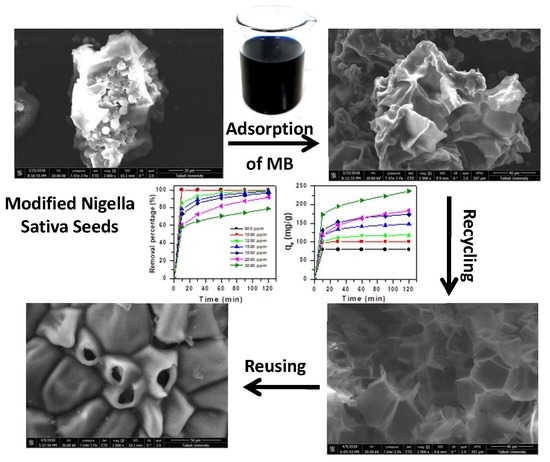
Modified Nigella Sativa Seeds as a Novel Efficient Natural Adsorbent for Removal of Methylene Blue Dye
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Nigella Sativa Treatment
2.3. Removal Studies
2.4. Regeneration Method
2.5. Characterization
3. Results and Discussion
3.1. Removal of MB Dye onto NS Adsorbent
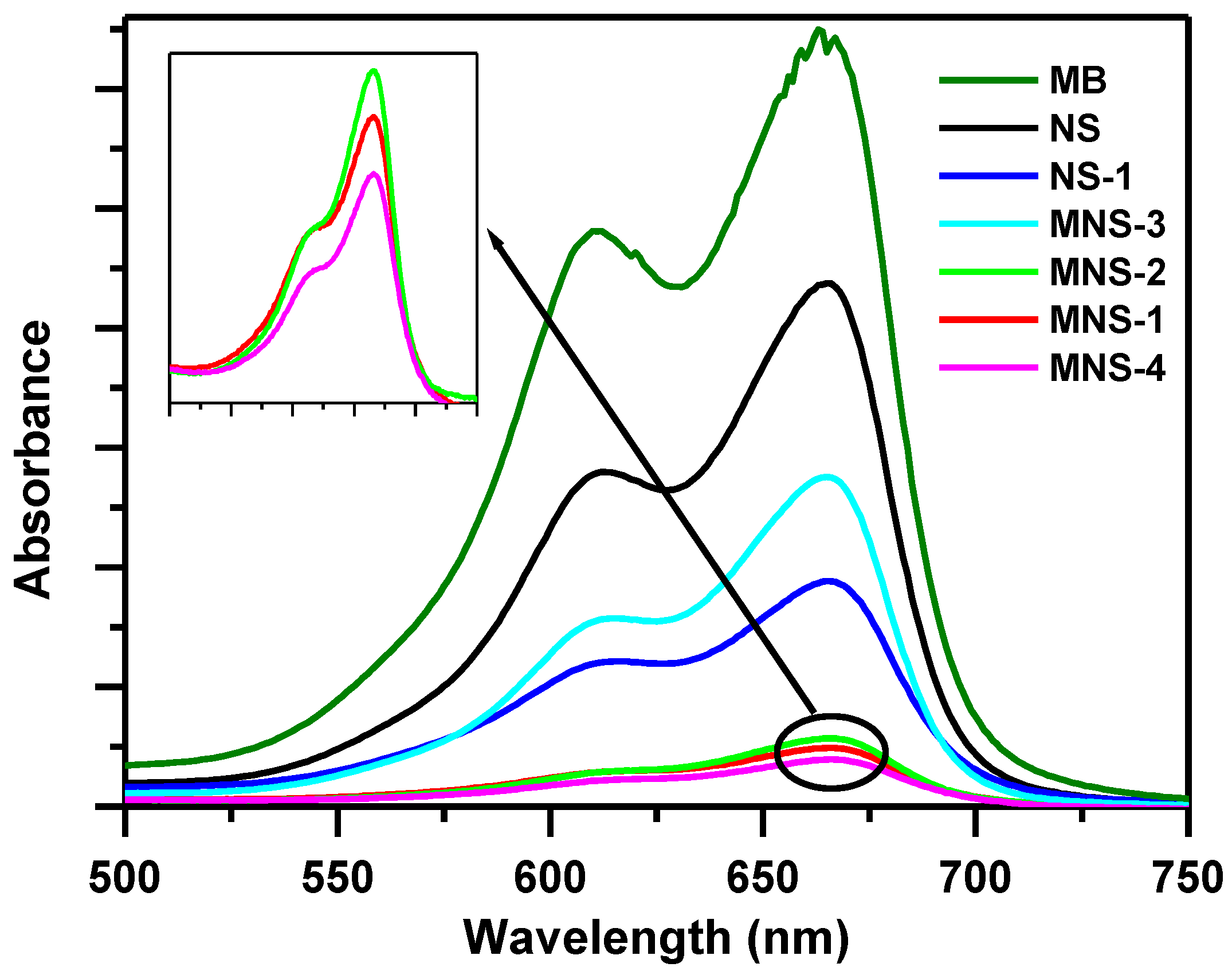
3.1.1. Effect of NS Treatment on Removal of MB dye
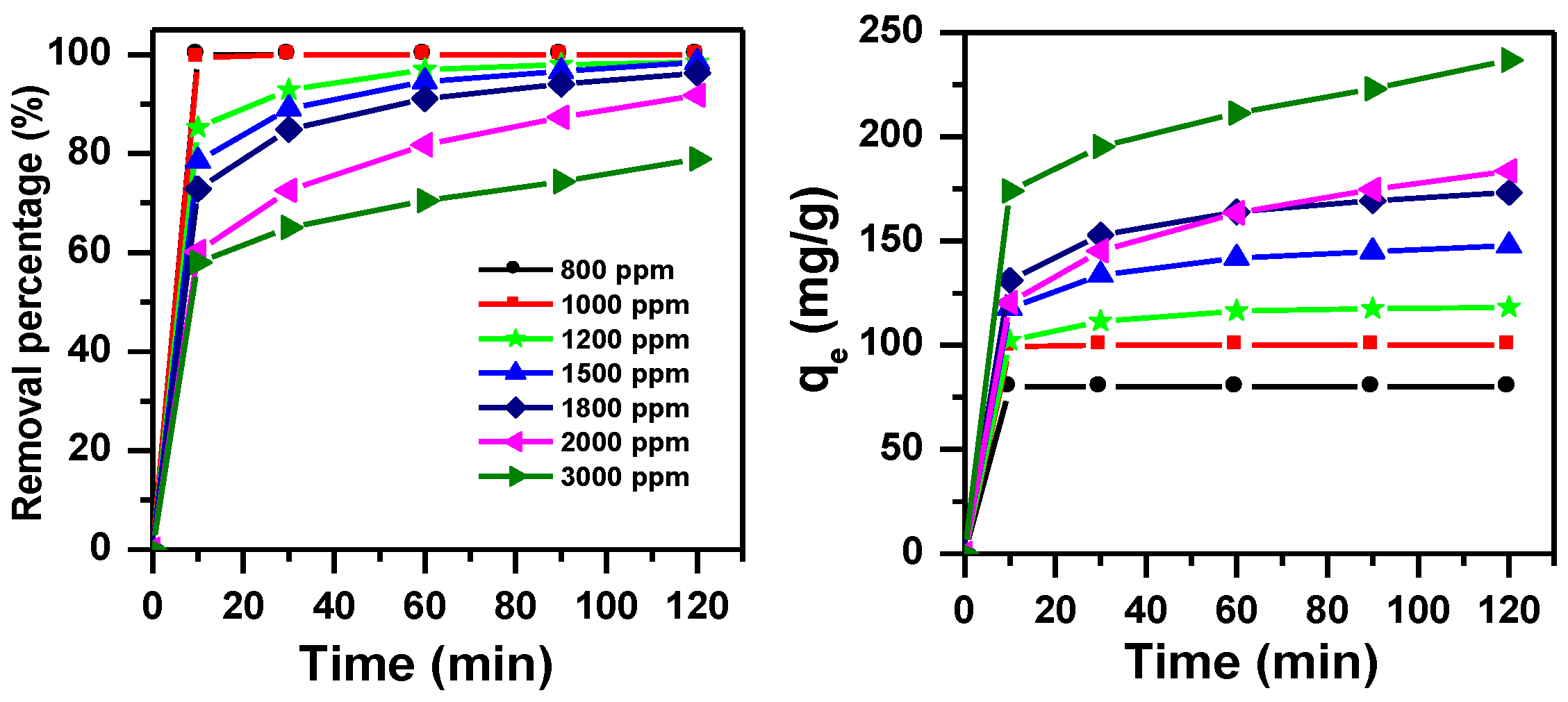
3.1.2. Effect of Initial Dye Concentration and Contact Time without pH Adjustment
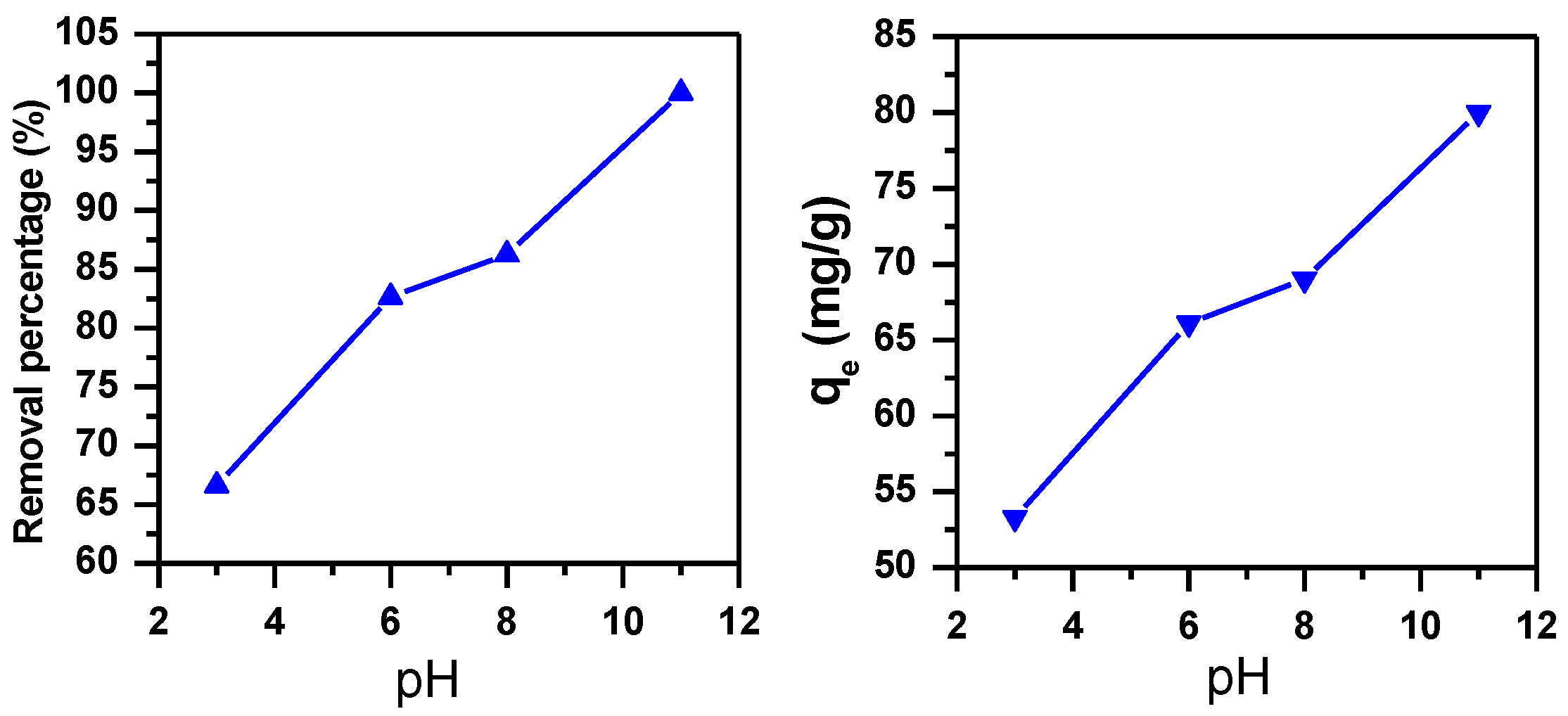
3.1.3. Effect of pH
3.1.4. Effect of Initial Dye Concentration and Contact Time with pH Adjustment
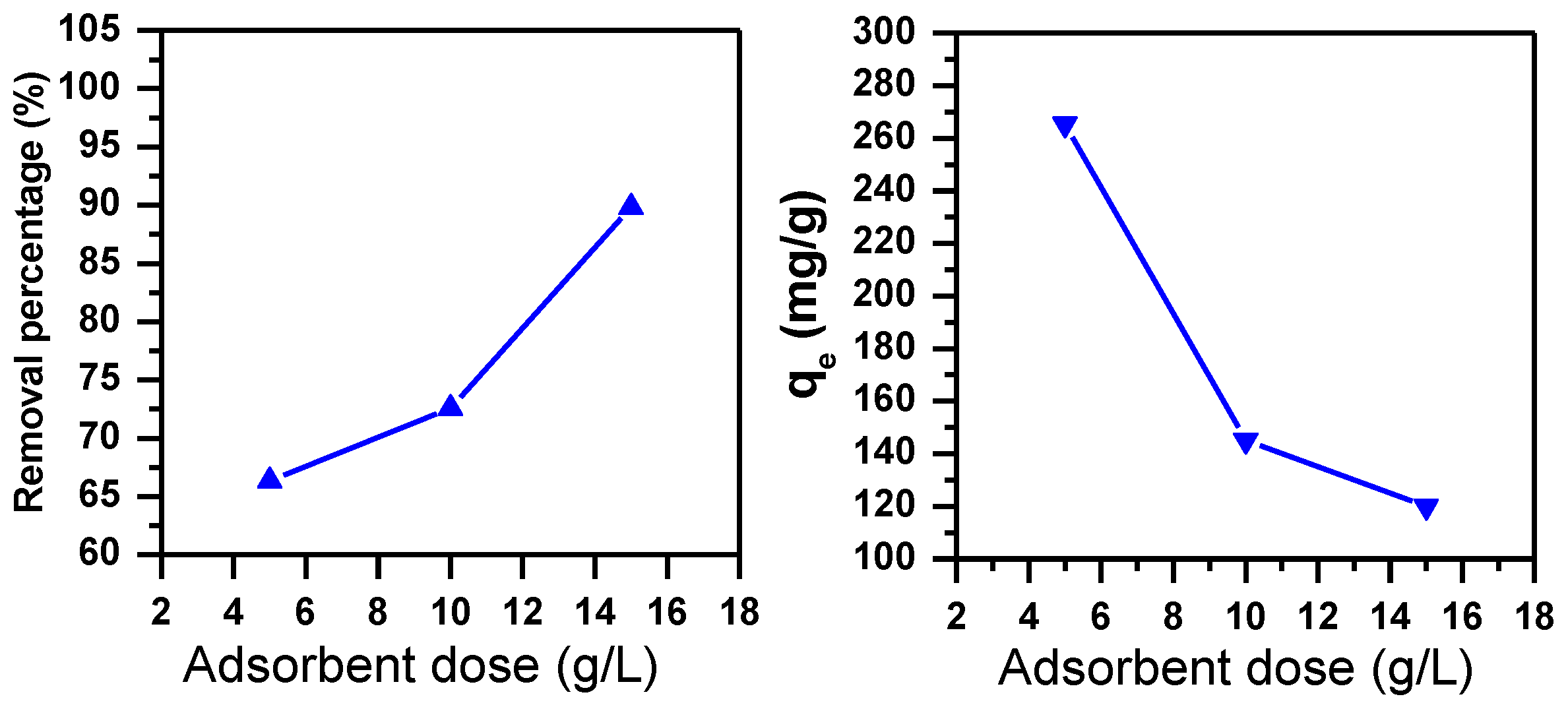
3.1.5. Effect of Adsorbent Dose
3.1.6. Effect of Temperature and Thermodynamic Parameters
3.2. Kinetics of Adsorption
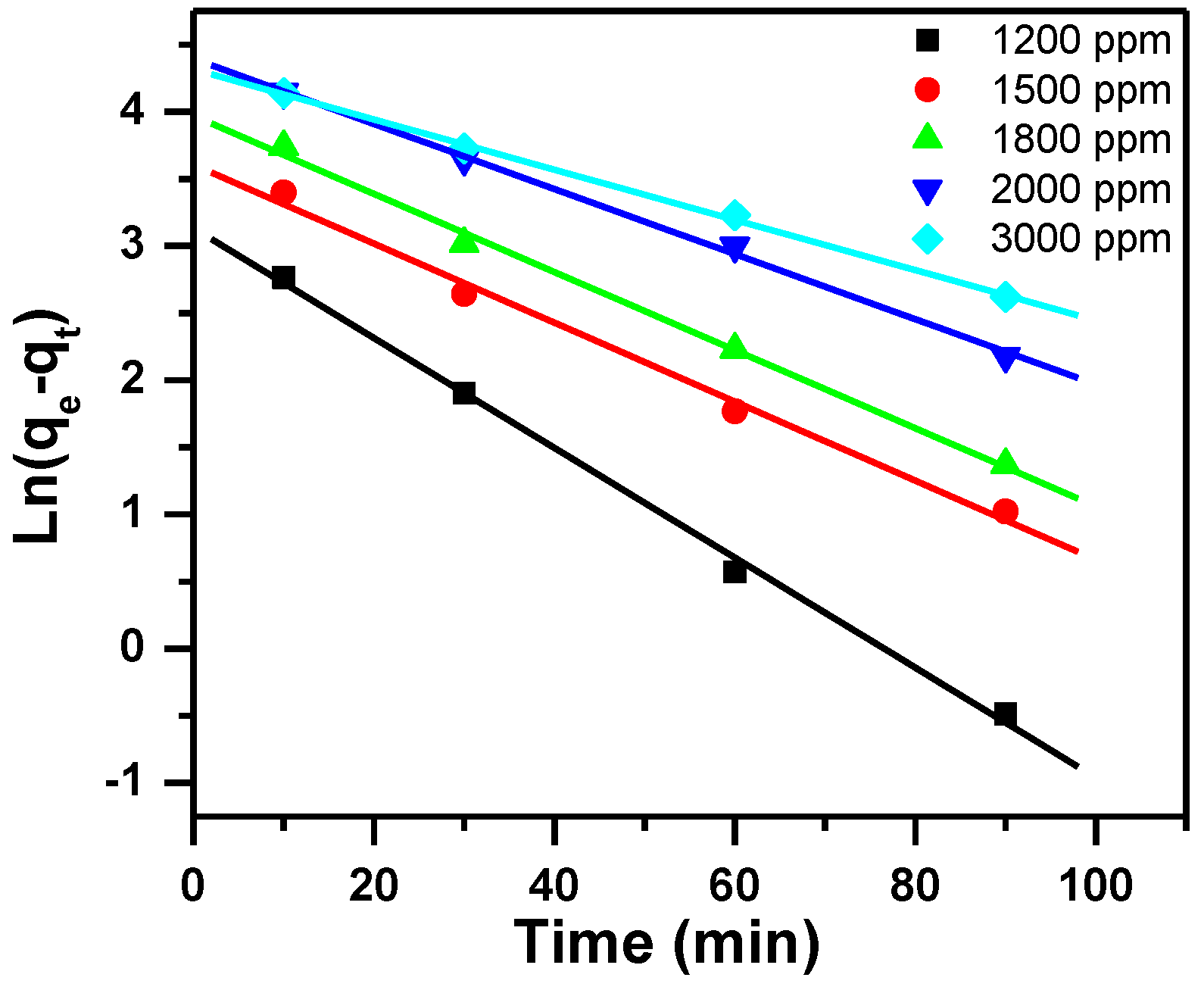
3.2.1. Pseudo-First-Order Kinetic Model
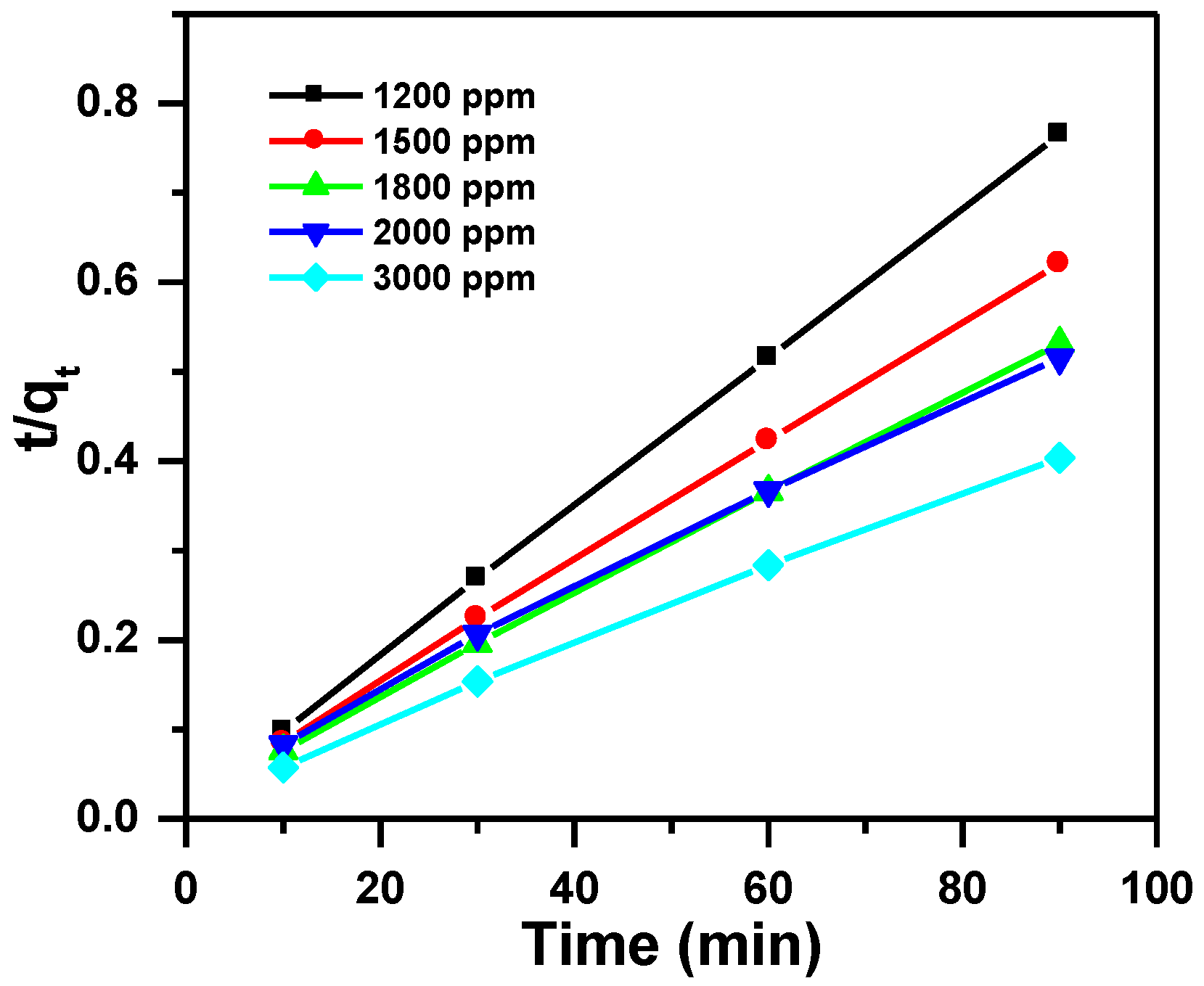
3.2.2. Pseudo-Second-Order Kinetic Model
3.2.3. Intraparticle Diffusion Process
3.3. Adsorption Isotherm Models
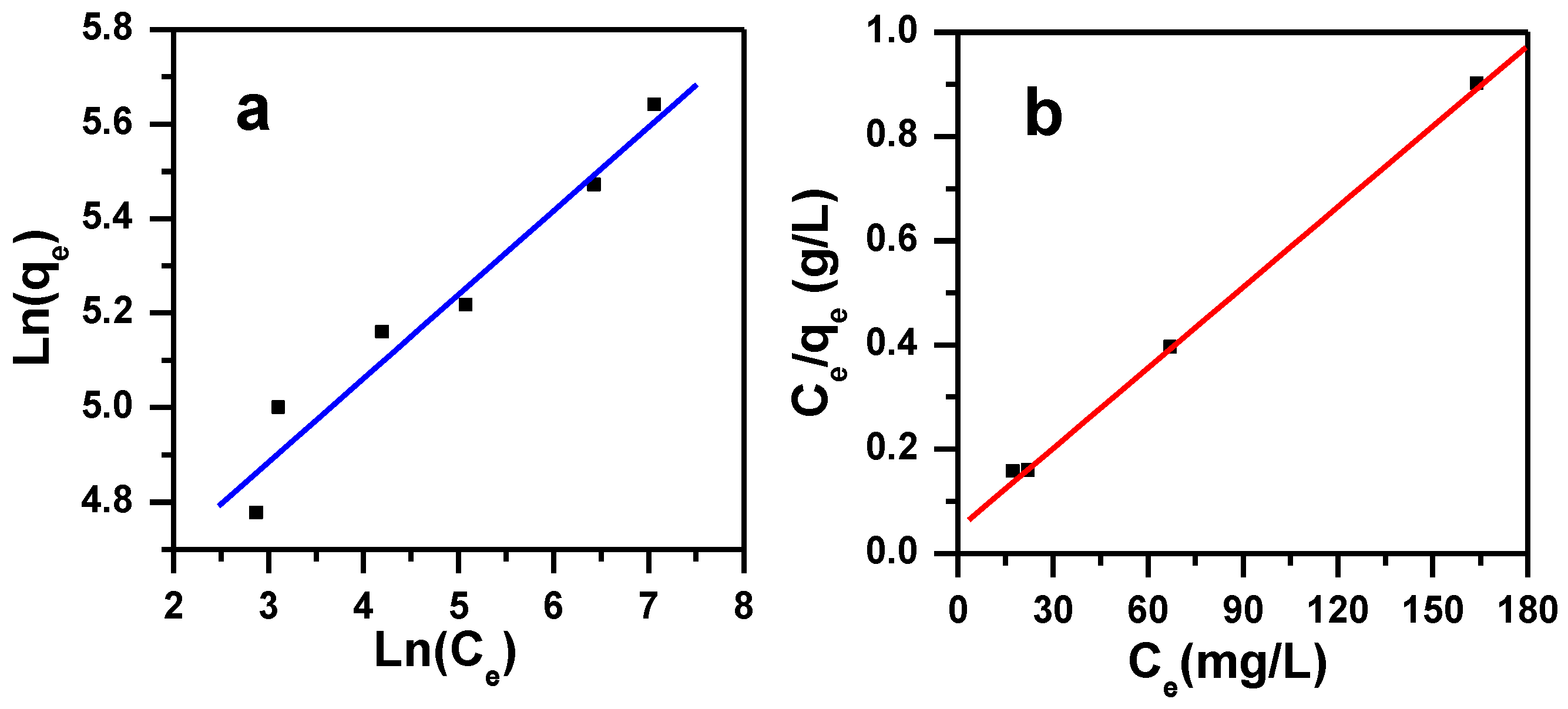
3.3.1. Langmuir Isotherm
3.3.2. Freundlich Isotherm
3.3.3. The Dubinin-Radushkevich (D-R) Isotherm
3.3.4. Temkin Model
3.4. Characterization and Recycling of the MNS-4 Adsorbent
3.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.2. Regeneration Efficiency
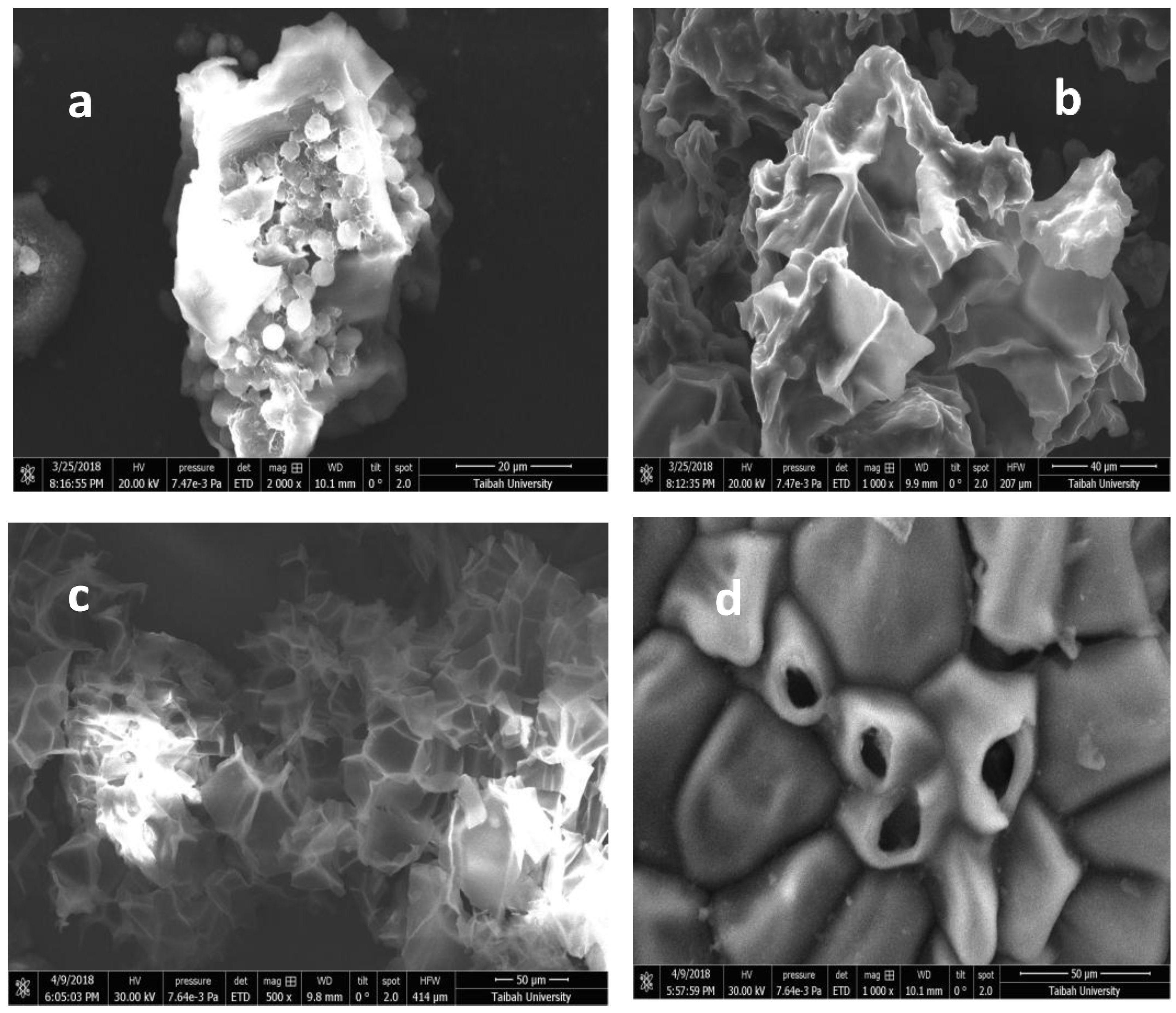
3.4.3. Scanning Electron Microscope (SEM)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ulson de Souza, S.M.A.G.; Forgiarini, E.; Ulson de Souza, A.A. Toxicity of textile dyes and their degradation by the enzyme horseradish peroxidase (HRP). J. Hazard. Mater. 2007, 147, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Sureyya, M.; Deniz, K.; Tugba, O. Color and COD removal from wastewater containing Reactive Black 5 using Fenton’s oxidation process. Chemosphere 2004, 54, 435–441. [Google Scholar]
- Ramabrahmam, V. Environmental effects of India due to water pollution. Int. J. Eng. Res.-Online 2016, 4, 145–148. [Google Scholar]
- Sucharita, A. Textile Dyes: It’s Impact on Environment and its Treatment. J. Bioremed. Biodeg. 2014, 5, 3. [Google Scholar]
- Esther, F.; Tibor, C.; Gyula, O. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar]
- Rita, K. Textile dyeing industry an environmental hazard. Nat. Sci. 2012, 4, 22–26. [Google Scholar]
- Boon, H.T.; Tjoon, T.T.; Omar, A.M. Removal of dyes and industrial dye waste by magnesium chloride. Wat. Res. 2000, 34, 597–601. [Google Scholar]
- Sampa, C.; Binay, K.D. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar]
- Mustafa, T.Y.; Tushar, K.S.; Sharmeen, A.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interfac 2014, 209, 172–184. [Google Scholar]
- Tim, R.; Geoff, M.; Roger, M.; Poonam, N. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar]
- Yahya, S.A.; Musa, I.E.; Amjad, H.E.; Gavin, M.W. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigments 2008, 77, 16–23. [Google Scholar]
- Jian-Hui, S.; Sheng-Peng, S.; Guo-Liang, W.; Li-Ping, Q. Degradation of azo dye Amido black 10B in aqueous solution by Fenton oxidation process. Dyes Pigments 2007, 74, 647–652. [Google Scholar]
- Sadeghzadeh-Attar, A. Efficient photocatalytic degradation of methylene blue dye by SnO2 nanotubes synthesized at different calcination temperatures. Sol. Energy Mater. Sol. Cells 2018, 183, 16–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Liu, J.; Wang, T.; Wang, X.; Liu, B.; Liu, Y.; Huo, Q.; Chu, Z. Synthesis of hierarchical hollow sodium titanate microspheres and their application for selective removal of organic dyes. J. Colloid Interface Sci. 2018, 528, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Martinez, A.I.; Oliva, A.I.; Garcia, C.R.; Martinez-Luevanos, A.; Garcia-Lobato, M.; Ochoa-Valiente, R.; Berlanga, A. Flexible graphene composites for removal of methylene blue dye-contaminant from water. Appl. Surf. Sci. 2018, 436, 739–746. [Google Scholar] [CrossRef]
- Bayat, M.; Javanbakht, V.; Esmaili, J. Synthesis of zeolite/nickel ferrite/sodium alginate bionanocomposite via a co-precipitation technique for efficient removal of water-soluble methylene blue dye. Int. J. Biol. Macromol. 2018, 116, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Shahdad, N.R.M.; Lim, Y.C.; Pace, A. Magnetic hybrid TiO2/Alg/FeNPs triads for the efficient removal of methylene blue from water. Sustain. Chem. Pharm. 2018, 8, 50–62. [Google Scholar] [CrossRef]
- Oudghiri-Hassani, H.; Rakass, S.; Abboudi, M.; Mohmoud, A.; Al Wadaani, F. Preparation and characterization of α-Zinc molybdate catalyst: Efficient sorbent for methylene blue and reduction of 3-nitrophenol. Molecules 2018, 23, 1462. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.C.; Luo, X.P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Khalil, A.; Zerrouq, F. Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surf. Interfaces 2018, 11, 74–81. [Google Scholar] [CrossRef]
- Low, S.K.; Tan, M.C. Dye adsorption characteristic of ultrasound pre-treated pomelo peel. J. Environ. Chem. Eng. 2018, 6, 3502–3509. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of methylene blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Removal of a cationic dye from aqueous solution by natural clay. Groundw. Sustain. Dev. 2018, 6, 255–262. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Akil, A.; Siti, H.M.S.; Chuo, S.C.; Asma, K.; Waseem, A.W.; Rajeev, K.; Mohd, R. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. J. RSC Adv. 2015, 5, 30801–30818. [Google Scholar]
- Grégorio, C. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar]
- Flàvio, A.P.; Ana, C.M.; Yoshitaka, G. Removal of methylene blue dye from aqueous solutions by adsorption using yellow passion fruit peel as adsorbent. Bioresour. Technol. 2008, 99, 3162–3165. [Google Scholar]
- Mohammed, M.A.; Shitu, A.; Ibrahim, A. Removal of methylene blue using low cost adsorbent: A review. Res. J. Chem. Sci. 2014, 4, 91–102. [Google Scholar]
- Gong, R.; Li, M.; Yang, C.; Sun, Y.; Chen, J. Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J. Hazard. Mater. 2005, 121, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Oladoja, N.A.; Aboluwoye, C.O.; Oladimeji, Y.B.; Ashogbon, A.O.; Otemuyiwa, I.O. Studies on castor seed shell as a sorbent in basic dye contaminated wastewater remediation. Desalination 2008, 227, 190–203. [Google Scholar] [CrossRef]
- Ponnusami, V.; Gunasekar, V.; Srivastava, S.N. Kinetics of methylene blue removal from aqueous solution using gulmohar (Delonix regia) plant leaf powder: Multivariate regression analysis. J. Hazard. Mater. 2009, 169, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Rafatullaha, M.; Sulaimana, O.; Hashima, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Mahmoudi, M. Removal of azo and anthraquinone reactive dyes from industrial wastewaters using MgO nanoparticles. J. Hazard. Mater. 2009, 168, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghani, N.T.; El-Chaghaby, G.; Rawash, S.; Lima, E. Adsorption of coomassie brilliant blue R-250 dye onto novel activated carbon prepared from Nigella sativa L. waste: Equilibrium, kinetics and thermodynamics Running Title: Adsorption of brilliant blue dye onto Nigella sativa L. waste actiated carbon. J. Chil. Chem. Soc. 2017, 62, 3505–3511. [Google Scholar] [CrossRef]
- Benkhaya, S.; El Harfi, S.; El Harfi, A. Classifications, properties and applications of textile dyes: A review. Appl. J. Environ. Eng. Sci. 2017, 3, 311–320. [Google Scholar]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 181–182, 449–457. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Hubicki, Z. Removal of tartrazine from aqueous solutions by strongly basic polystyrene anion exchange resins. J. Hazard. Mater. 2009, 164, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Karuppasamy, K. Low cost adsorbents for the removal of phenyl aceticacid from aqueous solution. Indian J. Environ. Prot. 1998, 18, 683–690. [Google Scholar]
- Krishnan, K.; Anirudhan, T.S. A Preliminary examination of the adsorption characteristics of Pb(II) ions using sulphurised activated carbon prepared from bagasse pith. Indian J. Chem. Technol. 2002, 9, 32–40. [Google Scholar]
- Karaer, H.; Kaya, I. Synthesis, characterization of magnetic chitosan/active charcoal composite and using at the adsorption of methylene blue and reactive blue4, Micropor. Mesopor. Mat. 2016, 232, 26–38. [Google Scholar] [CrossRef]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 90–97. [Google Scholar] [CrossRef]
- Patil, S.; Renukdas, S.; Patel, N. Removal of methylene blue, a basic dye from aqueous solutions by adsorption using teak tree (Tectona grandis) bark powder. Int. J. Environ. Sci. 2011, 1, 711–726. [Google Scholar]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Furusawa, T.; Smith, J.M. Intraparticle mass transport in slurries by dynamic adsorption studies. AIChE J. 1974, 20, 88–93. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, freundlich, temkin and dubinin–radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Gupta, V.K.; Ali, I. Removal of lead and chromium from wastewater using bagasse fly ash-a sugar industry waste. J. Colloid Interface Sci. 2004, 271, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ahalya, N.; Kanamadi, R.D.; Ramachandra, T.V. Biosorption of chromium (VI) from aqueous solutions by the husk of Bengal gram (Cicer arientinum). Electron. J. Biotechnol. 2005, 8, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.M.; Hamada, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of elovich equation to the kinetics of phosphate release and sorption in soils. Soil. Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Shawabkeh, R.A.; Tutunji, M.F. Experimental study and modeling of basic dye sorption by diatomaceous clay. Appl. Clay Sci. 2003, 24, 111–120. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Shahwan, T.; Erten, H.N. Temperature effects in barium sorption on natural kaolinite and chlorite-illite clays. J. Radioanal. Nucl Chem. 2004, 260, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Ozcan, A.S.; Erdem, B.; Ozcan, A. Adsorption of acid blue 193 from aqueous solutions onto BTMA-bentonite. Colloids Surf. A Physicochem. Eng. Asp. 2005, 266, 73–81. [Google Scholar] [CrossRef]
- El-Sayed, G.O. Removal of methylene blue and crystal violet from aqueous solutions by palm kernel fiber. Desalination 2011, 272, 225–232. [Google Scholar] [CrossRef]
- Belala, Z.; Jeguirim, M.; Belhachemi, M.; Addoun, F.; Trouvé, G. Biosorption of basic dye from aqueous solutions by date stones and palm-trees Waste: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 271, 80–87. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, J.; Ma, D.; Han, R. Characterization of bio-char from pyrolysis of wheat straw and its evaluation on methylene blue adsorption. Desalin. Water Treat. 2012, 46, 115–123. [Google Scholar] [CrossRef]
- Boumehdi-Toumi, L.; Hamdi, L.; Salem, Z.; Allia, K. Batch adsorption of methylene blue from aqueous solutions by untreated alfa grass. Desalin. Water Treat. 2015, 53, 806–817. [Google Scholar] [CrossRef]
- Deng, H.; Lu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Varadarajan, P.R.; Porkodi, K.; Subbhuraam, C.V. Adsorption of methylene blue onto jute fiber carbon : Kinetics and equilibrium studies. J. Colloid Interface Sci. 2005, 284, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Dewani, R.; Pervez, M.K.; Mahboob, S.J.; Soomro, S.A. Non-destructive FT-IR analysis of mono azo dyes. Bulg. Chem. Commun. 2016, 48, 71–77. [Google Scholar]
- Kooli, F.; Liu, Y.; Al-Faze, R.; Al Suhaimi, A. Effect of acid activation of Saudi local clay mineral on removal properties of basic blue 41 from an aqueous solution. Appl. Clay Sci. 2015, 116–117, 23–30. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Nigella sativa are available from the authors. |


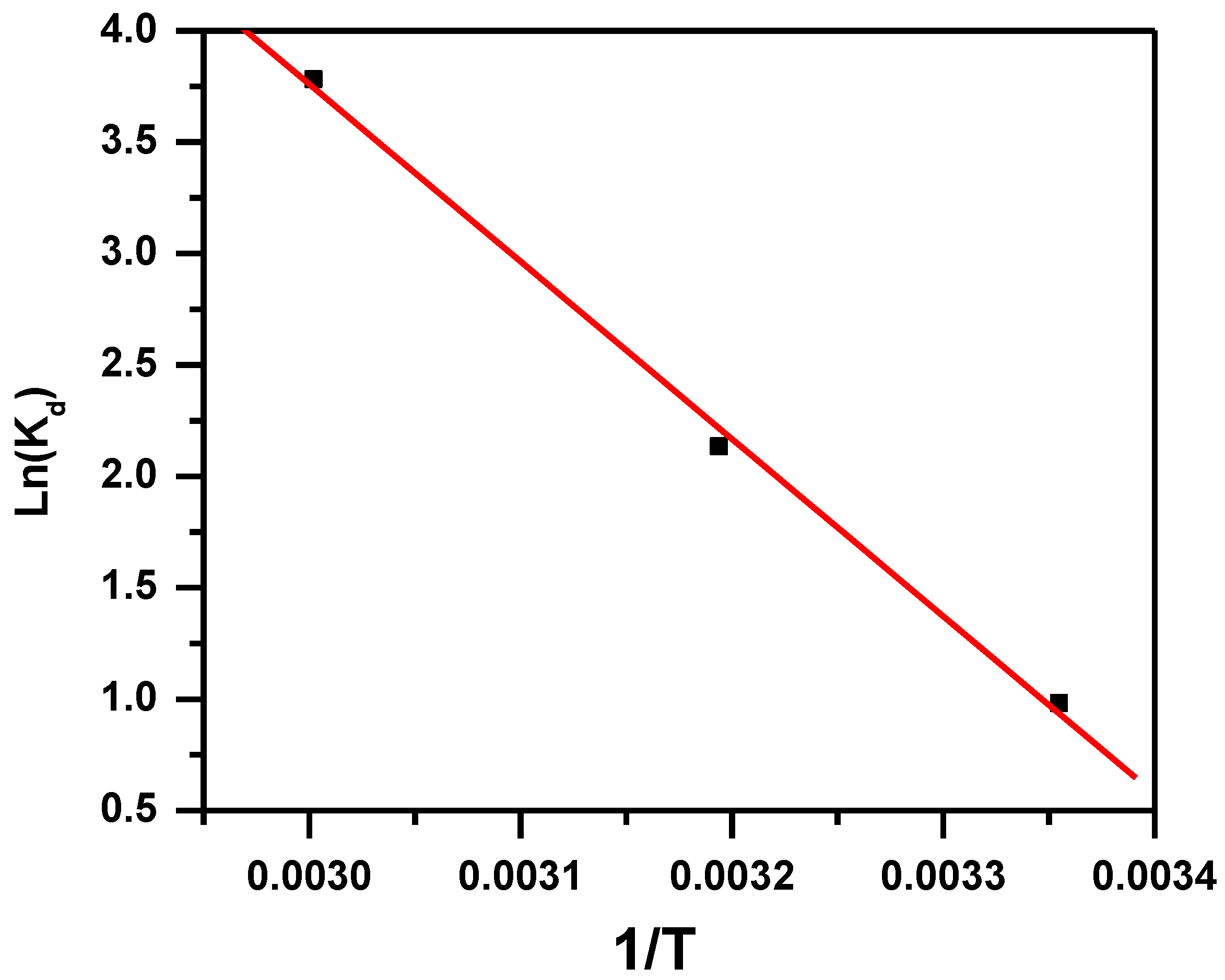



| Adsorbent | Adsorbate | ∆H° (KJ·mol−1) | ∆S° (KJ·mol−1·K) | ∆G° (KJ·mol−1) | ||
|---|---|---|---|---|---|---|
| MNS-4 | MB | 66 | 0.230 | 298K | 313K | 333K |
| −2.411 | −5.707 | −10.761 | ||||
| Dye Ci (ppm) | Pseudo-first Order | Pseudo-Second Order | Intra-Particle Diffusion Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qexp (mg/g) | qe (mg/g) | K1 (1/min) | R12 | qe (mg/g) | K2 (g/mg min) | R22 | I (mg/g) | KI (mg/g min0.5) | R32 | |
| 1200 | 118 | 23 | 0.041 | 0.993 | 120 | 0.00423 | 1.000 | 99 | 5 | 0.889 |
| 1500 | 148 | 37 | 0.029 | 0.993 | 150 | 0.00214 | 1.000 | 107 | 4 | 0.937 |
| 1800 | 173 | 53 | 0.029 | 0.997 | 176 | 0.00142 | 1.000 | 116 | 6 | 0.948 |
| 2000 | 184 | 81 | 0.024 | 0.998 | 186 | 0.00077 | 0.998 | 95 | 9 | 0.989 |
| 3000 | 237 | 75 | 0.019 | 0.998 | 232 | 0.00094 | 0.999 | 151 | 8 | 0.994 |
| Langmuir | Freundlich | Temkin | Dubinin–Radushkevich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | Range RL | qF (mg(1−1/n)L1/ng−1) | 1/n | R2 | AT (L/g) | BT | R2 | qm (mg/g) | R2 | E (Kj/mol) |
| 194 | 0.109 | 0.999 | 0.0023–0.0076 | 77 | 0.178 | 0.948 | 4.4E-11 | 0.029 | 0.937 | 85347 | 0.822 | 0.716 |
| Biosorbent | Q max (mg/g) | pH | Reference |
|---|---|---|---|
| Modified nigella sativa | 194 | 11 | Present work |
| Palm kernel fiber | 95 | 10–11 | [59] |
| Date stones | 44 | 6.3 | [60] |
| Bio-char from pyrolysis of wheat straw | 12 | 8–9 | [61] |
| Untreated alfa grass | 200 | 12 | [62] |
| Cotton stalk | 111 | 7 | [63] |
| Jute fiber carbon | 23 | 5–10 | [64] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakass, S.; Mohmoud, A.; Oudghiri Hassani, H.; Abboudi, M.; Kooli, F.; Al Wadaani, F. Modified Nigella Sativa Seeds as a Novel Efficient Natural Adsorbent for Removal of Methylene Blue Dye. Molecules 2018, 23, 1950. https://doi.org/10.3390/molecules23081950
Rakass S, Mohmoud A, Oudghiri Hassani H, Abboudi M, Kooli F, Al Wadaani F. Modified Nigella Sativa Seeds as a Novel Efficient Natural Adsorbent for Removal of Methylene Blue Dye. Molecules. 2018; 23(8):1950. https://doi.org/10.3390/molecules23081950
Chicago/Turabian StyleRakass, Souad, Ahmed Mohmoud, Hicham Oudghiri Hassani, Mostafa Abboudi, Fethi Kooli, and Fahd Al Wadaani. 2018. "Modified Nigella Sativa Seeds as a Novel Efficient Natural Adsorbent for Removal of Methylene Blue Dye" Molecules 23, no. 8: 1950. https://doi.org/10.3390/molecules23081950
APA StyleRakass, S., Mohmoud, A., Oudghiri Hassani, H., Abboudi, M., Kooli, F., & Al Wadaani, F. (2018). Modified Nigella Sativa Seeds as a Novel Efficient Natural Adsorbent for Removal of Methylene Blue Dye. Molecules, 23(8), 1950. https://doi.org/10.3390/molecules23081950