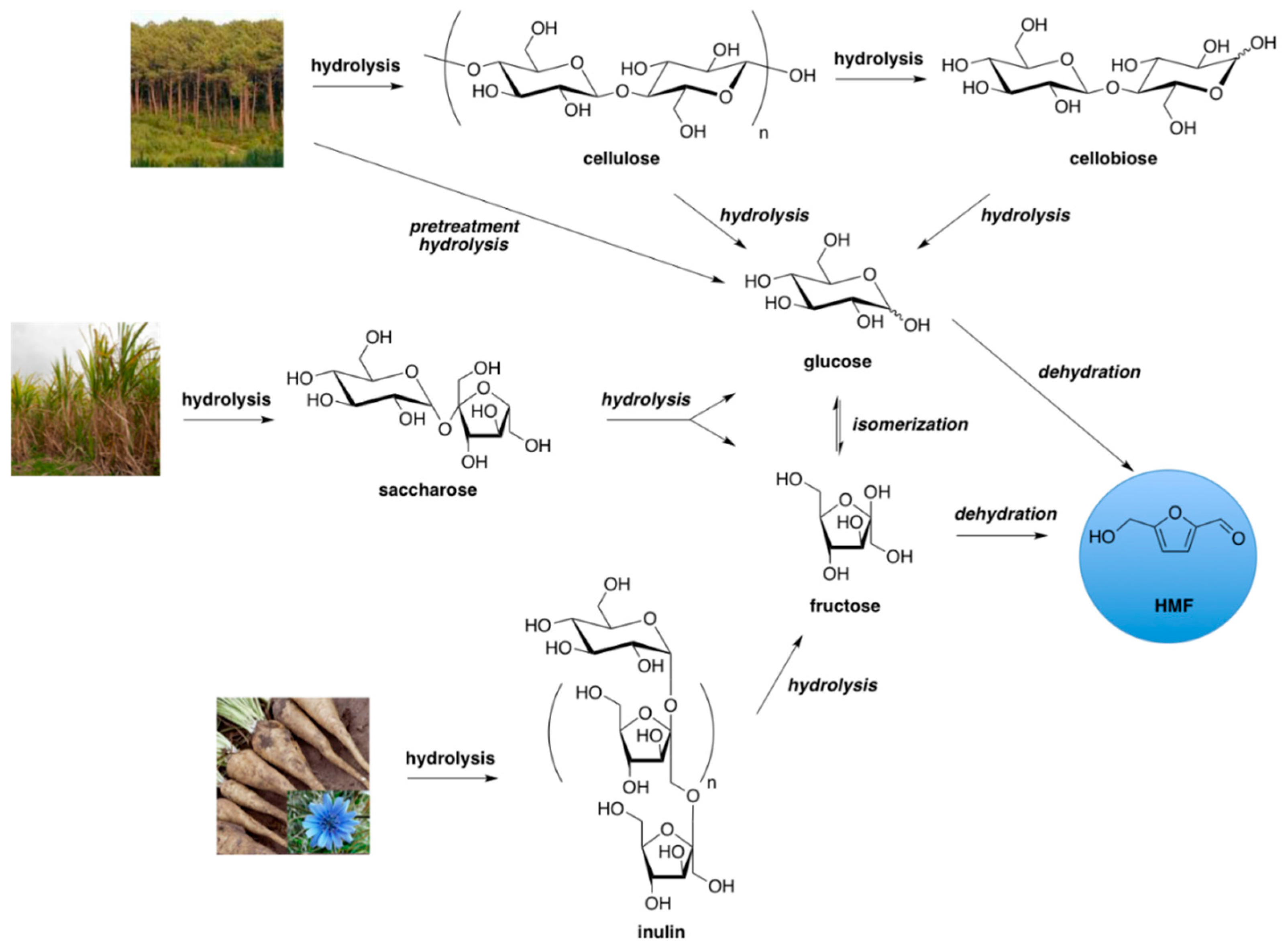
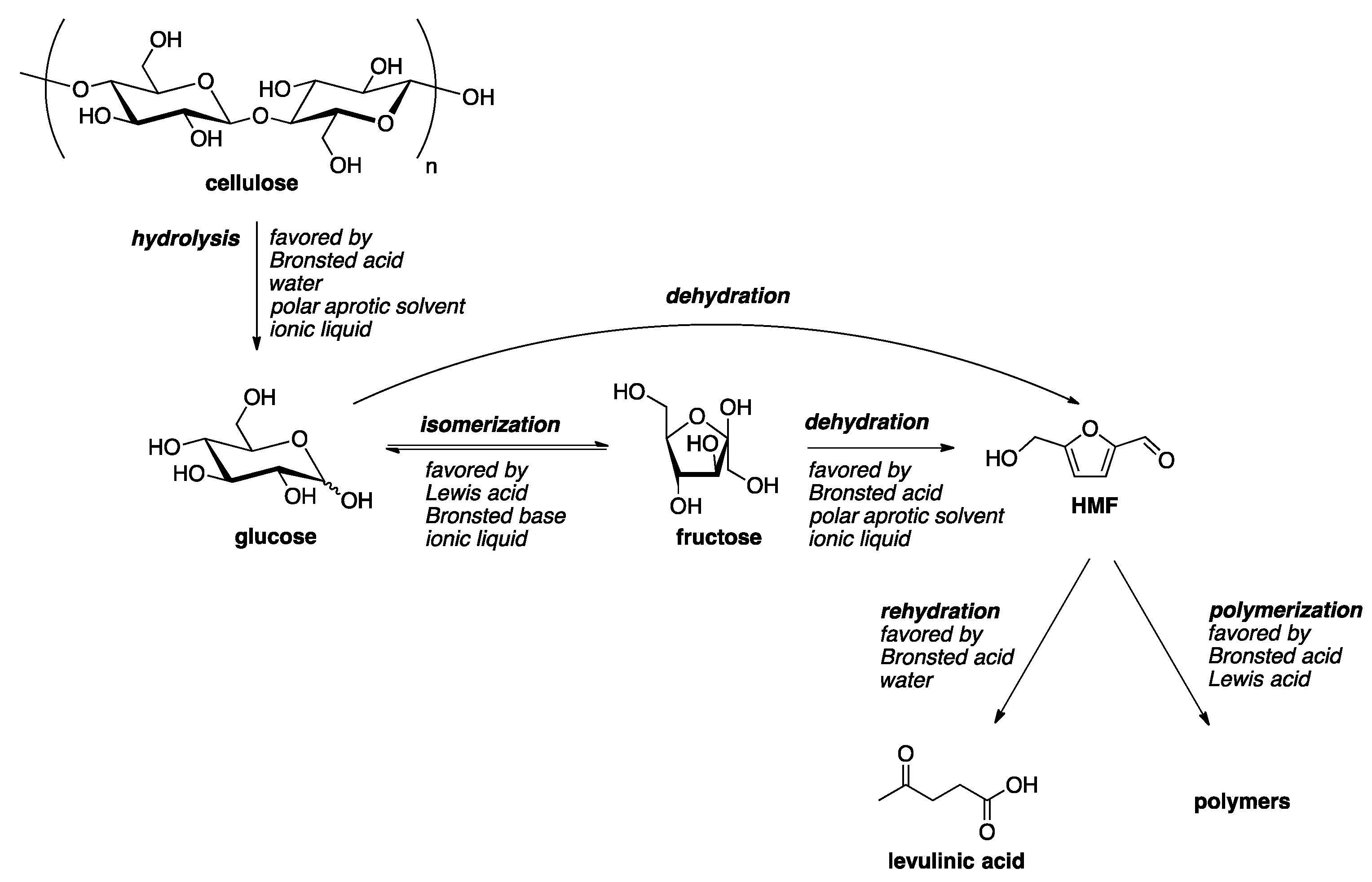
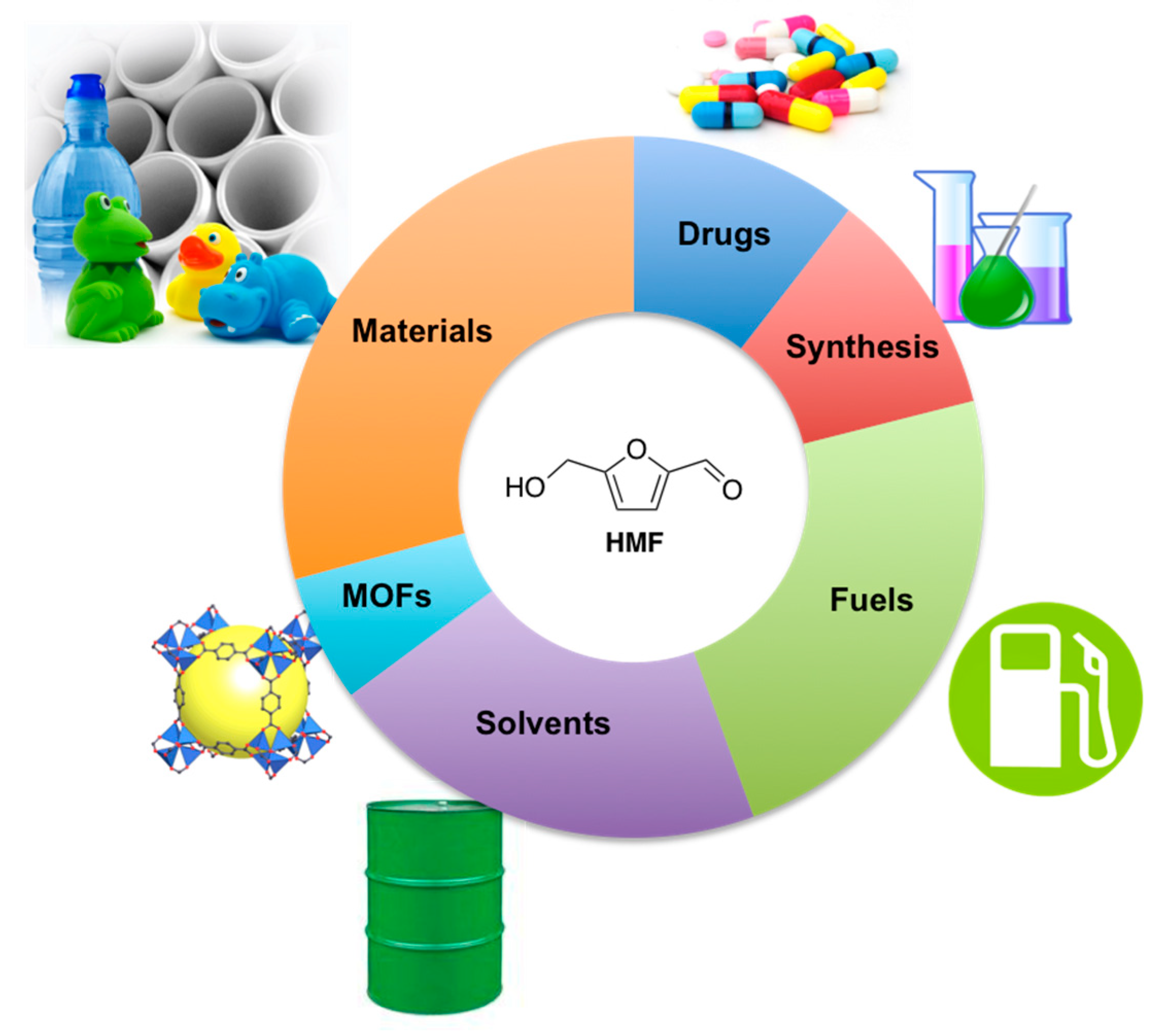
:1. Introduction
2. Homogeneous Catalyst in Liquid
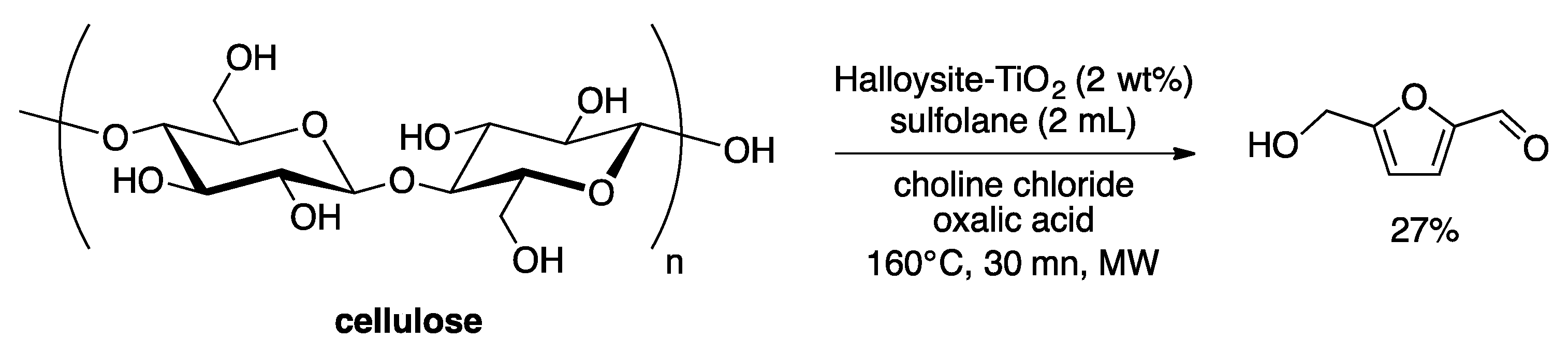
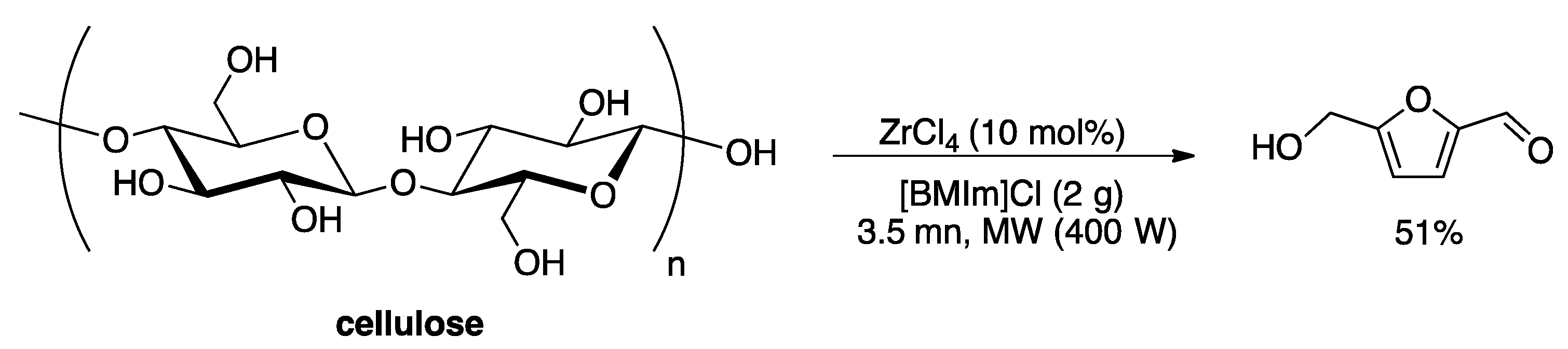
3. Heterogeneous Catalyst in Liquids
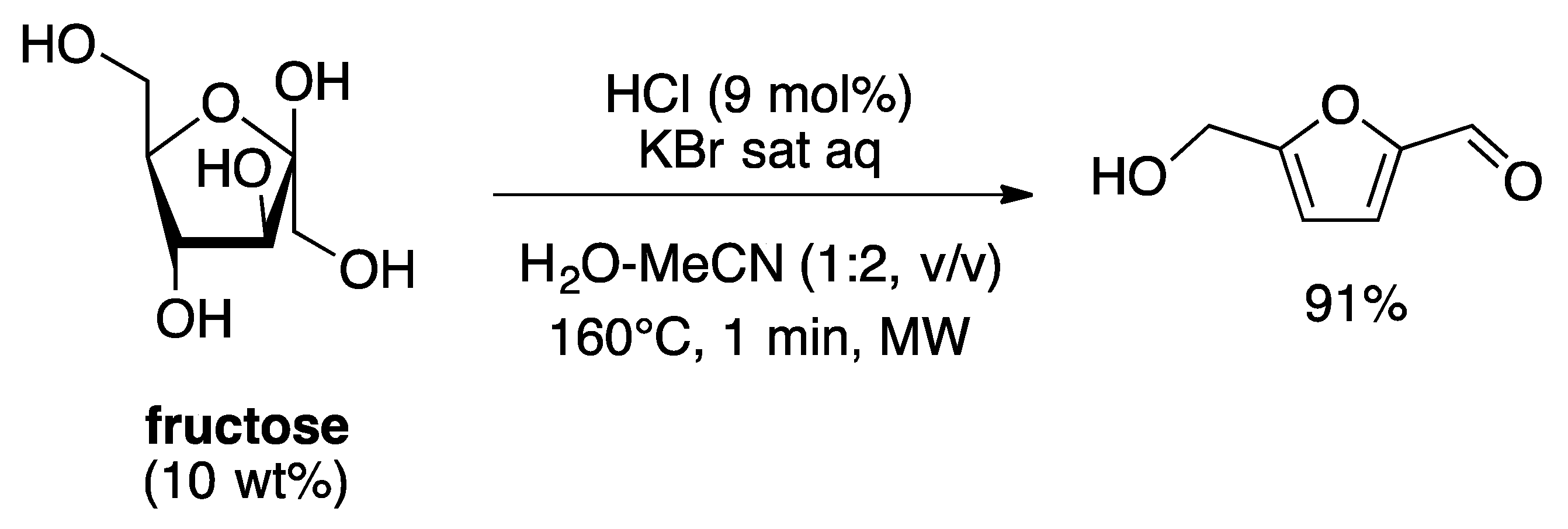
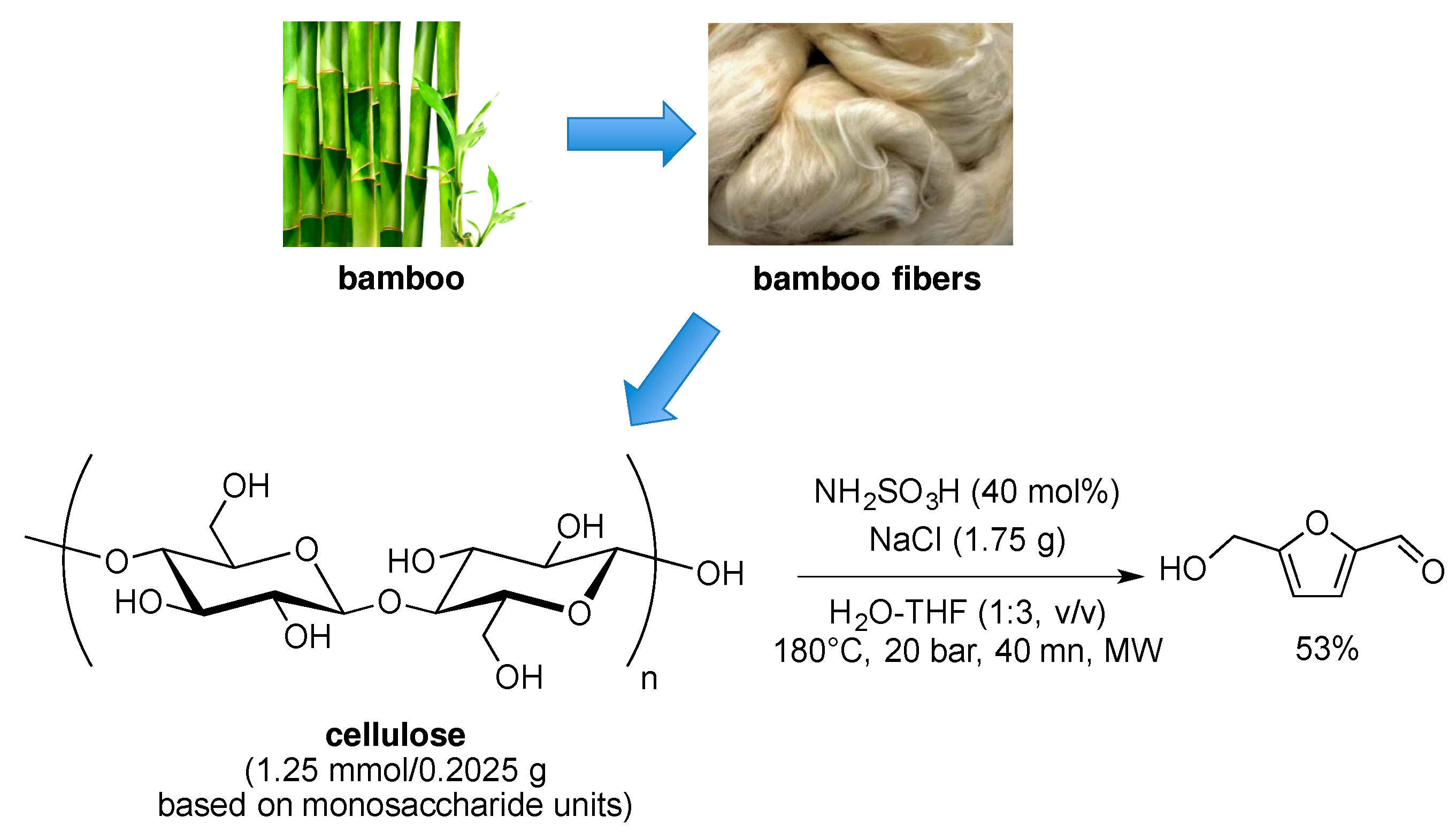
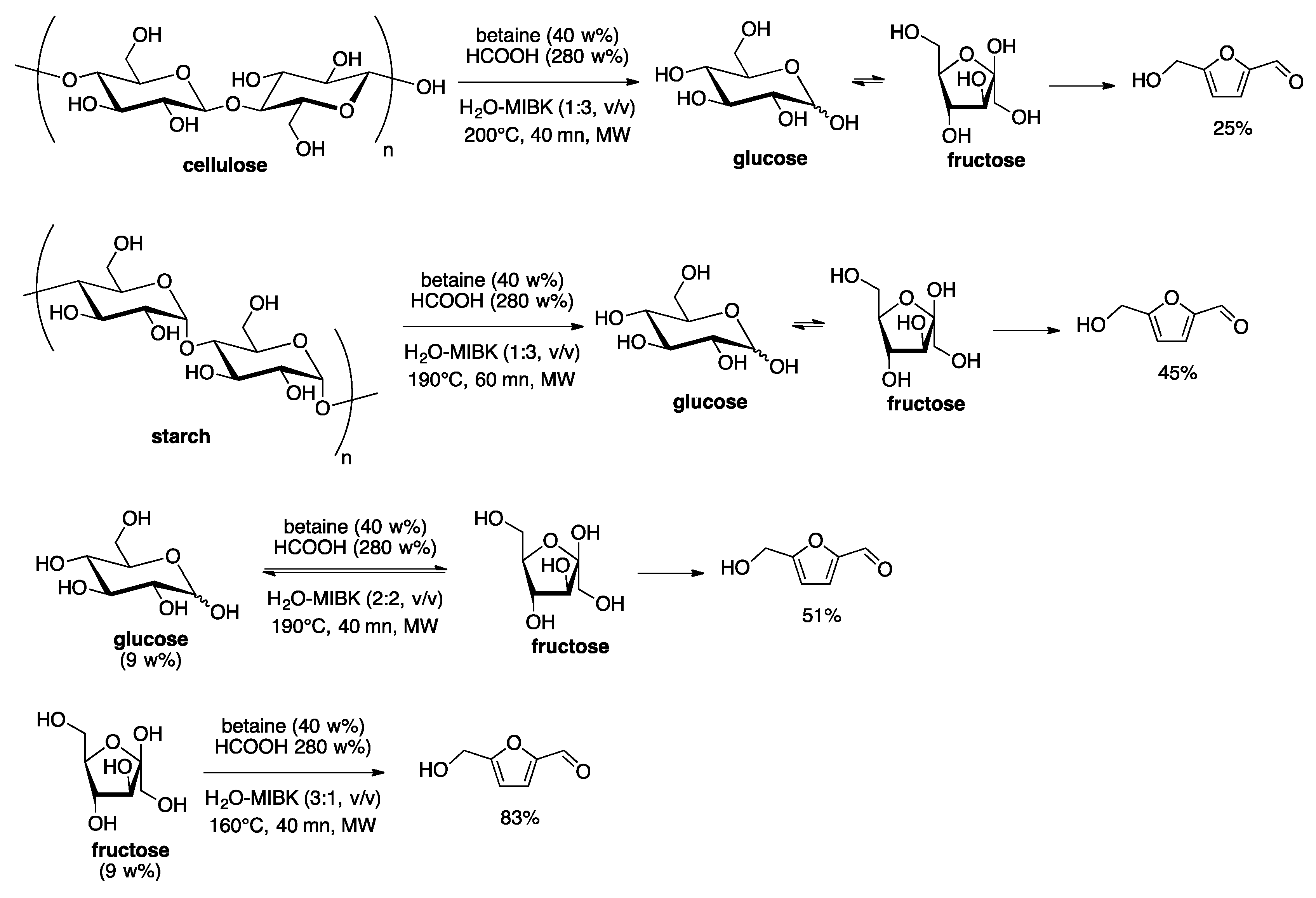
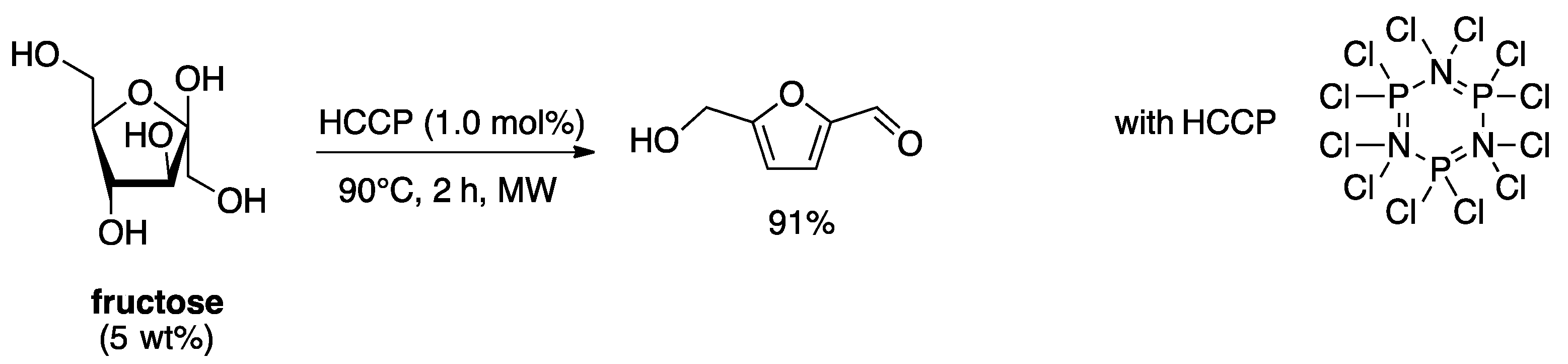
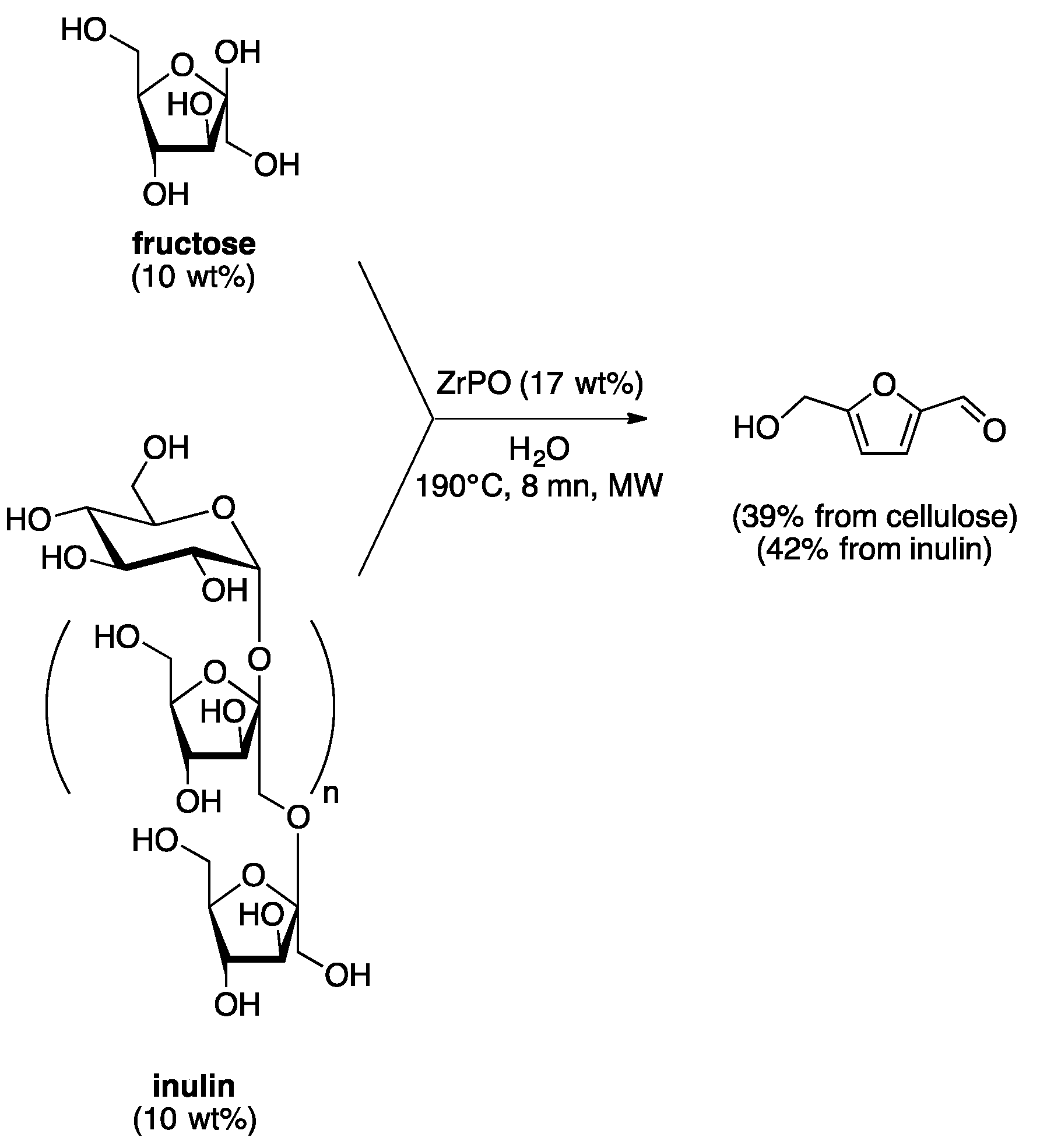
4. Free-Catalyst in Liquid
5. Conclusions
Authors Contributions
Funding
Conflicts of Interest
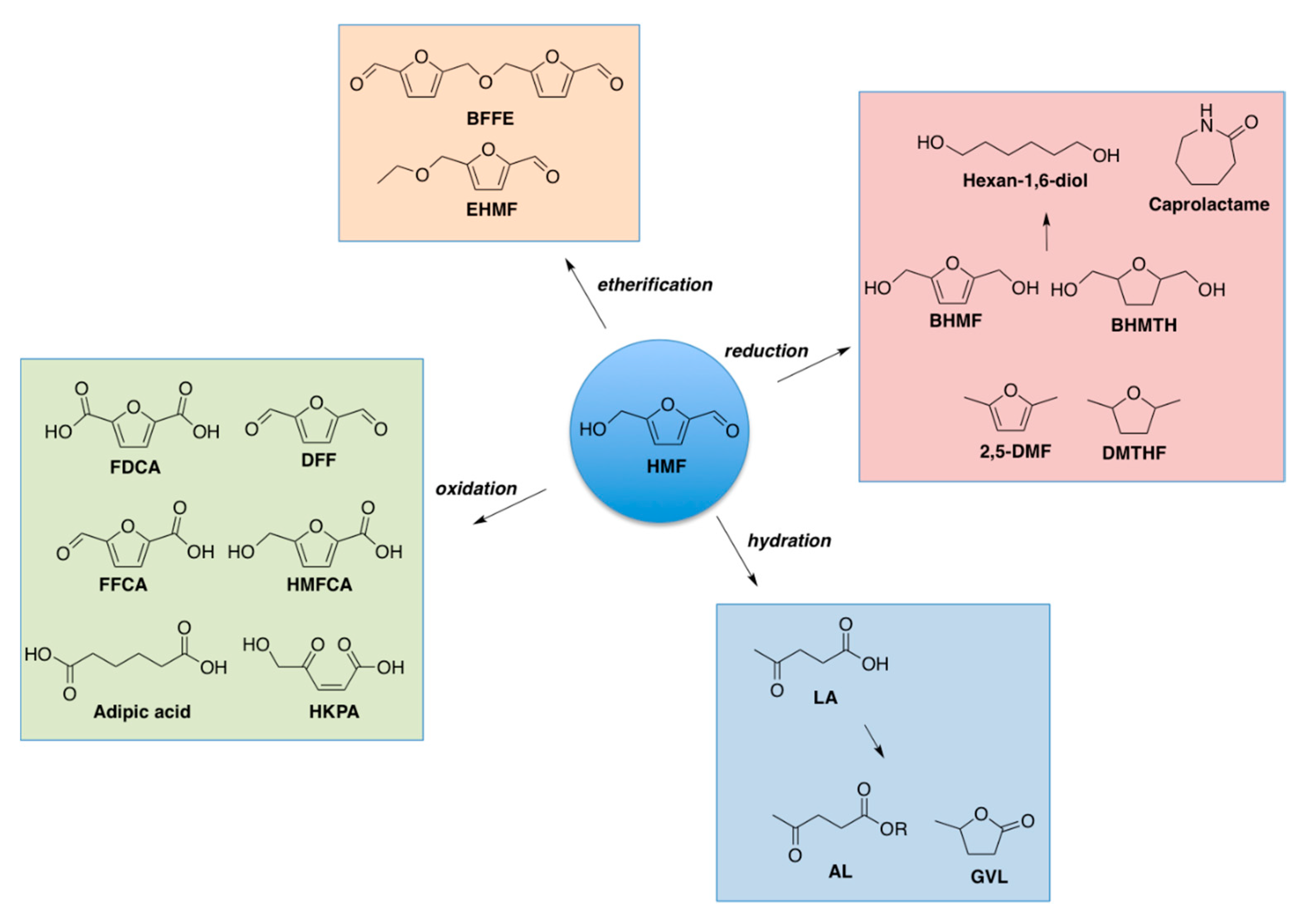
Abbreviations
| AL | alkyl levulinate |
| BFFE | bis (5-formyfurfuryl) ether |
| BHMF | 2,5-bis (hydroxymethyl) furan |
| BHMTHF | 2,5-bis (hydroxymethyl) tetrahydrofuran |
| [BMIm]Br | 1-butyl-3-methylimidazolium bromide |
| [BMIm]Cl | 1-butyl-3-methylimidazolium chloride |
| CNC | cyanuric chloride |
| CNT | carbon nanotube |
| DFF | 2,5-diformylfuran |
| DICA-T-1 | acronyme from the authors |
| DMAc | dimethylacetamide |
| 2,5-DMF | 2,5-dimethylfuran |
| DMSO | dimethyl sulfoxide |
| DMTHF | 2,5-dimethyltetrahydrofuran |
| EHMF | 5-ethoxymethylfurfural |
| [EMIm]Br | 1-ethyl-3-methylimidazolium bromide |
| FDCA | 2,5-furandicarboxylic acid |
| FFCA | 5-formylfuran-2-carboxylic acid |
| GVL | γ-valerolactone |
| HCCP | hexachlorocyclo triphosphazine |
| HKPA | 5-hydroxy-4-keto-pent-3-enoic acid |
| 5-HMF | 5-hydroxymethylfurfural |
| 5-HMFCA | 5-(hydroxymethyl)furan-2-carboxylic acid |
| IL | ionic liquid |
| LA | levulinic acid |
| MAOS | microwave-assisted organic synthesis |
| [MMIm]Cl | 1-methyl-3-methylimidazolium chloride |
| MIBK | methyl isobutyl ketone |
| MOF | metal organic framework |
| NbPO | niobium phosphate |
| PANI | polyaniline |
| PVA | polyvinyl alcohol |
| SiC | silicon carbide |
| SnPO | tin phosphate |
| TMG | tetramethylguanidine |
| ZrPO | zirconium phosphate |
References
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of hemicellulose and derivatives—A review of recent advances in the production of furfural. Front. Chem. 2018, 6, 146–174. [Google Scholar] [CrossRef] [PubMed]
- Kucherov, F.A.; Romashov, L.V.; Galkin, K.I.; Ananikov, V.P. Chemical transformations of biomass-derived C6-furanic platform chemicals for sustainable energy research, material science, and synthetic building blocks. ACS Sustain. Chem. Eng. 2018, 6, 8064–8092. [Google Scholar] [CrossRef]
- Agarwal, B.; Kailasam, K.; Sangwan, R.S.; Elumalai, S.; Kamalakannan, K. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sustain. Energy Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, A.; Baskar, C.; Kim, H. Biomass into chemicals: Green chemical conversion of carbohydrates into 5-hydroxymethylfurfural in ionic liquids. RSC Adv. 2016, 6, 63991–64002. [Google Scholar] [CrossRef]
- Zhou, P.; Zhnag, Z. One-pot catalytic conversion of carbohydrates into furfural and 5-hydroxymethylfurfural. Catal. Sci. Technol. 2016, 6, 3694–3712. [Google Scholar] [CrossRef]
- Zheng, X.; Gu, X.; Ren, Y.; Zhi, Z.; Lu, X. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulose in aqueous solution and different solvents. Biofuels Bioprod. Bioref. 2016, 10, 917–931. [Google Scholar] [CrossRef]
- Rout, P.K.; Nannaware, A.D.; Prakash, O.; Kalra, A.; Rajasekharan, R. Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem. Eng. Sci. 2016, 142, 318–346. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; De, S.; Saha, B. Advances in biomass transformation to 5-hydroxymethylfurfural and mechanistic aspects. Biomass Bioenergy 2013, 55, 355–369. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, L.; Liu, S. Efficient production of furan derivatives from a sugar mixture by catalytic process. Energy Fuels 2012, 26, 4560–4567. [Google Scholar] [CrossRef]
- Lange, J.P.; Van Der Heide, E.; Van Buijtenen, J.; Price, R. Furfural—A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydromethylfurfural (5-HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Karinen, R.; Vilonen, K.; Niemela, M. Biorefining: Heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural. ChemSusChem 2011, 4, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Dee, S.J.; Bell, A.T. A study of the acid-catalyzed hydrolysis of cellulose dissoved in ionic liquids and the factors influencing the dehydration of glucose and the formation of humins. ChemSusChem 2011, 4, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.K.R.; Heltzel, J.; Lund, C.R.F. Comparison of structural features of humins formed catalytically from glucose, fructose, and 5-hydroxymethylfurfuraldehyde. Energy Fuels 2012, 26, 5281–5293. [Google Scholar] [CrossRef]
- Cheng, Z.; Everhart, J.L.; Tsilomelekis, G.; Nikolakis, V.; Saha, B.; Vlachos, D.G. Structural analysis of humins formed in the Bronsted acid catalyzed dehydration of fructose. Green Chem. 2018, 20, 997–1006. [Google Scholar] [CrossRef]
- Yang, L.; Tsilomelekis, G.; Caratzoulas, S.; Vlachos, D.G. Mechanism of Bronsted acid-catalyzed glucose dehydration. ChemSusChem 2015, 8, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Gallagher-Duval, S.; Herve, G.; Sartori, G.; Enderlin, G.; Len, C. Improved microwave-assisted ligand-free Suzuki-Miyaura cross-coupling of 5-iodo-2’-deoxyuridine in pure water. New J. Chem. 2013, 37, 1989–1995. [Google Scholar] [CrossRef]
- Saggadi, H.; Luart, D.; Thiebault, N.; Polaert, I.; Estel, L.; Len, C. Toward the synthesis of 6-hydroxyquinoline starting from glycerol via improved microwave-assisted modified Skraup reaction. Catal. Commun. 2014, 44, 15–18. [Google Scholar] [CrossRef]
- Saggadi, H.; Luart, D.; Thiebault, N.; Polaert, I.; Estel, L.; Len, C. Quinoline and phenanthroline preparation starting from glycerol via improved microwave-assisted modified Skraup reaction. RSC Adv. 2014, 4, 21456–21464. [Google Scholar] [CrossRef]
- Herve, G.; Len, C. First ligand-free microwaves-assisted Heck cross-coupling reaction in sole water on nucleoside—Application to the synthesis of antiviral BVDU. RSC Adv. 2014, 4, 46926–46929. [Google Scholar] [CrossRef]
- Lussier, T.; Herve, G.; Enderlin, G.; Len, C. Original access to 5-aryluracils from 5-iodo-2’-deoxyuridine via a microwave assisted Suzuki-Miyaura cross-coupling/deglycosydation sequence in pure water. RSC Adv. 2014, 4, 46218–46223. [Google Scholar] [CrossRef]
- Hassine, A.; Bouhrara, M.; Sebti, S.; Solhy, A.; Luart, D.; Len, C.; Fihri, A. Natural phosphate-supported palladium: A highly efficient and recyclable catalyst for the Suzuki-Miyaura coupling under microwave irradiation. Curr. Org. Chem. 2014, 18, 3141–3148. [Google Scholar] [CrossRef]
- Saggadi, H.; Polaert, I.; Luart, D.; Len, C.; Estel, L. Microwaves under pressure for the continuous production of quinoline from glycerol. Catal. Today 2015, 255, 66–74. [Google Scholar] [CrossRef]
- Le Guenic, S.; Ceballos, C.; Len, C. Synthesis of phenylacetaldehyde from 1-phenylethan-1,2-diol by microwave-assisted dehydration in water. Catal. Lett. 2015, 145, 1851–1855. [Google Scholar] [CrossRef]
- Le Guenic, S.; Delbecq, F.; Ceballos, C.; Len, C. Microwave-aided dehydration of D-xylose into furfural by diluted inorganic salts solution in a biphasic system. J. Mol. Catal. A Chem. 2015, 410, 1–7. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.K.; Bahadur, V.; Len, C.; Richards, N.G.J.; Parmar, V.S.; Van Der Eycken, E.; Singh, B.K. Microwave assisted metal free, base mediated C-N bond formation/cleavage: Synthesis of benzimidazo[1,2-a]quinazoline derivatives. ACS Sustain. Chem. Eng. 2016, 4, 2206–2210. [Google Scholar] [CrossRef]
- Le Guenic, S.; Gergela, D.; Ceballos, C.; Delbecq, F.; Len, C. Furfural production from D-xylose and xylan by using stable Nafion NR50 and NaCl in a microwave-assisted biphasic reaction. Molecules 2016, 21, 1102. [Google Scholar] [CrossRef] [PubMed]
- Polaert, I.; Estel, L.; Luart, D.; Len, C.; Delmotte, M. A new and original microwave continuous reactor under high pressure for future chemistry. AIChE J. 2017, 63, 192–199. [Google Scholar] [CrossRef]
- Nguyen, R.; Galy, N.; Singh, A.; Paulus, F.; Stoebener, D.; Schlesser, C.; Sharma, S.K.; Haag, R.; Len, C. A simple and efficient process for large scale glycerol oligomerization by microwave irradiation. Catalysts 2017, 7, 123. [Google Scholar] [CrossRef]
- Wang, Y.; Delbecq, F.; Kwapinski, W.; Len, C. Application of sulfonated carbon-based catalyst for the furfural production from D-xylose and xylan in a microwave-assisted biphasic reaction. Mol. Catal. 2017, 438, 167–172. [Google Scholar] [CrossRef]
- Wang, Y.; Delbecq, F.; Varma, R.S.; Len, C. Comprehensive study on expeditious conversion of pre-hydrolyzed alginic acid to furfural in Cu(II) biphasic systems using microwaves. Mol. Catal. 2018, 445, 73–79. [Google Scholar] [CrossRef]
- Danoun, K.; Essamlali, Y.; Amadine, O.; Tabit, R.; Fihri, A.; Len, C.; Zahouily, M. Nanostructured pyrophosphate Na2PdP2O7: A highly efficient and recyclable catalyst for the Suzuki-Miyaura coupling under microwave irradiation. Appl. Organometal. Chem. 2018, 32, e4232. [Google Scholar] [CrossRef]
- Balint, E.; Keglevich, G. The spread of the application of the microwave technique in organic synthesis. In Milestones in Microwave Chemistry; Keglevich, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–10. ISBN 978-3-319-30630-8. [Google Scholar]
- Kiss, N.Z.; Balint, E.; Keglevich, G. Microwave-assisted syntheses in organic chemistry. In Milestones in Microwave Chemistry; Keglevich, E., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 11–45. ISBN 978-3-319-30630-8. [Google Scholar]
- Delbecq, F.; Wang, Y.; Len, C. Various carbohydrates precursors dehydration to 5-HMF in an acidic biphasic system under microwave heating using betaine as a co-catalyst. Mol. Catal. 2017, 434, 80–85. [Google Scholar] [CrossRef]
- Wrigstedt, P.; Keskivali, J.; Repo, T. Microwave-enhanced aqueous biphasic dehydration of carbohydrates to 5-hydroxymethylfurfural. RSC Adv. 2016, 6, 18973–18979. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.S.; Woodley, J.M.; Riisager, A. Efficient microwave-assisted synthesis of 5-hydroxymethylfurfural from concentrated aqueous fructose. Carbohydr. Res. 2009, 344, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- Delbecq, F.; Wang, Y.; Len, C. Conversion of xylose, xylan and rice husk into furfural via betaine and formic acid mixture as novel homogeneous catalyst in biphasic system by microwave-assisted dehydration. J. Mol. Catal. A Chem. 2016, 423, 520–525. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, X.; Shen, Y.; Yi, Y.; Wang, B.; Xu, F.; Sun, R. Conversion of Bamboo fiber into 5-hydroxymethylfurfural catalyzed by sulfamic acid with microwave assistance in biphasic system. Ind. Crops Prod. 2015, 70, 266–271. [Google Scholar] [CrossRef]
- Qu, Y.; Wei, Q.; Li, H.; Oleskowicz-Popiel, P.; Huang, C.; Xu, J. Microwave-assisted conversion of microcrystalline cellulose to 5-hydroxymethylfurfural catalyzed by ionic liquids. Bioresour. Technol. 2014, 162, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.O.; Fayet, G.; Len, C.; Marlair, G. Evaluation of heats of combustion of ionic liquids through use of existing and purpose built models. Ind. Eng. Chem. Res. 2012, 51, 3149–3156. [Google Scholar] [CrossRef]
- Diallo, A.O.; Len, C.; Morgan, A.B.; Marlair, G. Revisiting phys.-chem. Related safety issues of ionic liquids. Sep. Purif. Technol. 2012, 97, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Diallo, A.O.; Morgan, A.B.; Len, C.; Marlair, G. An innovative experimental approach aiming to understand and quantifiy the actual fire hazards of ionic liquids. Energy Environ. Sci. 2013, 6, 699–710. [Google Scholar] [CrossRef] [Green Version]
- Chancelier, L.; Diallo, A.O.; Santini, C.C.; Marlair, G.; Gutel, T.; Mailley, S.; Len, C. Targeting adequate thermal stability and fire safety in selecting ionic liquid-based electrolytes for energy storage. Phys. Chem. Chem. Phys. 2014, 16, 1967–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bado-Nilles, A.; Diallo, A.O.; Marlair, G.; Pandard, P.; Chabot, L.; Geffard, A.; Len, C.; Porcher, J.M.; Sanchez, W. Coupling of OECD standardized test and immunomarkers to select the most environmentally benign ionic liquid option—Towards an innovative “safety by design” approach. J. Hazard. Mater. 2015, 283, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yan, Y.; Zhang, Y.; Tang, Y. Microwave-assisted highly efficient transformation of ketose/aldose to 5-hydroxymethylfurfural (5-HMF) in a simple phosphate buffer system. RSC Adv. 2012, 2, 7652–7655. [Google Scholar] [CrossRef]
- Sweygers, N.; Alewaters, N.; Dewil, R.; Appels, L. Microwave effects in the dilute acid hydrolysis of cellulose to 5-Hydroxymethylfurfural. Sci. Rep. 2018, 8, 7719–7729. [Google Scholar] [CrossRef] [PubMed]
- Finn, M.; Ridenour, J.A.; Heltzel, J.; Cahill, C.; Voutchkova-Kostal, A. Next-generation water-soluble homogeneous catalysts for conversion of glycerol to lactic acid. Organometallics 2018, 37, 1400–1409. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, Y.; Chao, Y.; Shen, W.; Wang, C.; Xu, M. Triazaheterocyclic compound as an efficient catalyst for dehydration of fructose into 5-hydroxymethylfurfural. RSC Adv. 2014, 4, 13434–13437. [Google Scholar] [CrossRef]
- Da silva Lacerda, V.; Lopez-Sotelo, J.B.; Correa-Guimeraes, A.; Hernandez-Navarro, S.; Sanchez-Bascones, M.; Naras-Gracia, L.M.; Martin-Ramos, P.; Perez-Lebena, E.; Martin-Gil, J. A kinetic study on microwave-assisted conversion of cellulose and lignocellulose waste into hydroxymethylfurfural/furfural. Bioresour. Technol. 2015, 180, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Z.; Liu, B.; Xu, Z.; Deng, Z. Microwave-assisted rapid conversion of carbohydrates into 5-hydroxymethylfurfural by ScCl3 in ionic liquids. Carbohydr. Res. 2013, 375, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, Z.; Zhao, Z.K. Microwave-assisted catalytic conversion of cellulose into 5-hydroxymethylfurfural in ionic liquids. Chem. Eng. J. 2013, 215–216, 517–521. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Ok, Y.S.; Poon, C.S. Valorization of starchy, cellulosic, and sugary food waste into hydroxymethylfurfural by one-pot catalysis. Chemosphere 2017, 184, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.R.; Dumont, M.-J.; Raghavan, G.S.V. Microwave assisted synthesis of 5-hydroxymethylfurfural from starch in AlCl3, 6 H2O/DMSO/[BMIM]Cl system. Ind. Eng. Chem. Res. 2016, 55, 4473–4481. [Google Scholar] [CrossRef]
- De, S.; Datta, S.; Saha, B. Microwave assisted conversion of carbohydrates and biopolymers to 5-hydroxymethylfurfural with aluminium chloride as catalyst in water. Green Chem. 2011, 13, 2859–2868. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.K. Microwave-assisted conversion of lignocellulosic biomass into furans in ionic liquids. Bioresour. Technol. 2010, 101, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Z.; Zhao, Z.K. Direct conversion of glucose and cellulose to 5-hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 2009, 50, 5403–5405. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Aria, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L., Jr. Catalytical conversion of fructose and glucose into 5-hydroxymethylfurfural in hot compressed water by microwave heating. Catal. Commun. 2008, 86, 2244–2249. [Google Scholar] [CrossRef]
- Antonetti, C.; Melloni, M.; Licursi, D.; Fulignati, S.; Ribechini, E.; Rivas, S.; Parajo, J.C.; Cavani, F.; Raspoli Galleti, A.M. Microwave-assisted dehydration of fructose and inulin to 5-HMF catalyzed by niobium and zirconium phosphate catalysts. Appl. Catal. B 2017, 206, 364–377. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Liu, L.; Chen, Y.; Huang, F.; Zhang, S.; Li, W.; Ju, M. Tin phosphate as a heterogeneous catalyst for efficient dehydration of glucose into 5-hydroxymethylfurfural in ionic liquid. Appl. Catal. B 2018, 224, 183–193. [Google Scholar] [CrossRef]
- Wang, J.; Qu, T.; Liang, M.; Zhao, Z. Microwave-assisted rapid conversion of fructose into 5-HMF over solid acid catalysts. RSC Adv. 2015, 5, 106053–106060. [Google Scholar] [CrossRef]
- Antonetti, C.; Raspolli Galletti, A.M.; Fulignati, S.; Licursi, D. Amberlyst A-70: A surprisingly active catalyst for the MW-assisted dehydration of fructose and inulin to 5-HMF in water. Catal. Commun. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Pawar, A.H.; Lali, S.M. Polyvinyl Alcohol Functionalized Solid Acid Catalyst DICAT-1 for Microwave-Assisted Synthesis of 5-hydroxymethylfurfural in Green Solvent. Energy Technol. 2016, 4, 823–834. [Google Scholar] [CrossRef]
- Ji, T.; Tu, R.; Mu, L.; Lu, X.; Zhu, J. Enhancing Energy Efficiency in Saccharide-5-HMF Conversion with Core/Shell Structured Microwave Responsive Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 4352–4358. [Google Scholar] [CrossRef]
- Ji, T.; Tu, R.; Mu, L.; Lu, X.; Zhu, J. Structurally tuning microwave absorption of core/shell structured CNT/polyaniline catalysts for energy efficient saccharide-5-HMF conversion. Appl. Catal. B 2018, 220, 581–588. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.K.; Cai, H.; Wang, A.; Zhang, T. Microwave-promoted conversion of concentrated fructose into 5-hydroxymethylfurfural in ionic liquids in the absence of catalysts. Biomass Bioenergy 2011, 35, 2013–2017. [Google Scholar] [CrossRef] [Green Version]
- Despax, S.; Maurer, C.; Estrine, B.; Le Bras, J.; Hoffmann, N.; Marinkovic, S.; Muzart, J. Fast and efficient DMSO-mediated dehydration of carbohydrates into 5-hydroxymethylfurfural. Catal. Commun. 2014, 51, 5–9. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delbecq, F.; Len, C. Recent Advances in the Microwave-Assisted Production of Hydroxymethylfurfural by Hydrolysis of Cellulose Derivatives—A Review. Molecules 2018, 23, 1973. https://doi.org/10.3390/molecules23081973
Delbecq F, Len C. Recent Advances in the Microwave-Assisted Production of Hydroxymethylfurfural by Hydrolysis of Cellulose Derivatives—A Review. Molecules. 2018; 23(8):1973. https://doi.org/10.3390/molecules23081973
Chicago/Turabian StyleDelbecq, Frederic, and Christophe Len. 2018. "Recent Advances in the Microwave-Assisted Production of Hydroxymethylfurfural by Hydrolysis of Cellulose Derivatives—A Review" Molecules 23, no. 8: 1973. https://doi.org/10.3390/molecules23081973