Ultrasound Assisted Synthesis of 4-(Benzyloxy)-N-(3-chloro-2-(substitutedphenyl)-4-oxoazetidin-1-yl) Benzamide as Challenging Anti-Tubercular Scaffold
Abstract
:1. Introduction
2. Results and Discussion
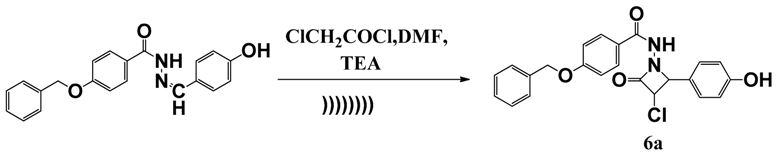
2.1. Chemistry
2.2. Biological Activity
In Vitro Anti-Tubercular Activity
2.3. In Vitro Cytotoxicity Study
2.4. Molecular Docking
Admet Predication
3. Materials and Methods
3.1. General Information
3.2. Biological Activity
Control − absorbance of Blank)] × 100
3.3. In Vitro Cytotoxicity Studies by MTT Assay
compound)/(absorbance of control − absorbance of blank)] × 100
3.4. Molecular Docking
3.5. In Silico ADMET Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Report 2013. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- World Health Organization. WHO Report 2014. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- World Health Organization. Tuberculosis Control in the South-East Asia Region: Annual Report 2015; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. WHO Report 2015. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Shah, N.S.; Abigail, W.; Gill-Han, B.; Lucia, B.; Fadila, B.; Nuria, M.C.; Francis, D.; Chris, G.; Marta, H.; Rosario, L.; et al. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg. Infect. Dis. 2007, 13, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.R.; Nunn, P.; Dheda, K.; Schaaf, H.S.; Zignol, M.; Van Soolingen, D.; Jensen, P.; Bayona, J. Multidrug-resistant and extensively drug-resistant tuberculosis: A threat to global tuberculosis. Lancet 2010, 375, 1830–1843. [Google Scholar] [CrossRef]
- Young, D.B.; Perkins, M.D.; Duncan, K.; Barry, C.E. Confronting the scientific obstacles to globa control of tuberculosis. J. Clin. Investig. 2008, 118, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.; Andrew, V.; Raviglione, M.C. New drugs and new regimens for the treatment of tuberculosis: Review of the drug development pipeline and implications for national programmes. Curr. Opin. Pulm. Med. 2010, 16, 186–193. [Google Scholar]
- Sankar, M.M.; Singh, J.; Diana, S.C.A.; Singh, S. Molecular characterization of Mycobacterium tuberculosis isolates from North Indian patients with extrapulmonar tuberculosis. Tuberculosis 2013, 93, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Migliori, G.B.; De Laco, G.; Besozzi, G.; Centis, R.; Cirillo, D.M. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill. 2007, 12, 3194. [Google Scholar] [CrossRef]
- Fauci, A.S. NIAID Tuberculosis Working Group. Multidrug-resistant and extensively drug-resistant tuberculosis: The national institute of allergy and infectious diseases research agenda and recommendations for priority research. J. Infect. Dis. 2008, 197, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Velayati, A.A.; Masjedi, M.R.; Farnia, P.; Tabarsi, P.; Ghanavi, J.; ZiaZarifi, A.H. Emergence of new forms of totally drug-resistant tuberculosis bacilli: Super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest 2009, 136, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Loewenberg, S. India reports cases of totally drug-resistant tuberculosis. Lancet 2012, 379, 205. [Google Scholar] [CrossRef]
- Ahirrao, P. Recent developments in antitubercular drugs. Mini Rev. Med. Chem. 2008, 8, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, A.M. Tuberculosis drug development: Progress, challenges, and the road ahead. Tuberculosis 2010, 90, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T.; Riccardi, G. New tuberculosis drugs on the horizon. Curr. Opin. Microbiol. 2011, 14, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.newtbdrugs.org/pipeline.php.
- Villemagne, B.; Crauste, C.; Flipo, M.; Baulard, A.R.; Deprez, B.; Willand, N. Tuberculosis: The drug development pipeline at a glance. Eur. J. Med. Chem. 2012, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Donald, P.R.; Pym, A.; Grobusch, M.; Patientia, R.F.; Mahanyele, R.; Bantubani, N.; Narasimooloo, R.; De Marez, T.; van Heeswijk, R.; et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: Long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob. Agents Chemother. 2012, 56, 3271–3276. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R. Bedaquiline: First FDA-approved tuberculosis drug in 40 years. Int. J. Appl. Basic Med. Res. 2013, 3, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.J. The penicillins. Mayo Clin. Proc. 1999, 74, 290–307. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S. Emerging trends in chemistry and pharmacology of β-lactams. Mod. Chem. Appl. 2013, 1, e108. [Google Scholar] [CrossRef]
- Patel, P.S.; Gor, D.G.; Patel, P.A. Synthesis, characterization and anti-microbial activity of 3-{4-[3-chloro-2-(substituted phenyl)-4-oxoazetidin-1yl] phenyl}-6-bromo-2-methylquinazoline-4-one. Int. J. Pharm. Res. Sch. 2012, 1, 12–15. [Google Scholar]
- Patel, R.B.; Desai, P.S.; Desai, K.S.; Chikhalia, K.H. Synthesis of pyrimidine based thiazolidinones and azetidinones: Antimicrobial and antitubercular agent. Indian J. Chem. 2006, 45B, 773–778. [Google Scholar] [CrossRef]
- Parikh, K.A.; Oza, P.S.; Parikh, A.R. Synthesis of some new 2-azetidinones as potential antitubercular agents. Indian J. Chem. 2000, 39B, 716–722. [Google Scholar]
- Bhat, I.K.; Chaitanya, S.K.; Satyanarayan, P.D.; Kalluraya, B. The synthesis and antimicrobial study of some azetidinone derivatives with the para-anisidine moiety. J. Serb. Chem. Soc. 2007, 72, 437–442. [Google Scholar] [CrossRef]
- Narute, A.S.; Khedekar, P.B.; Bhusari, K.P. QSAR studies on 4-thiazolidinones and 2-azetidinones bearing benzothiophene nucleus as potential anti-tubercular agents. Indian J. Chem. 2008, 47B, 586–591. [Google Scholar]
- Bhusnure, O.G.; Mokale, S.S.; Nalwar, Y.S.; Vibhute, Y.B. Microwave assisted and conventional synthesis, characterization and biological activity of 2-azetidinones and 4-thiazolidinone. J. Pharm. Biomed. Sci. 2011, 6, 1–8. [Google Scholar]
- Basavaraj, K.; Asifiqbal, K. Synthsis, docking and antitubercular activity of some newer azetidinones. Unique J. Pharm. Biol. Sci. 2014, 2, 43–50. [Google Scholar]
- Chavan, A.A.; Pai, N.R. Synthesis and biological activity of N-Substituted-3-chloro-2-azetidinones. Molecules 2007, 12, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Havaldar, F.H.; Mishra, S.K. Synthesis of some azetidin-2-ones and thiazolidin-4-ones as potential antimicrobial agents. Indian J. Heterocycl. Chem. 2004, 13, 197–200. [Google Scholar]
- Sulthana Munavar, R.; Darlin Quine, S. Synthesis, molecular structure, spectroscopic (FT-IR and NMR), NLO, HOMO-LUMO and NLO properties of 2-Azetidinone derivatives:a comprehensive experimental and DFT Study. Int. J. Curr. Res. Chem. Pharm. Sci. 2016, 3, 21–31. [Google Scholar]
- Dhingra, A.K.; Chopra, B.; Dass, R.; Mittal, S.K. Synthesis and biological evaluation of some new quinazolone fused azetidine analogs as potential anti-inflammatory agents. Der Pharm. Chem. 2015, 7, 103–109. [Google Scholar]
- Aakash, D.; Pradeep, K.; Balasubrmanian, N.; Lim, S.M.; Kalavathy, R.; Rakesh, K.M.; Vasudevan, M. Synthesis, antimicrobial and anticancer evaluation of 2-azetidinones clubbed with quinazolinone. Pharm. Chem. J. 2016, 50, 24–28. [Google Scholar]
- Sulthana, M.R.; Quine, S.D. Synthesis, characterization, antibacterial evaluation and molecular docking studies of 2-azetidinone derivatives as novel DNA gyrase inhibitors. Int. Lett. Chem. Phys. Astron. 2015, 47, 94–108. [Google Scholar] [CrossRef]
- Jetti, V.; Chidurala, P.; Pagadala, R.; Meshram, J.S.; Ramakrishna, C. Ultrasound-assisted one-pot synthesis of bis-azetidinones in the presence of zeolite. J. Heterocycl. Chem. 2014, 51, E183–E188. [Google Scholar] [CrossRef]
- Mason, T.J.; Cintas, P. Handbook of Green Chemistry and Technology; Clark, J., Macquarrie, D., Eds.; Blackwell Science: Oxford, UK, 2002. [Google Scholar]
- Mason, T.J. Sonochemistry and the environment-providing a “green” link between chemistry, physics and engineering. Ultrason. Sonochem. 2007, 14, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Luche, J.L. Synthetic Organic Sonochemistry; Plenum Press: New York, NY, USA, 1999; Volume 2, pp. 55–56. [Google Scholar]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. J. Chem. Soc. 2006, 35, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Muravyova, E.A.; Desenko, S.M.; Musatov, V.I.; Knyazeva, I.V.; Shishkina, S.V.; Shishkin, O.V.; Chebanov, V.A. Ultrasonic-promoted three-component synthesis of some biologically active 1,2,5,6-tetrahydropyrimidines. J. Comb. Chem. 2007, 9, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Li, J.T.; Yin, Y.; Li, L.; Sun, M.X. A convenient and efficient protocol for the synthesis of 5-aryl-1,3-diphenylpyrazole catalyzed by hydrochloric acid under ultrasound irradiation. Ultrason. Sonochem. 2010, 17, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S. Comprehensive Coordination Chemistry 2; Elsevier: New York, NY, USA, 2003; pp. 731–739. [Google Scholar]
- Cella, R.; Stefani, H.A. Ultrasound in heterocycles chemistry. Tetrahedron 2009, 65, 2619–2641. [Google Scholar] [CrossRef]
- Dandia, A.; Bhati, D.S.; Jain, A.K.; Sharma, G.N. Ultrasound promoted clay catalyzed efficient and one pot synthesis of substituted oxindoles. Ultrason. Sonochem. 2011, 18, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Rodríguez, H.; Coro, J.; Salfrán, E.; Suárez, M.; Martínez-Alvarez, R.; Martín, N. Ultrasound-assisted one-pot, four component synthesis of 4-aryl 3,4-dihydropyridone derivatives. Ultrason. Sonochem. 2011, 18, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Lengauer, T.; Rarey, M. Computational methods for biomolecular docking. Curr. Opin. Struct. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef]
- Tasdemir, D.; Topaloglu, B.; Perozzo, R.; Brun, R.; O’Neill, R.; Carballeira, N.M.; Zhang, X.; Tonge, P.J.; Linden, A.; Rüedi, P. Marine natural products from the turkish sponge agelas oroides that inhibit the enoyl reductase from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Biorg. Med. Chem. 2007, 5, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Vasconcelos, I.B.; Moreira, I.S.; Santos, D.S.; Basso, L.A. Enoyl reductases as targets for the development of anti-tubercular and anti-malarial agents. Curr. Drug Targets 2007, 8, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 2, 3–26. [Google Scholar] [CrossRef]
- Nimbalkar, U.D.; Tupe, S.G.; Seijas Vazquez, J.A.; Kalam Khan, F.A.; Sangshetti, J.N.; Nikalje, A.G. Ultrasound and molecular sieves-assisted synthesis, molecular docking 3-((phenylamino)methyl)-1,3,4-oxadiazole-2(3H)-thiones. Molecules 2016, 21, 484. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, A.G.; Tiwari, S.V.; Sarkate, A.P.; Karnik, K.S. Imidazole-thiazole coupled derivatives as novel lanosterol 14-α demethylase inhibitors: Ionic liquid mediated synthesis, biological evaluation and molecular docking study. Med. Chem. Res. 2018, 27, 592–606. [Google Scholar] [CrossRef]
- Chollet, A.; Mourey, L.; Lherbet, C.; Delbot, A.; Julien, S.; Baltas, M.; Bernadou, J.; Pratviel, G.; Maveyraud, L.; Génisson, V.B. Crystal structure of the enoyl-ACP reductase of mycobacterium tuberculosis (InhA) in the apo-form and in complex with the active metabolite of isoniazid pre-formed by a biomimetic approach. J. Struct. Biol. 2015, 190, 328–337. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Alian, A.; Stroud, R.; Ortiz de Montellano, P.R. Pyrrolidine carboxamides as a novel class of inhibitors of enoyl acyl carrier protein reductase from mycobacterium tuberculosis. J. Med. Chem. 2006, 49, 6308–6323. [Google Scholar] [CrossRef] [PubMed]
- Rozwarski, D.A.; Vilche, C.; Sugantino, M.; Bittman, R.; Sacchettini, J.C. Crystal structure of the mycobacterium tuberculosis enoyl-ACP reductase, InhA, in complex with NAD+ and a C16 Fatty acyl substrate. J. Biol. Chem. 1999, 274, 15582–15589. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hickey, R.P.; Yeh, J.L.; Liu, D.; Dadak, A.; Young, L.H.; Johnson, R.S.; Giordano, F.J. Cardiac myocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J. 2004, 18, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, M.B.; Dhumal, S.T.; Deshmukh, A.R.; Nawale, L.U.; Khedkar, V.; Sarkar, D.; Mane, R.A. New bithiazolyl hydrazones: Novel synthesis, characterization and anti-tubercular evaluation. Bioorg. Med. Chem. Lett. 2017, 27, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Somani, H.; Trivedi, A.; Bhatt, K.; Nawale, L.; Khedkar, V.M.; Jha, P.C.; Sarkar, D. Synthesis, biological evaluation and molecular docking study of some novel indole and pyridine based 1,3,4-oxadiaz ole derivatives as potential anti-tubercular agents. Bioorg. Med. Chem. 2016, 26, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, S.T.; Deshmukh, A.R.; Manisha, R.B.; Vijay, M.K.; Nawale, L.U.; Dhiman, S.; Mane, R.A. Synthesis and antitubercular activity of new 1,3,4-oxadiazoles bearing pyridyl and thiazolyl scaffolds. Bioorg. Med. Chem. 2016, 26, 3646–3651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Luo, Y.; He, L.Q.; Liu, Z.J.; Zhu, H.L. Synthesis, biological evaluation, and molecular docking studies of novel 1,3,4-oxadiazole derivatives possessing benzotriazole moiety as FAK inhibitors with anticancer activity. Bioorg. Med. Chem. 2013, 21, 3723–3729. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [PubMed]
- Sangshetti, J.; Khan, F.; Chouthe, R.; Damale, M.; Shinde, D. Synthesis, docking and ADMET prediction of novel 5-((5-substituted-1-H.-1,2,4-triazol-3-yl)methyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine as antifungal agents. Chin. Chem. Lett. 2014, 25, 1033–1038. [Google Scholar] [CrossRef]
- Lagorce, D.; Sperandio, H.; Miteva, M.; Villoutreix, B.O. FAF-Drugs2: Free ADME/tox filtering tool to assist drug discovery and chemical biology project. BMC Bioinform. 2008, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 46, 3–26. [Google Scholar] [CrossRef]
- Singh, U.; Akhtar, S.; Mishra, A.; Sarkar, D. A novel screening method based on menadione mediated rapid reduction of tetrazolium salt for testing of anti-mycobacterial agents. J. Microbiol. Methods 2011, 84, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Wagh, M.A.; Baravkar, S.B.; Jedhe, G.S.; Borkute, R.; Choudhari, A.S.; Sarkar, D.; Sanjayan, G.J. Design and synthesis of 2-amino-thiophene-tethered ureidopenicillin analogs with potent antibacterial and antitubercular activity. ChemistrySelect 2018, 3, 3122–3126. [Google Scholar] [CrossRef]
- Luckner, S.R.; Liu, N.; am Ende, C.W.; Tonge, P.J.; Kisker, C.A. A slow, tight binding inhibitor of InhA, the enoyl-acyl carrier protein reductase from mycobacterium tuberculosis. J. Biol. Chem. 2010, 285, 14330–14337. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
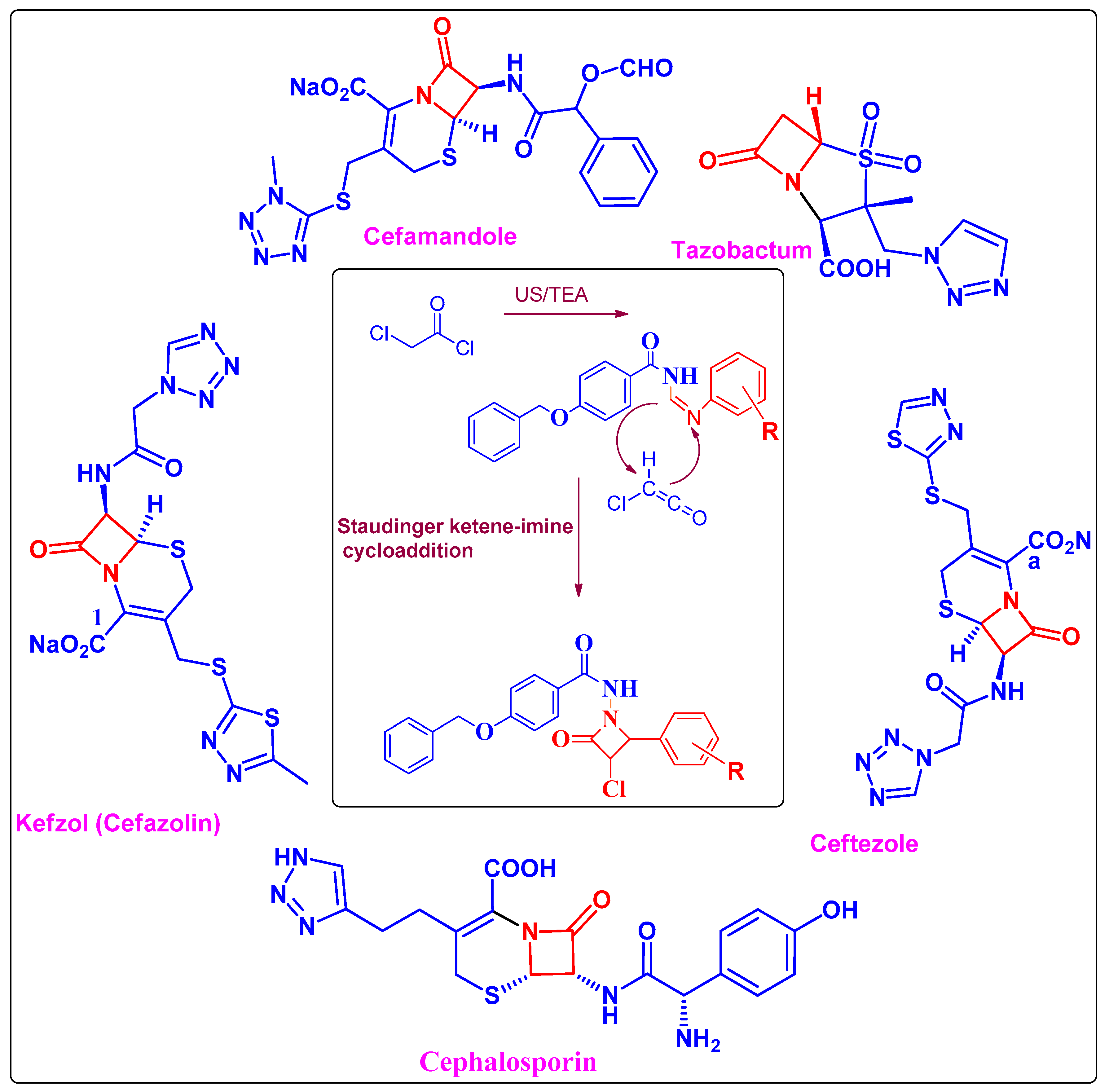
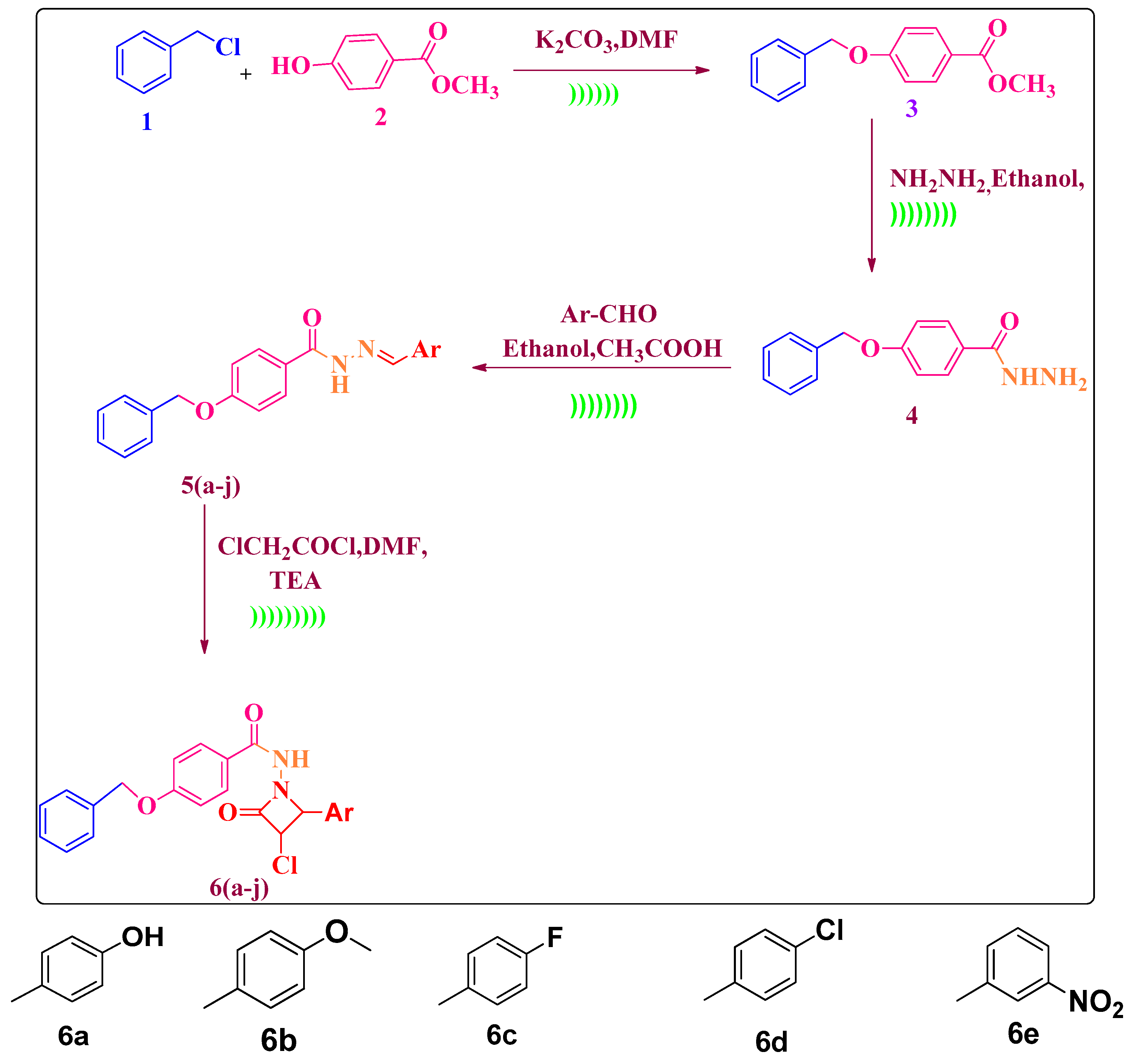
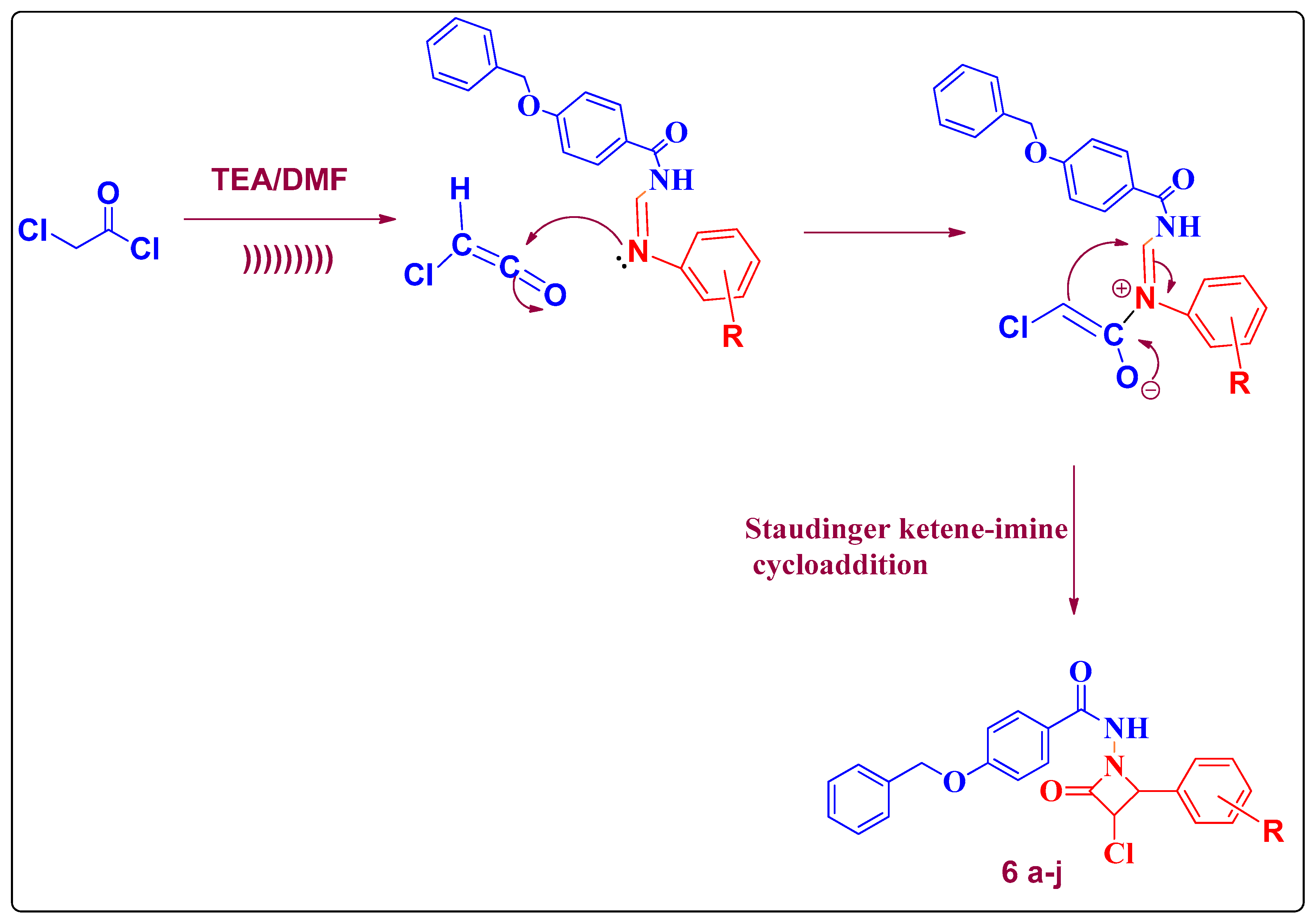
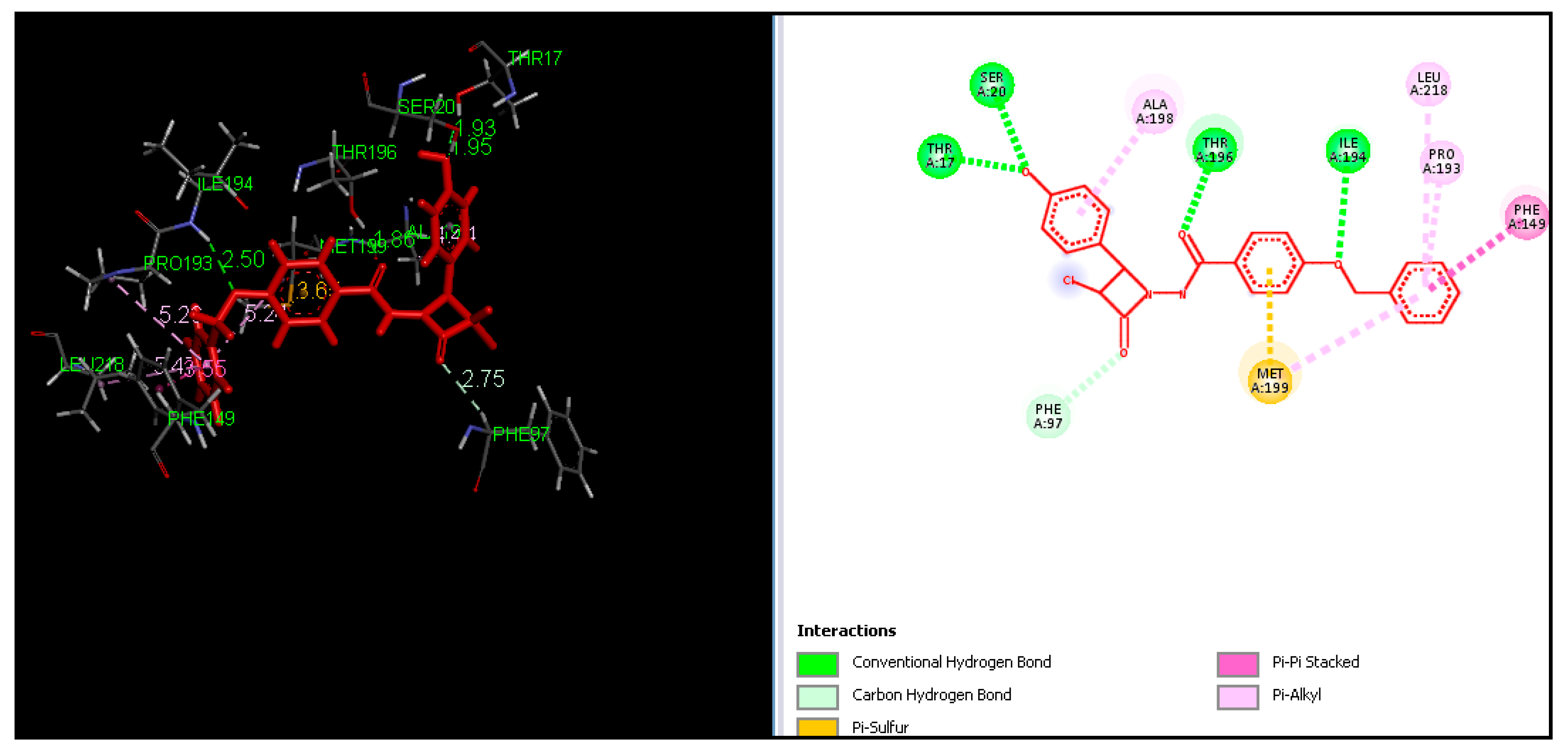
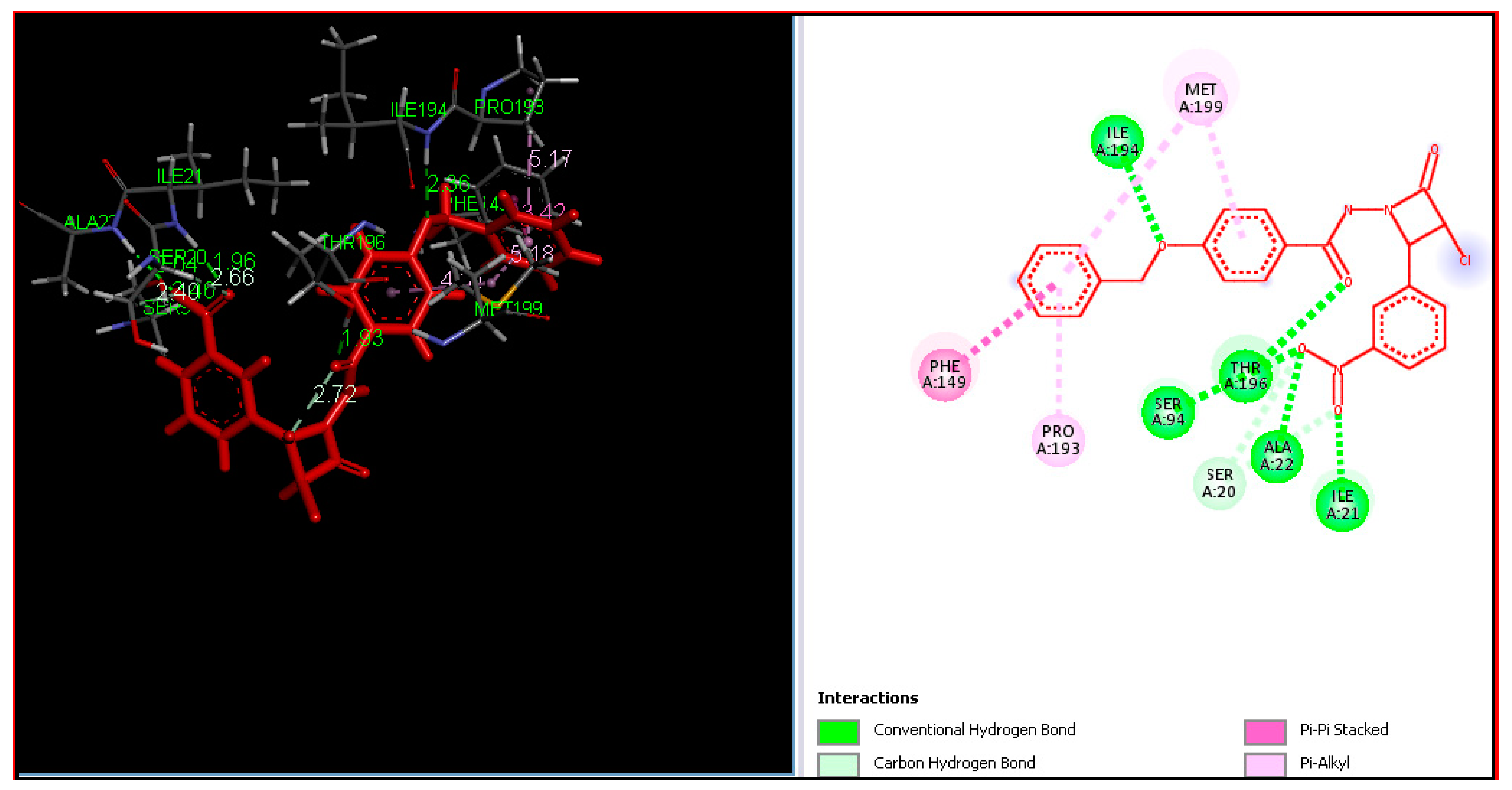
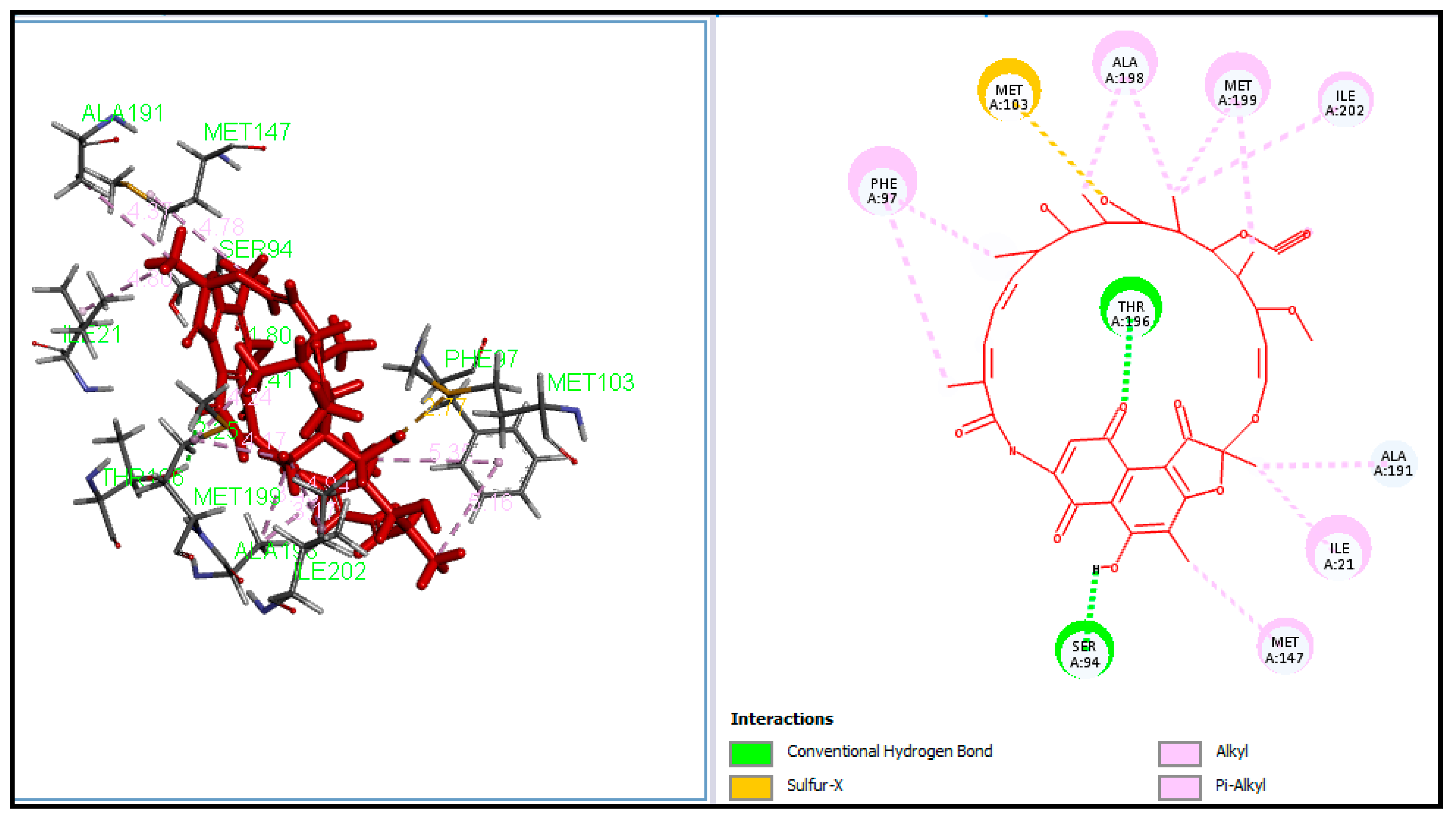
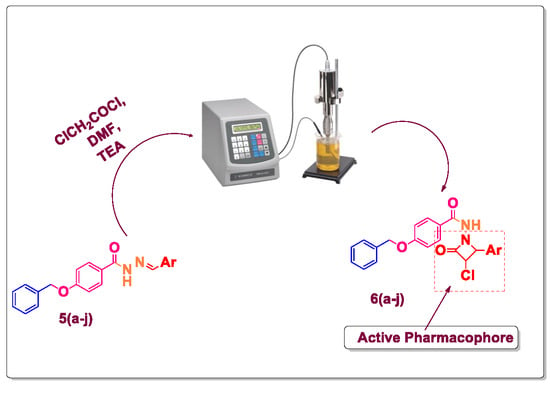
| Entry | Catalyst | Solvent | Method A Conventional Reflux | Method B Ultrasound Assisted | ||
|---|---|---|---|---|---|---|
| Time (h) | Yield (%) | Time (h) | Yield (%) | |||
| 1 | No Catalyst | Benzene | 22 | - | 6 | - |
| 2 | No Catalyst | 1,4-Dioxane | 15 | - | 6 | - |
| 3 | No Catalyst | DMF | 12 | - | 5 | - |
| 4 | Triethyl amine(TEA) | Benzene | 16 | 55 | 4 | 65 |
| 5 | Triethyl amine(TEA) | 1,4-Dioxane | 12 | 60 | 3 | 75 |
| 6 | Triethyl amine(TEA) | DMF | 08 | 70 | 2 | 88 |
| Entry | Conventional Refluxing | Ultrasonic Irradiation | ||
|---|---|---|---|---|
| Time (h) | Yield (%) | Time (h) | Yield (%) | |
| 6a | 08.00 | 70 | 02.00 | 88 |
| 6b | 06.00 | 65 | 02.00 | 82 |
| 6c | 06.50 | 72 | 02.10 | 80 |
| 6d | 07.00 | 70 | 02.20 | 79 |
| 6e | 07.50 | 71 | 02.15 | 80 |
| 6f | 08.00 | 65 | 02.00 | 79 |
| 6g | 07.50 | 67 | 02.00 | 86 |
| 6h | 07.50 | 61 | 02.30 | 81 |
| 6i | 08.00 | 66 | 02.00 | 78 |
| 6j | 08.00 | 65 | 02.00 | 79 |
| Sr. No | Mycobacterium tuberculosis H37Ra (ATCC 25177) IC50 (μg/mL) | Hela Cells IC50 μg/mL | Molecular Docking Score |
|---|---|---|---|
| Total Score against 4TZK (-log Ki) | |||
| 6a | 0.652 | 82.62 | 8.647 |
| 6b | 0.918 | 54.855 | 6.9851 |
| 6c | 0.85 | 31.612 | 7.5334 |
| 6d | 1.343 | 70.36 | 6.9795 |
| 6e | 0.654 | >100 | 8.6156 |
| 6f | 1.715 | 44.087 | 6.34 |
| 6g | 0.718 | >100 | 8.476 |
| 6h | 0.85 | >100 | 7.082 |
| 6i | 1.309 | 89.187 | 6.9839 |
| 6j | 0.786 | 33.182 | 8.0345 |
| Albendazole | NA | NA | 6.073 |
| Paclitaxel | NA | 0.0056 | NA |
| Rifampicin | 0.004 | NA | NA |
| ID | MW | % ABS | LogP | PSA | Rot. B | RigidB | HBD | HBA | Ratio H/C | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| 6a | 422.861 | 81.7899 | 4.1357 | 78.87 | 6 | 25 | 2 | 4 | 0.304 | Non Toxic |
| 6b | 436.888 | 85.5849 | 4.4387 | 67.87 | 7 | 25 | 1 | 4 | 0.291 | Non Toxic |
| 6c | 424.852 | 88.7692 | 4.5692 | 58.64 | 6 | 25 | 1 | 3 | 0.304 | Non Toxic |
| 6d | 441.307 | 88.7692 | 5.0835 | 58.64 | 6 | 25 | 1 | 3 | 0.304 | Non Toxic |
| 6e | 451.867 | 73.3477 | 4.7529 | 103.34 | 7 | 26 | 2 | 5 | 0.391 | Non Toxic |
| 6f | 466.914 | 82.4005 | 4.4473 | 77.1 | 8 | 25 | 1 | 5 | 0.32 | Non Toxic |
| 6g | 452.887 | 78.6055 | 4.1443 | 88.1 | 7 | 25 | 2 | 5 | 0.333 | Non Toxic |
| 6h | 466.914 | 78.6055 | 4.5344 | 88.1 | 8 | 25 | 2 | 5 | 0.32 | Non Toxic |
| 6i | 512.984 | 85.5849 | 6.0091 | 67.87 | 9 | 31 | 1 | 4 | 0.233 | Non Toxic |
| 6j | 412.889 | 79.0264 | 4.4916 | 86.88 | 6 | 24 | 1 | 4 | 0.333 | Toxic |
| Albendazole | 281.331 | 73.365 | 3.1975 | 103.29 | 5 | 13 | 2 | 4 | 0.583 | Non Toxic |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimbalkar, U.D.; Seijas, J.A.; Borkute, R.; Damale, M.G.; Sangshetti, J.N.; Sarkar, D.; Nikalje, A.P.G. Ultrasound Assisted Synthesis of 4-(Benzyloxy)-N-(3-chloro-2-(substitutedphenyl)-4-oxoazetidin-1-yl) Benzamide as Challenging Anti-Tubercular Scaffold. Molecules 2018, 23, 1945. https://doi.org/10.3390/molecules23081945
Nimbalkar UD, Seijas JA, Borkute R, Damale MG, Sangshetti JN, Sarkar D, Nikalje APG. Ultrasound Assisted Synthesis of 4-(Benzyloxy)-N-(3-chloro-2-(substitutedphenyl)-4-oxoazetidin-1-yl) Benzamide as Challenging Anti-Tubercular Scaffold. Molecules. 2018; 23(8):1945. https://doi.org/10.3390/molecules23081945
Chicago/Turabian StyleNimbalkar, Urja D., Julio A. Seijas, Rachna Borkute, Manoj G. Damale, Jaiprakash N. Sangshetti, Dhiman Sarkar, and Anna Pratima G. Nikalje. 2018. "Ultrasound Assisted Synthesis of 4-(Benzyloxy)-N-(3-chloro-2-(substitutedphenyl)-4-oxoazetidin-1-yl) Benzamide as Challenging Anti-Tubercular Scaffold" Molecules 23, no. 8: 1945. https://doi.org/10.3390/molecules23081945