An Ultra-High Performance Liquid Chromatography with Tandem Mass Spectrometry Method for Determination of 10 Alkaloids in Beagle Dog Plasma after the Oral Administration of the Corydalis yanhusuo W.T. Wang Extract and Yuanhuzhitong Tablets
Abstract
:1. Introduction
2. Results
2.1. Optimization of UHPLC-ESI-MS/MS Condition
2.2. Method Validation
2.2.1. Selectivity
2.2.2. Linearity and LLOQs
2.2.3. Precision and Accuracy
2.2.4. Extraction Recovery and I.S.-Normalized Matrix Factor
2.2.5. Stability
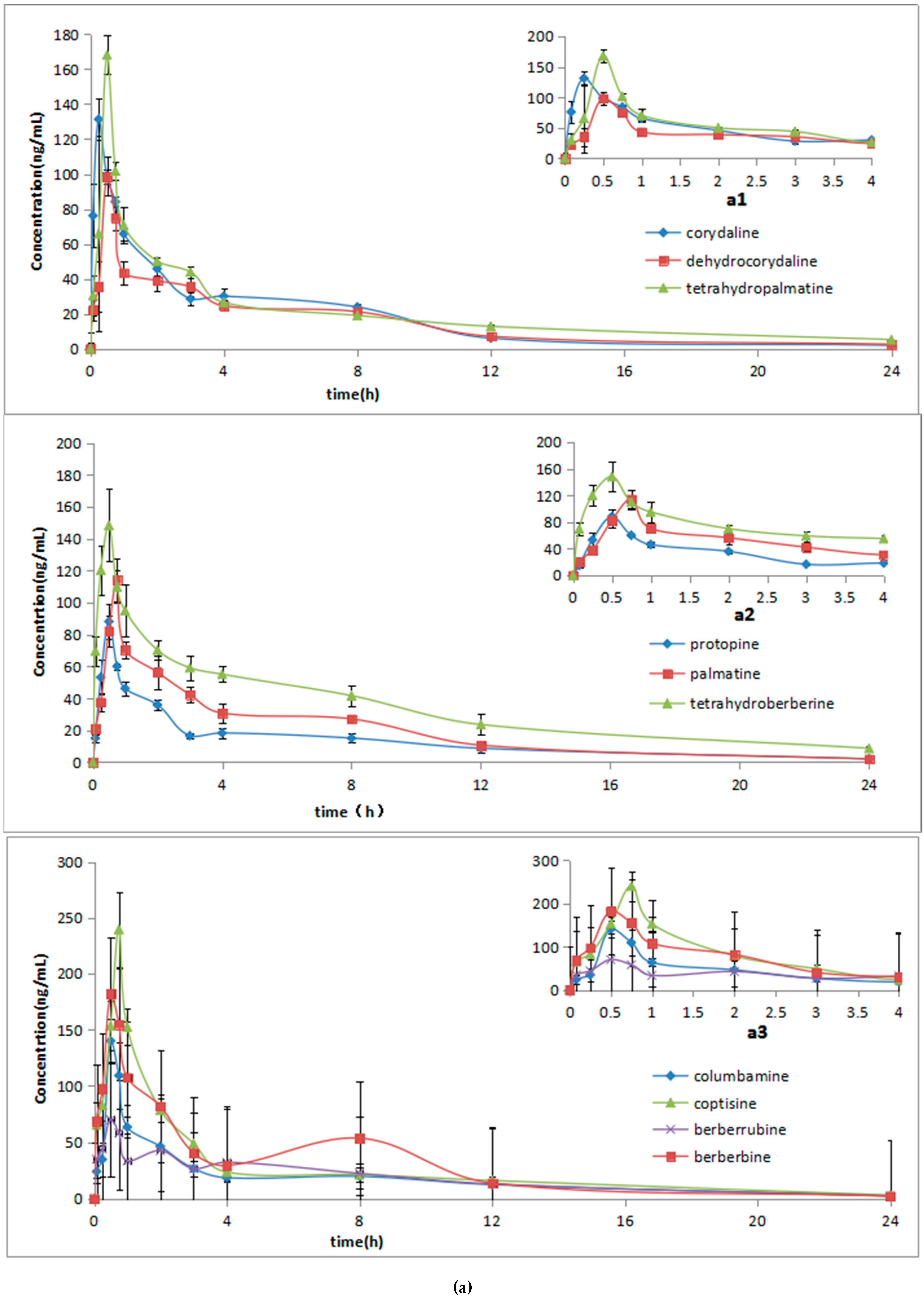
2.3. Pharmacokinetic Studies
3. Discussion
3.1. Selection of Extraction Method
3.2. Selection of I.S.
3.3. Pharmacokinetic Studies
4. Materials and Methods
4.1. Materials
4.2. UHPLC-ESI-MS/MS Conditions
4.3. Preparation of Standard and QC Solutions
4.4. Preparation of C. yanhusuo Extract and YHZT
4.5. Biosample Preparation
4.6. Method Validation
4.6.1. Selectivity
4.6.2. Linearity and LLOQ
4.6.3. Precision and Accuracy
4.6.4. Extraction Recovery and I.S.-Normalized Matrix Factor
4.6.5. Stability
4.7. Application to Pharmacokinetic Studies
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- China Pharmacopoeia Committee. Pharmacopoeia of the People’s Prpublic of China; China Chemical Industry Press: Beijing, China, 2015; p. 139. [Google Scholar]
- Zhang, J.; Jin, Y.; Dong, J.; Xiao, Y.S.; Feng, J.T.; Zhang, X.L. Systematic screening and characterization of tertiary and quaternary alkaloids from corydalis yanhusuo W.T. Wang using ultra-performance liquid chromatography–quadrupoletime-of-flight mass spectrometry. Talanta 2009, 78, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.J.; Li, X.D.; Gu, X.Z.; Cheng, L.P.; Mao, S.J. Effects of different types and standard of processing vinegaron inherent constituents in rhizoma of Corydalis yanhusuo. China J. Chinese Mater. Med. 2006, 31, 465–467. [Google Scholar]
- Wu, H.; Waldbauer, K.; Tang, L.; Xie, L.; McKinnon, R.; Yang, H.; Xu, H.; Kopp, B. Influence of vinegar and wine processing on the alkaloid content and composition of the traditional Chinese medicine Corydalis Rhizoma (Yanhusuo). Molecules 2014, 19, 11487–11504. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Yue, P.F.; Wu, B.; Hu, P.Y.; Wu, Z.F.; Yang, M. Pharmacokinetics comparative study of a novel Chinese traditional herbal formula and its compatibility. J. Ethnopharmacol. 2011, 137, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Zhang, K.R.; Lin, X.; Miao, Y.Q.; Meng, L.K.; Chen, W.; Tang, X. Pharmacokinetic comparisons of single herb extract of Fufang Danshen preparation with different combinations of its constituent herbs in rats. Pharm. Biomed. Anal. 2012, 67, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.H.; Tong, L.; Zhou, S.P.; Sun, H.; Bi, K.S.; Zhang, B.L. Simultaneous determinationof active flavonoids and alkaloids of Tang-Min-Ling-Pill in rat plasma by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2012, 904, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Yu, B.Y. Comparative studies on chemistry and pharmacodynamics of the compatibility of Yuanhu Zhitong prescription. Chin. Pharm. Univ. 2003, 34, 461–464. [Google Scholar]
- Chen, D.D.; Zhou, P.; Bai, G.G.; Li, X.; Chen, J.W. Discussion of HPLC fingerprint of traditional Chinese medicine of Corydalis yanhusuo and its preparation. China J. Chin. Mater. Med. 2015, 40, 2470–2473. [Google Scholar]
- Zhou, Y.; Gao, X.; Wu, C.; Wu, Y. Bioaccessibility and safety assessment of trace elements from decoction of “Zhebawei” herbal medicines by in vitro digestion method. Trace Elem. Med. Biol. 2014, 28, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Wang, Z.; Tong, N.; Kweon, T.D.; Vo, B.C.; Wang, X.; Zhang, J.Y.; Chung, A.; Alachkar, X.; et al. The antinociceptive properties of the Corydalis yanhusuo extract. PLoS ONE 2016, 11, e0162875. [Google Scholar]
- Wu, L.; Ling, H.; Li, L.; He, M. Beneficial effects of the extract from Corydalis yanhusuo in rats with heart failure following myocardial infarction. J. Pharm. Pharmacol. 2007, 59, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Wu, L.; Li, L. Corydalis yanhusuo, rhizoma extract reduces infarct size and improves heart function during myocardial ischemia/reperfusion by inhibiting apoptosis in rats. Phytother. Res. 2006, 20, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; Shi, Y. Analysis of chemical constituents of anti-myocardial ischemia fraction of Corydalis yanhusuo. China J. Chin. Mater. Med. 2008, 33, 1717–1719. [Google Scholar]
- Hu, T.; Zhang, X.; Ma, S.; Cheng, Y.; Yao, X. Chemical Constituents from Corydalis yanhusuo. China J. Chin. Mater. Med. 2009, 34, 1917–1920. [Google Scholar]
- Guo, Z.; Man, Y.; Wang, X.; Jin, H.; Sun, X.; Su, X.; Hao, J.; Mi, W. Levo-tetrahydropalmatine attenuates oxaliplatin-induced mechanical hyperalgesia in mice. Sci. Rep. 2014, 4, 3905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Liang, L.; Zhang, Q.; Li, X.; Fu, Z. Preclinical pharmacokinetics, tissue distribution and excretion studies of a potential analgesics-corydaline using an ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013, 30, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Ando, T.; Maeda, O.; WSTANABE, O.; Goto, H. Dehydrocorydaline inhibits elevated mitochondrial membrane potential in lipopolysaccharide-stimulated macrophages. Int. Immunopharmacol. 2011, 11, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Lapish, C.C.; Belardetti, F.; Ashby, D.M.; Ahn, S.; Butts, K.A.; So, K.; Macrae, C.M.; Hynd, J.J.; Miller, J.J.; Phillips, A.G. A preclinical assessment of D, L-govadine as a potential antipsychotic and cognitive enhancer. Int. J. Neuropsychopharmacol. 2011, 15, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Still, P.C.; Yi, B.; González-Cestari, T.F.; Pan, L.; Pavlovicz, R.E.; Chai, H.B.; Ninh, T.N.; Soejarto, D.D.; McKay, D.B.; Kinghorm, A.D. Alkaloids from Microcos paniculata with cytotoxic and nicotinic receptor antagonistic activities. J. Nat. Prod. 2013, 76, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Severina, I.I.; Muntyan, M.S.; Lewis, K.; Skulachev, V.P. transfer of cationic antibacterial agents berberine, palmatine, and benzalkonium through bimolecular planar phospholipid film and staphylococcus aureus membrane. Iubmb. Life 2001, 52, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shen, H.; Wang, L.; Meng, Q.; Li, W. Analyses of total alkaloid extract of corydalis yanhusuo by comprehensive RP × RP liquid chromatography with pH difference. Anal. Method. Chem. 2016, 2016, 9752735. [Google Scholar]
- Cheng, X.Y.; Yue, S.; Zhen, S.L.; Sun, H.; Jin, W. HPLC-MS analysis of ethanol extract of Corydalis yanhusuo and simultaneous determination of eight protoberberine quaternary alkaloids by HPLC-DAD. J. Chromatogr. Sci. 2010, 48, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Zhou, T.; Fan, G.; Hong, Z.; Wu, Y. Qualitative and quantitative determination of ten alkaloids in traditional Chinese medicine Corydalis yanhusuo W.T. Wang by LC-MS/MS and LC-DAD. J. Pharm. Biomed. Anal. 2007, 45, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Li, K.T.; Sun, H.; Jin, W.; Shi, J.W.; Shi, Y. LC-MS/MS determination and pharmacokinetic study of dehydrocorydaline in rat plasma after oral administration of dehydrocorydaline and Corydalis yanhusuo extract. Molecules 2014, 19, 16312–16326. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.Y.; Le, J.; Lin, M.; Fan, G.R.; Chai, Y.F.; Yin, X.P.; Wu, Y.T. Comparative studies on pharmacokinetic fates of tetrahydropalmatine enantiomers in different chemical environments in rats. Chirality 2008, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, Y.; Guo, T.; He, Z.; Chang, X.; Pu, X. Simultaneous determination of tetrahydropalmatine, protopine, and palmatine in rat plasma by LC-ESI-MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2009, 49, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hu, S.; Zhang, M.; Li, L.; Lin, Y. Simultaneous determination of four alkaloids in mice plasma and brain by LC-MS/MS for pharmacokinetic studies after administration of Corydalis Rhizoma and Yuanhu Zhitong extracts. J. Pharm. Biomed. Anal. 2014, 92, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; Shi, Y.; Sun, H.; Jin, W.; Zheng, S.L.; Li, K.T.; Yang, S. Identification and analysis of absorbed components in rat plasma after oral administration of active fraction of Corydalis yanhusuo by LC-MS/MS. Acta Pharm. Sin. B 2009, 44, 167–174. [Google Scholar]
- Murata, S.; Ueda, S.; Shimojo, F.; Tokunaga, Y.; Hata, T.; Ohnishi, N. In vivo performance of time-controlled explosion system (TES) in GI physiology regulated dogs. Int. J. Pharm. 1998, 161, 161–168. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Guo, X.; Sun, J.; Lin, L.; Wu, L. Simultaneous determination of seven anthraquinones in rat plasma by Ultra High Performance Liquid Chromatography-tandem Mass Spectrometry and pharmacokinetic study after oral administration of Semen Cassiae extract. J. Ethnopharmacol. 2015, 169, 305–313. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368107.pdf (accessed on 1 September 2013).
Sample Availability: Samples of the corydaline, dehydrocorydaline, tetrahydropalmatine, protopine, palmatine, tetrahydroberberine, columbamine, berberine, coptisine and berberrubine are available from the authors. |


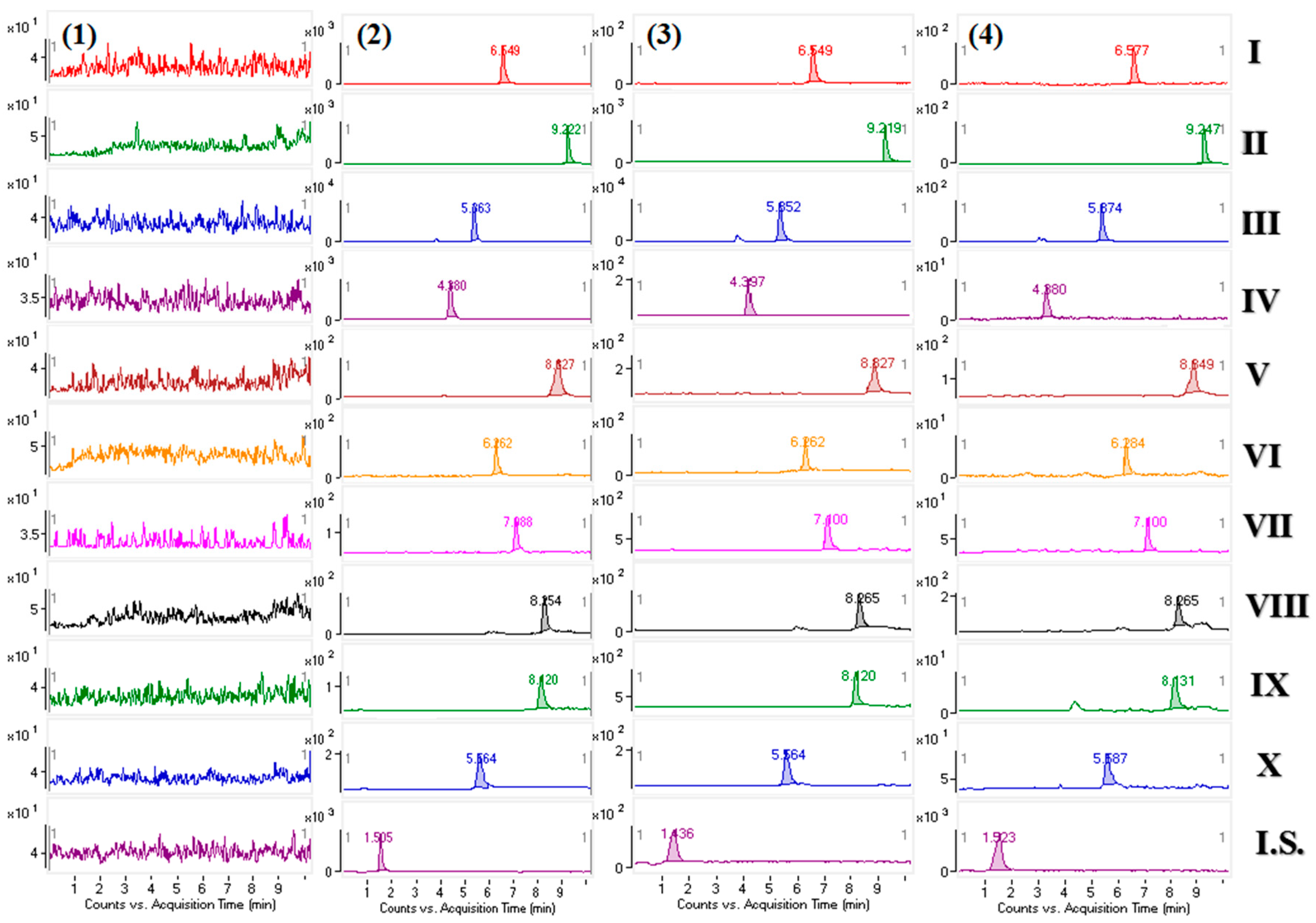

| Compound | Regression Equation | r2 | Linear Range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|
| corydaline | Y = 1.395 × 10−3X + 1.874 × 10−3 | 0.9938 | 0.54–557.0 | 0.54 |
| dehydrocorydaline | Y = 9.668 × 10−4X + 3.035 × 10−3 | 0.9912 | 0.25–257.5 | 0.25 |
| tetrahydropalmatine | Y = 1.368 × 10−3X − 2.608 × 10−4 | 0.9810 | 0.50–507.0 | 0.50 |
| protopine | Y = 4.086 × 10−4X − 3.221 × 10−5 | 0.9870 | 0.53–540.0 | 0.53 |
| palmatine | Y = 4.409 × 10−4X − 7.801 × 10−5 | 0.9868 | 0.51–525.0 | 0.51 |
| tetrahydroberberine | Y = 6.810 × 10−4X + 1.381 × 10−4 | 0.9801 | 0.41–420.0 | 0.41 |
| columbamine | Y = 4.941 × 10-4X − 1.679 × 10−4 | 0.9936 | 0.39–400.0 | 0.39 |
| berberine | Y = 1.667 × 10−3X + 6.950 × 10−3 | 0.9888 | 0.53–540.0 | 0.53 |
| coptisine | Y = 3.263 × 10−4X + 5.393 × 10−3 | 0.9934 | 0.49–500.0 | 0.49 |
| berberrubine | Y = 1.623 × 102X − 2.685 × 10−3 | 0.9816 | 0.22–230.0 | 0.22 |
| Compound | Spiked Concentration (ng/mL) | Measured Concentration (ng/mL) | Accuracy (RE%) | Intra-Day Precision (RSD%) | Inter-Day Precision (RSD%) |
|---|---|---|---|---|---|
| corydaline | 0.5 | 0.53 ± 0.03 | −7.50 | 10.90 | 6.00 |
| 1.1 | 0.96 ± 0.07 | −10.75 | 8.22 | 2.53 | |
| 34.8 | 32.87 ± 1.69 | −5.56 | 5.18 | 4.82 | |
| 445.6 | 432.80 ± 18.00 | −2.86 | 3.99 | 5.24 | |
| dehydrocorydaline | 0.3 | 0.21 ± 0.04 | −12.10 | 12.10 | 12.40 |
| 0.5 | 0.43 ± 0.05 | −4.95 | 4.43 | 8.30 | |
| 16.1 | 14.59 ± 1.45 | −9.34 | 9.94 | 10.13 | |
| 206.0 | 196.20 ± 8.40 | −4.75 | 3.81 | 6.79 | |
| tetrahydropalmatine | 0.5 | 0.45 ± 0.03 | −6.90 | 8.80 | 12.10 |
| 1.0 | 0.93 ± 0.05 | −5.82 | 5.00 | 7.95 | |
| 31.7 | 30.04 ± 1.50 | −5.18 | 5.19 | 2.72 | |
| 405.6 | 401.50 ± 20.26 | −3.84 | 4.15 | 4.22 | |
| protopine | 0.5 | 0.48 ± 0.02 | −11.90 | 9.20 | 8.80 |
| 1.1 | 0.94 ± 0.04 | −5.96 | 4.97 | 1.53 | |
| 33.8 | 31.54 ± 0.88 | −6.56 | 2.76 | 2.96 | |
| 432.0 | 403.30 ± 14.58 | −6.64 | 3.61 | 3.64 | |
| palmatine | 0.5 | 0.47 ± 0.02 | −9.60 | 7.00 | 12.00 |
| 1.0 | 0.91 ± 0.11 | −12.32 | 10.51 | 7.09 | |
| 32.8 | 30.56 ± 1.48 | −6.83 | 5.02 | 3.42 | |
| 420.0 | 401.90 ± 12.13 | −4.29 | 2.94 | 3.54 | |
| tetrahydroberberine | 0.4 | 0.39 ± 0.03 | −12.90 | 7.79 | 7.30 |
| 0.8 | 0.78 ± 0.06 | −4.46 | 7.57 | 4.67 | |
| 26.3 | 24.17 ± 1.80 | −7.92 | 7.66 | 5.50 | |
| 336.0 | 306.40 ± 24.86 | −8.81 | 8.02 | 8.80 | |
| columbamine | 0.4 | 0.38 ± 0.02 | −10.50 | 11.40 | 13.70 |
| 0.8 | 0.76 ± 0.04 | −3.19 | 5.04 | 4.61 | |
| 25.0 | 24.02 ± 1.41 | −3.92 | 6.19 | 2.35 | |
| 320.0 | 295.20 ± 7.17 | −7.74 | 2.16 | 3.91 | |
| berberine | 0.5 | 0.42 ± 0.05 | −14.40 | 11.20 | 14.90 |
| 1.1 | 0.87 ± 0.09 | −13.22 | 10.66 | 3.28 | |
| 33.8 | 32.04 ± 0.74 | −5.07 | 2.40 | 1.35 | |
| 432.0 | 411.60 ± 17.07 | −4.73 | 4.22 | 3.60 | |
| coptisine | 0.5 | 0.47 ± 0.04 | −5.90 | 8.80 | 13.40 |
| 1.0 | 0.92 ± 0.06 | −7.71 | 6.13 | 7.29 | |
| 31.3 | 30.28 ± 0.89 | −3.10 | 2.78 | 3.97 | |
| 400.0 | 389.40 ± 9.20 | −2.66 | 2.30 | 2.78 | |
| berberrubine | 0.2 | 0.18 ± 0.04 | −16.09 | 19.40 | 18.20 |
| 0.5 | 0.41 ± 0.02 | −8.70 | 5.93 | 6.70 | |
| 14.4 | 13.48 ± 1.15 | −6.18 | 7.89 | 12.47 | |
| 184.0 | 185.50 ± 10.41 | 0.83 | 5.15 | 8.30 |
| Compound | Spiked Concentration (ng/mL) | IS-Normalized Matrix Factor | Extraction Recovery | |
|---|---|---|---|---|
| Mean (%) | RSD (%) | |||
| corydaline | 1.1 | 0.91 | 79.36 | 9.903 |
| 34.8 | 0.97 | 85.91 | 6.930 | |
| 445.6 | 1.00 | 79.11 | 9.320 | |
| dehydrocorydaline | 0.5 | 1.00 | 84.12 | 8.781 |
| 16.1 | 1.04 | 88.02 | 8.180 | |
| 206.0 | 1.14 | 86.84 | 5.280 | |
| tetrahydropalmatine | 1.0 | 0.94 | 90.72 | 9.730 |
| 31.7 | 0.98 | 87.25 | 12.68 | |
| 507.0 | 1.01 | 87.96 | 10.15 | |
| protopine | 1.1 | 1.15 | 84.33 | 12.67 |
| 33.8 | 1.08 | 91.22 | 13.75 | |
| 432.0 | 1.09 | 83.46 | 4.350 | |
| palmatine | 1.0 | 0.94 | 91.21 | 11.01 |
| 32.8 | 0.87 | 87.29 | 7.730 | |
| 420.0 | 0.74 | 84.82 | 7.230 | |
| tetrahydroberberine | 0.8 | 0.89 | 89.17 | 12.30 |
| 26.3 | 0.88 | 88.48 | 5.243 | |
| 336.0 | 0.92 | 93.00 | 8.870 | |
| columbamine | 0.8 | 0.96 | 96.58 | 11.31 |
| 25.0 | 0.91 | 91.61 | 11.58 | |
| 320.0 | 0.94 | 84.66 | 5.910 | |
| berberine | 1.1 | 0.90 | 90.06 | 6.380 |
| 33.8 | 0.94 | 84.58 | 5.520 | |
| 432.0 | 0.97 | 87.94 | 7.270 | |
| coptisine | 1.0 | 0.95 | 91.75 | 9.640 |
| 31.3 | 0.88 | 88.93 | 7.880 | |
| 400.0 | 0.95 | 85.60 | 6.890 | |
| berberrubine | 0.5 | 0.94 | 88.41 | 7.790 |
| 14.4 | 0.97 | 77.09 | 12.86 | |
| 184.0 | 0.91 | 98.55 | 5.000 | |
| Compound | Spiked Concentration (ng/mL) | Stability (% RE a) | |||
|---|---|---|---|---|---|
| Short-Term | Long-Term | Three Freeze-Thaw | Post-Preparation | ||
| corydaline | 1.1 | 3.86 | 4.90 | −2.72 | 2.30 |
| 34.8 | −4.30 | −4.52 | −4.83 | −4.36 | |
| 445.6 | −1.88 | −4.45 | −3.63 | −4.56 | |
| dehydrocorydaline | 0.5 | 8.52 | 2.85 | 3.90 | 3.87 |
| 16.1 | 4.15 | 5.16 | 10.41 | 13.40 | |
| 206.0 | 3.50 | 2.97 | 1.85 | 1.05 | |
| tetrahydropalmatine | 1.0 | −4.16 | −4.47 | −4.77 | −4.36 |
| 31.7 | −1.92 | −4.85 | −4.76 | 2.09 | |
| 507.0 | −2.69 | −3.38 | −3.36 | −4.54 | |
| protopine | 1.1 | −4.47 | −4.48 | −6.91 | −4.40 |
| 33.8 | −2.86 | −4.78 | −3.96 | −3.63 | |
| 432.0 | −4.96 | −4.93 | −4.38 | −3.92 | |
| palmatine | 1.0 | −4.06 | 2.25 | −2.08 | −4.98 |
| 32.8 | −3.35 | −4.59 | −3.35 | −3.35 | |
| 420.0 | −3.83 | −3.95 | −2.02 | −2.78 | |
| tetrahydroberberine | 0.8 | −2.54 | −2.59 | −4.69 | −3.54 |
| 26.3 | −3.38 | −4.95 | −3.22 | −4.74 | |
| 336.0 | −3.32 | −3.04 | −3.92 | −4.51 | |
| columbamine | 0.8 | −3.44 | −3.46 | −3.06 | −1.75 |
| 25.0 | −2.54 | −3.94 | −4.49 | −3.14 | |
| 320.0 | −3.99 | −4.88 | −4.29 | −4.42 | |
| berberine | 1.1 | −4.14 | −4.51 | −3.91 | −3.47 |
| 33.8 | −1.87 | −4.50 | −4.62 | −4.47 | |
| 432.0 | −2.88 | −4.28 | −3.62 | −4.08 | |
| coptisine | 1.0 | −5.42 | −4.76 | −3.90 | −1.88 |
| 31.3 | −3.34 | −4.31 | −1.37 | −4.31 | |
| 400.0 | −4.14 | −2.34 | −2.09 | −4.09 | |
| berberrubine | 0.5 | −12.10 | −6.88 | −7.75 | −11.52 |
| 14.4 | 10.68 | −3.02 | −13.61 | −5.61 | |
| 184.0 | 1.39 | −2.87 | 1.67 | 0.89 | |
| Compounds | Cmax (ng/mL) | Tmax (h) | t1/2 (h) | AUC0→t (ng·h/mL) | AUC0→∞ (ng·h/mL) | |
|---|---|---|---|---|---|---|
| (A) | corydaline | 138 ± 24.5 | 0.29 ± 0.10 | 4.90 ± 0.70 | 358.34 ± 39.02 | 401.61 ± 48.38 |
| dehydrocorydaline | 99.7 ± 12.3 | 0.54 ± 0.10 | 5.83 ± 1.75 | 342.28 ± 9.30 | 403.19 ± 30.18 | |
| tetrahydropalmatine | 169 ± 55.3 | 0.54 ± 0.10 | 9.09 ± 2.17 | 424.21 ± 20.94 | 591.20 ± 50.58 | |
| protopine | 89.4 ± 8.49 | 0.95 ± 0.13 | 5.75 ± 0.81 | 275.69 ± 18.43 | 349.18 ± 38.33 | |
| palmatine | 116 ± 13.9 | 0.95 ± 051 | 4.45 ± 0.71 | 397.05 ± 30.16 | 465.14 ± 34.69 | |
| tetrahydroberberine | 154 ± 24.9 | 0.50 ± 0.16 | 7.06 ± 1.35 | 711.63 ± 56.12 | 1044.40 ± 68.18 | |
| columbamine | 144 ± 11.01 | 0.54 ± 0.10 | 5.67 ± 1.95 | 404.21 ± 15.57 | 506.82 ± 40.75 | |
| berberine | 187 ± 23.4 | 0.54 ± 0.10 | 5.00 ± 0.90 | 593.56 ± 23.76 | 673.64 ± 29.62 | |
| coptisine | 242 ± 58.2 | 0.79 ± 0.10 | 5.89 ± 1.07 | 631.90 ± 86.03 | 748.34 ± 68.39 | |
| berberrubine | 72.1 ± 1.9 | 0.54 ± 0.10 | 5.01 ± 0.61 | 422.88 ± 23.26 | 440.26 ± 19.33 | |
| (B) | corydaline | 165 ± 21.3 * | 0.29 ± 0.10 | 11.24 ± 1.49 | 361.474 ± 43.83 | 435.90 ± 58.28 |
| dehydrocorydaline | 187 ± 23.8 * | 0.54 ± 0.10 | 5.61 ± 1.05 | 1306.88 ± 148.77 * | 1399.25 ± 142.40 * | |
| tetrahydropalmatine | 277 ± 23.7 * | 0.71 ± 0.10 | 9.34 ± 0.26 | 1268.11 ± 61.64 * | 1462.37 ± 98.46 * | |
| protopine | 139 ± 24.2 * | 0.38 ± 0.14 | 7.77 ± 0.81 | 601.27 ± 66.95 * | 683.25 ± 67.68 * | |
| palmatine | 127 ± 14.6 | 0.50 ± 0.16 | 6.09 ± 0.66 | 638.87 ± 43.52 * | 684.56± 60.57 * | |
| tetrahydroberberine | 145 ± 12.0 | 0.54 ± 0.10 | 7.65 ± 1.14 | 637.16 ± 59.08 | 718.80 ± 98.29 | |
| columbamine | 120 ± 83 * | 0.54 ± 0.10 | 11.73 ± 2.92 | 611.24 ± 53.13 * | 755.66 ± 95.47 * | |
| berberine | ± 28.0 * | 0.71 ± 0.10 | 8.48 ± 1.92 | 988.42 ± 116.18 * | 1140.97 ± 91.92 * | |
| coptisine | 133 ± 15.1 * | 0.46 ± 0.10 | 9.58 ± 1.81 | 685.60 ± 60.34 | 824.82 ± 106.82 | |
| berberrubine | 86.0 ± 6.7 * | 0.54 ± 0.10 | 5.05 ± 0.48 | 445.44 ± 27.44 | 464.11 ± 25.40 |
| Compound | Ion Pair (m/z) | Qualifier Ion (m/z) | Fragment (V) | CE (V) | Polarity |
|---|---|---|---|---|---|
| corydaline | 370.2→192.1 | 165.1 | 170 | 30 | Positive |
| dehydrocorydaline | 366.1→350.1 | 334.1 | 140 | 30 | Positive |
| tetrahydropalmatine | 356.0→192.0 | 165.0 | 159 | 27 | Positive |
| protopine | 354.1→188.0 | 149.0 | 170 | 30 | Positive |
| palmatine | 352.1→336.2 | 294.1 | 155 | 40 | Positive |
| tetrahydroberberine | 340.1→176.1 | 149.0 | 168 | 40 | Positive |
| columbamine | 339.2→323.2 | 295.1 | 160 | 29 | Positive |
| berberine | 336.2→320.1 | 292.2 | 136 | 30 | Positive |
| coptisine | 320.2→292.2 | 292.2 | 167 | 29 | Positive |
| berberrubine | 322.2→307.2 | 250.1 | 160 | 29 | Positive |
| theophyline (I.S.) | 181.2→124.0 | 108.9 | 120 | 14 | Positive |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Yang, J.; Wang, Z.; Wu, C.; Dong, H.; Ren, Y.; Yang, C. An Ultra-High Performance Liquid Chromatography with Tandem Mass Spectrometry Method for Determination of 10 Alkaloids in Beagle Dog Plasma after the Oral Administration of the Corydalis yanhusuo W.T. Wang Extract and Yuanhuzhitong Tablets. Molecules 2018, 23, 1925. https://doi.org/10.3390/molecules23081925
Cui B, Yang J, Wang Z, Wu C, Dong H, Ren Y, Yang C. An Ultra-High Performance Liquid Chromatography with Tandem Mass Spectrometry Method for Determination of 10 Alkaloids in Beagle Dog Plasma after the Oral Administration of the Corydalis yanhusuo W.T. Wang Extract and Yuanhuzhitong Tablets. Molecules. 2018; 23(8):1925. https://doi.org/10.3390/molecules23081925
Chicago/Turabian StyleCui, Binbin, Jing Yang, Zhibin Wang, Chengcui Wu, Hongrui Dong, Yixuan Ren, and Chunjuan Yang. 2018. "An Ultra-High Performance Liquid Chromatography with Tandem Mass Spectrometry Method for Determination of 10 Alkaloids in Beagle Dog Plasma after the Oral Administration of the Corydalis yanhusuo W.T. Wang Extract and Yuanhuzhitong Tablets" Molecules 23, no. 8: 1925. https://doi.org/10.3390/molecules23081925
APA StyleCui, B., Yang, J., Wang, Z., Wu, C., Dong, H., Ren, Y., & Yang, C. (2018). An Ultra-High Performance Liquid Chromatography with Tandem Mass Spectrometry Method for Determination of 10 Alkaloids in Beagle Dog Plasma after the Oral Administration of the Corydalis yanhusuo W.T. Wang Extract and Yuanhuzhitong Tablets. Molecules, 23(8), 1925. https://doi.org/10.3390/molecules23081925





