Effect of Heat Stress on Yield, Monoterpene Content and Antibacterial Activity of Essential Oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens
Abstract
1. Introduction
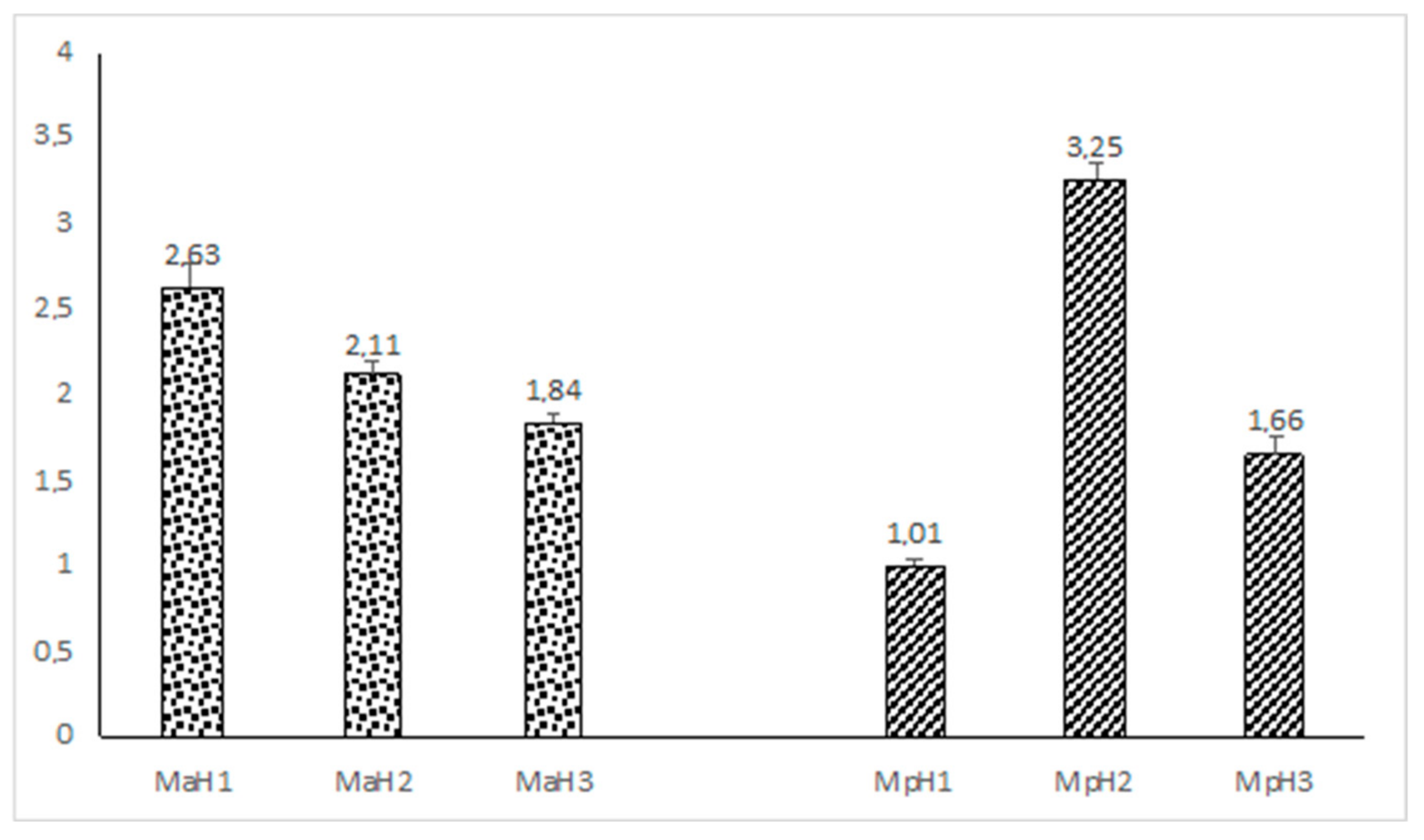
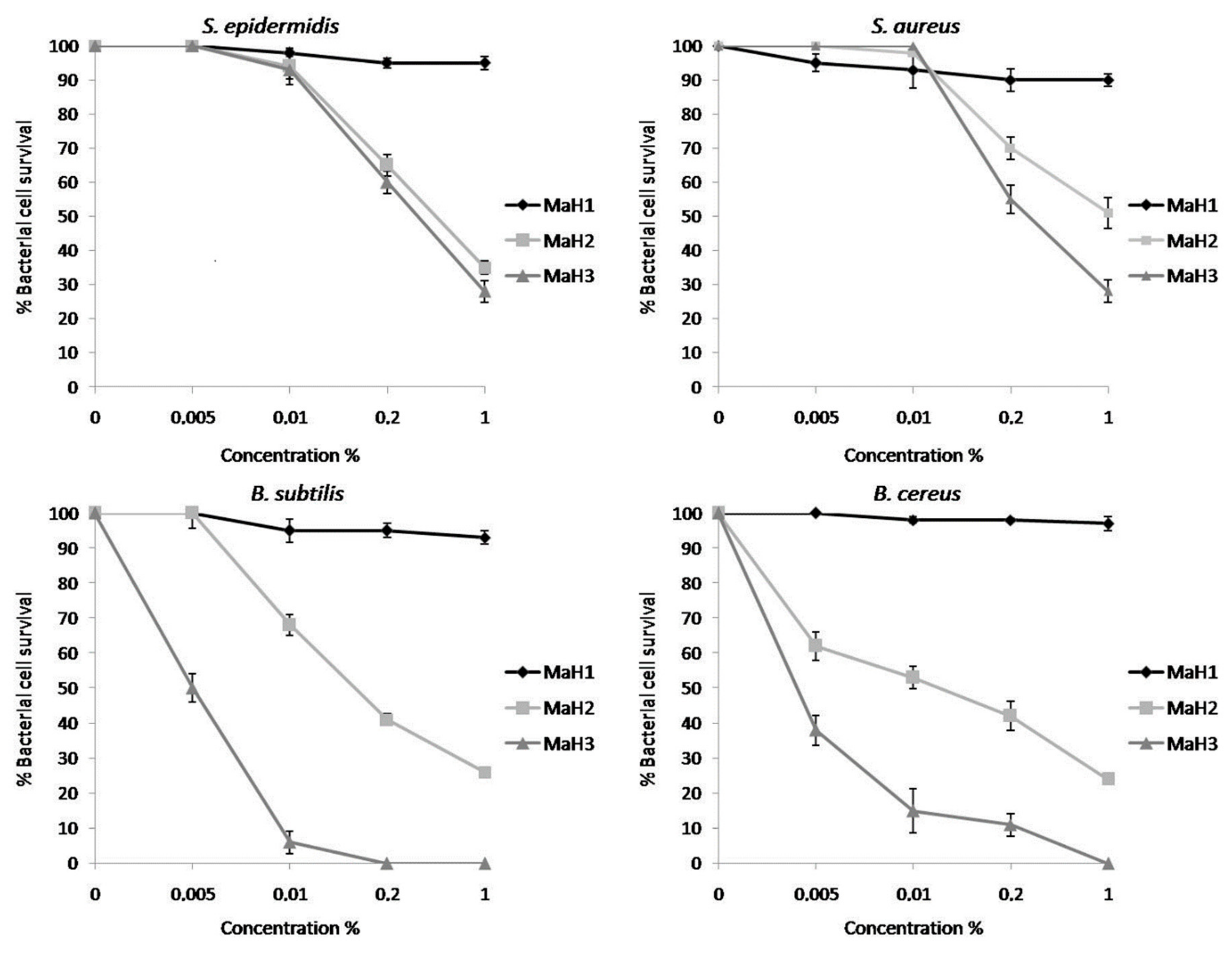
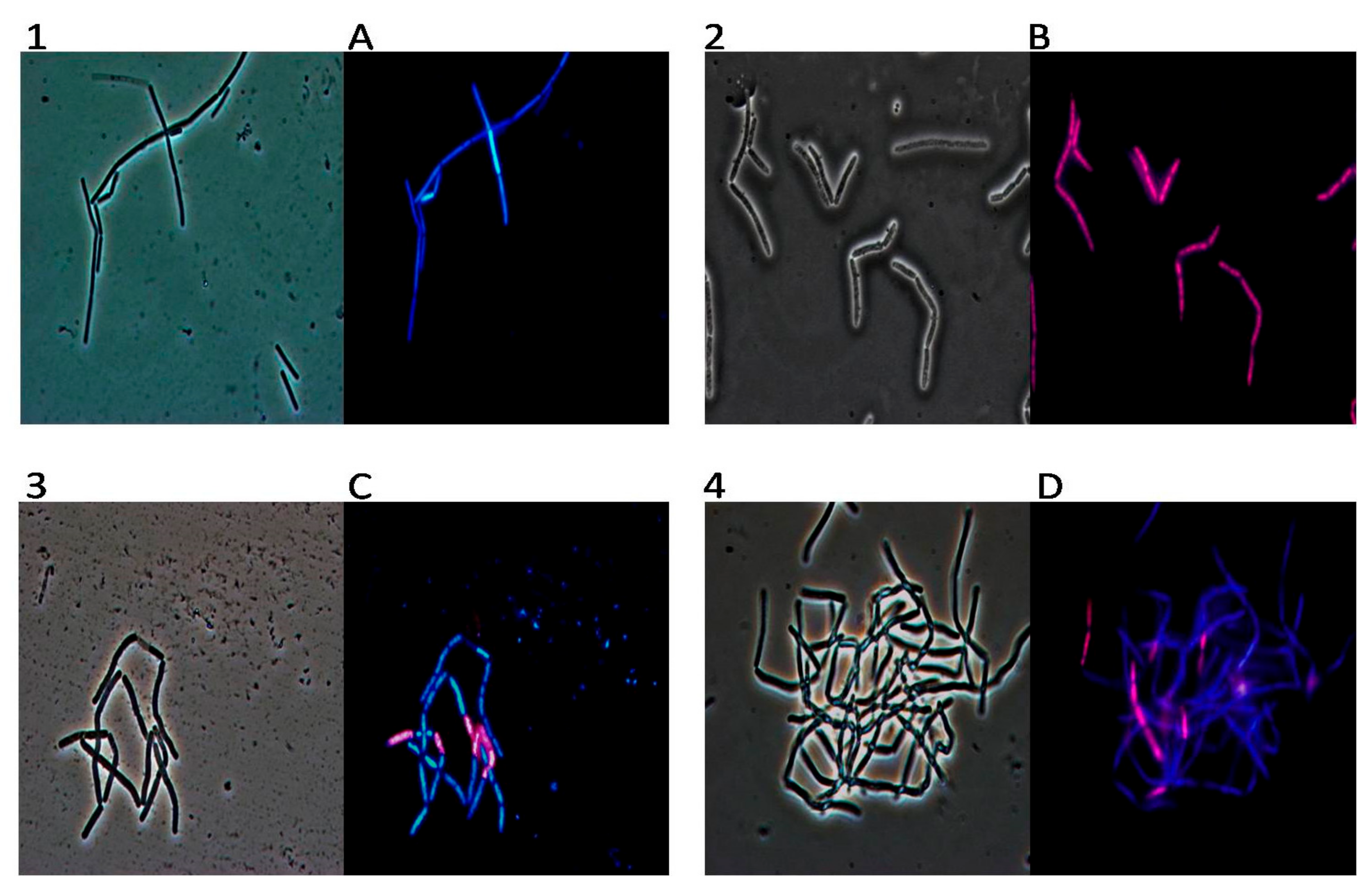
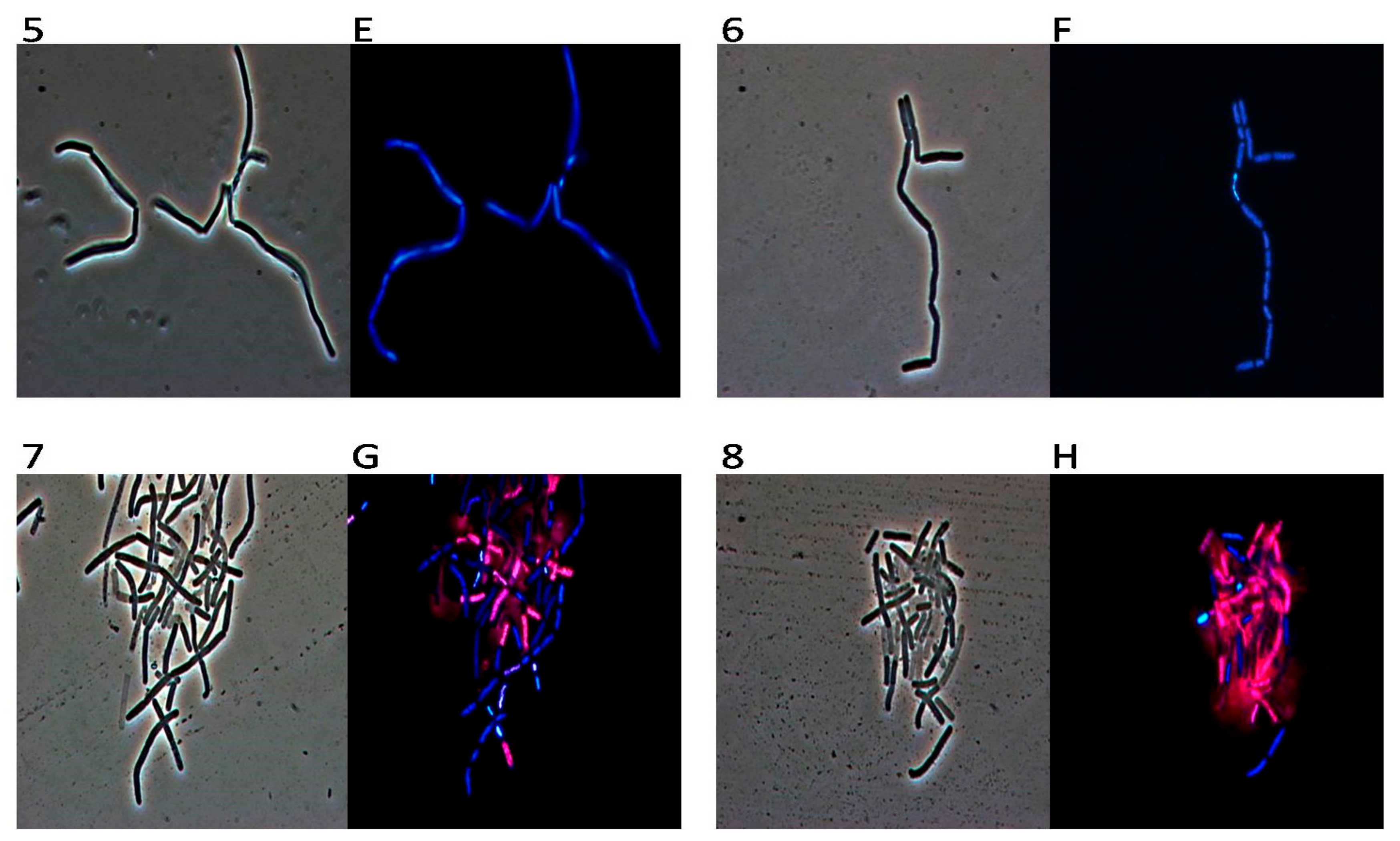
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material, Culture and Treatment
4.2. Isolation of Essential Oils
4.3. GC and GC-MS Analysis
4.4. Bacterial Strains
4.5. Antimicrobial Activity Assay
4.6. Determination of Minimal Inhibitory Concentration
4.7. DAPI/PI Dual Staining and Fluorescence Microscopy Image Acquisition
4.8. TEM (Transmission Electron Microscopy)
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R.I. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules 2017, 22, E263. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Yadav, R.K.; Mishra, S.; Sangwan, R.S.; Singh, A.K.; Mishra, B.; Srivastava, A.K.; Sangwan, N.S. Effect of gibberellic acid and calliterpenone on plant growth attributes, trichomes, essential oil biosynthesis and pathway gene expression in differential manner in Mentha arvensis L. Plant Physiol. Biochem. 2013, 66, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Croteau, R.B.; Davis, E.M.; Ringer, K.L.; Wildung, M.R. (-)-Menthol biosynthesis and molecular genetics. Naturwissenschaften 2005, 92, 562–577. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.Q.; Qamar, N.; Yadav, P.; Kulkarni, P.; Kumar, A.; Shasany, A.K. Comparative glandular trichome transcriptome-based gene characterization reveals reasons for differential (-)-menthol biosynthesis in Mentha species. Physiol. Plant. 2017, 160, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Maffei, M.; Croteau, R. Biochemical and histochemical localization of monoterpene biosynthesis in the glandular trichomes of spearmint (Mentha spicata). Plant Physiol. 1989, 89, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P. Recent advances and challenges in trichome research and essential oil biosynthesis in Mentha arvensis L. Ind. Crops Prod. 2016, 82, 141–148. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Ghasemi, M.; Modarresi, M.; Babaeian Jelodar, N.; Bagheri, N.; Jamali, A. The evaluation of exogenous application of salicylic acid on physiological characteristics, proline and essential oil content of chamomile (Matricaria chamomilla L.) under normal and heat stress conditions. Agriculture 2016, 6, 31. [Google Scholar] [CrossRef]
- Modhej, A.; Naderi, A.; Emam, Y.; Aynehband, A.; Normohamadi, G. Effects of post-anthesis heat stress and nitrogen levels on grain yield in wheat (T. durum and T. aestivum) genotypes. Int. J. Plant Prod. 2012, 2, 257–268. [Google Scholar]
- Modarresi, M.; Mohammadi, V.; Zali, A.; Mardi, M. Response of wheat yield and yield related traits to high temperature. Cereal Res. Commun. 2010, 38, 23–31. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Varcamonti, M.; Basile, A.; Bruno, M.; Maggi, F.; Zanfardino, A. Anti-Pseudomonas aeruginosa activity of hemlock (Conium maculatum, Apiaceae) essential oil. Nat. Prod. Res. 2018, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.W.; Zarowin, P. Does Income Smoothing Improve Earnings Informativeness? Social Science Research Network: Rochester, NY, USA, 2005. [Google Scholar]
- Guérout-Fleury, A.M.; Shazand, K.; Frandsen, N.; Stragier, P. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 1995, 167, 335–336. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Cardile, V.; Arnold, N.A.; Senatore, F. Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three lebanese Salvia species. Ind. Crops Prod. 2016, 83, 492–499. [Google Scholar] [CrossRef]
- Rigano, D.; Arnold, N.A.; Conforti, F.; Menichini, F.; Formisano, C.; Piozzi, F.; Senatore, F. Characterisation of the essential oil of Nepeta glomerata Montbret et Aucher ex Bentham from Lebanon and its biological activities. Nat. Prod. Res. 2011, 25, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Tagg, J.R.; McGiven, A.R. Assay system for bacteriocins. Appl. Microbiol. 1971, 21, 943. [Google Scholar] [PubMed]
- Iyapparaj, P.; Maruthiah, T.; Ramasubburayan, R.; Prakash, S.; Kumar, C.; Immanuel, G.; Palavesam, A. Optimization of bacteriocin production by Lactobacillus sp. MSU3IR against shrimp bacterial pathogens. Aquat. Biosyst. 2013, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Zanfardino, A.; Pizzo, E.; Di Maro, A.; Varcamonti, M.; D’Alessio, G. The bactericidal action on Escherichia coli of ZF-RNase-3 is triggered by the suicidal action of the bacterium OmpT protease. FEBS J. 2010, 277, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, I.; Higa, M.; Furugen, F.; Yamane, N. Evaluation of inoculum density prepared by prompt inoculation system and antimicrobial susceptibility test results by the automated MicroScan WalkAway system. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi 1999, 10, 83–89. [Google Scholar] [PubMed]
- Pizzo, E.; Zanfardino, A.; Di Giuseppe, A.M.A.; Bosso, A.; Landi, N.; Ragucci, S.; Varcamonti, M.; Notomista, E.; Di Maro, A. A new active antimicrobial peptide from PD-L4, a type 1 ribosome inactivating protein of Phytolacca dioica L.: A new function of RIPs for plant defence? FEBS Lett. 2015, 589, 2812–2818. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Cogoni, A.E.; Bassi, P.; Fabrizi, E.; Sorbo, S.; Giordano, S.; Castaldo Cobianchi, R. Accumulation of Pb and Zn in gametophytes and sporophytes of the moss Funaria hygrometrica (Funariales). Ann. Bot. 2001, 87, 537–543. [Google Scholar] [CrossRef]
Sample Availability: Samples of the essential oils are available from the authors. |


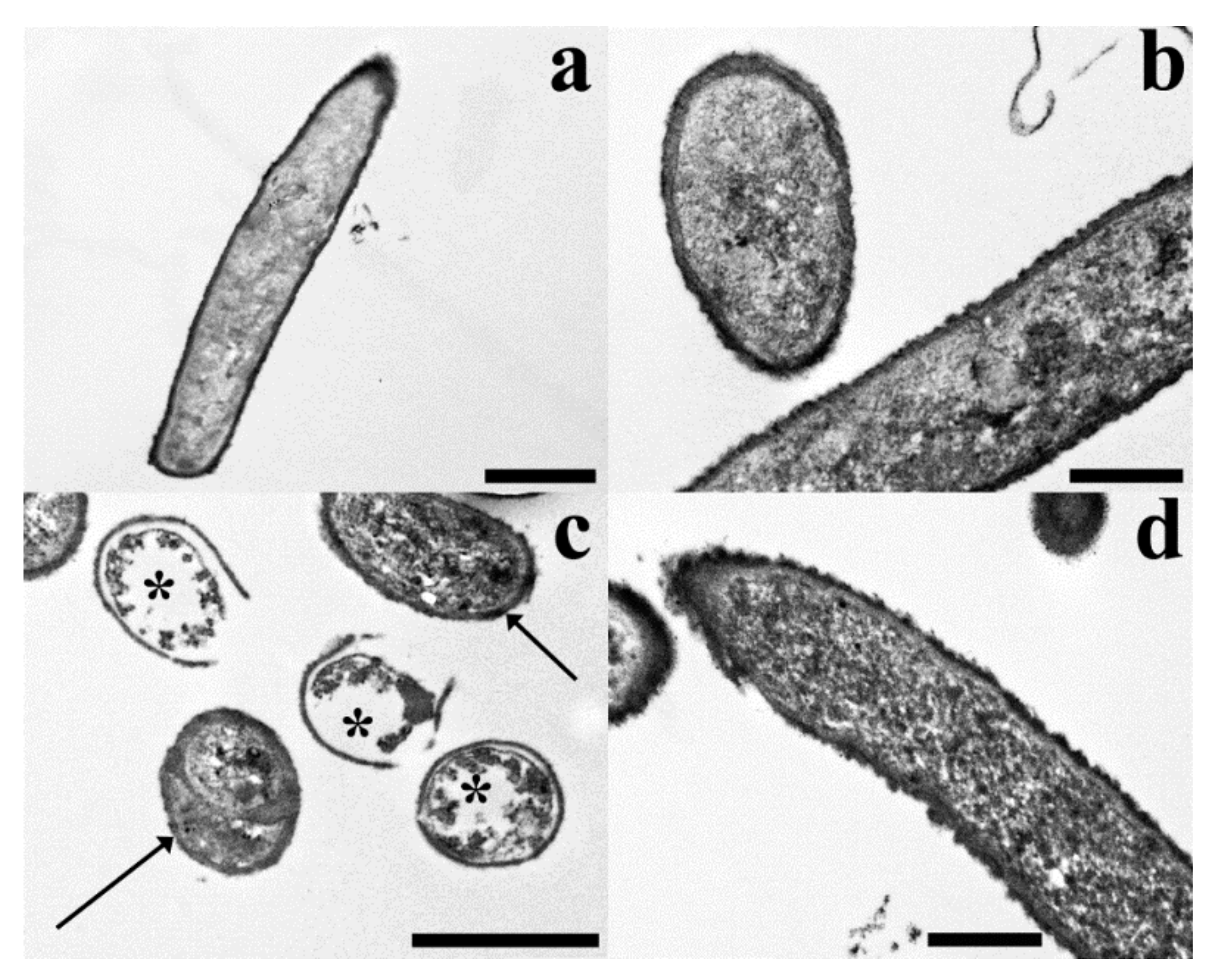
| LRI a | LRI b | Component | MpH1 | MpH2 | MpH3 | MaH1 | MaH2 | MaH3 |
|---|---|---|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 4.3 | 3.6 | 6.7 | 3.9 | 3.6 | 5.0 | ||
| 938 | 1075 | α-Pinene | 0.7 ± 0.01 | 0.6 ± 0.01 | 0.7 ± 0.02 | 0.4 ± 0.01 | 0.6 ± 0.01 | |
| 973 | 1132 | Sabinene | 0.3 ± 0.02 | 0.4 ± 0.01 | ||||
| 975 | p-2-menthene | 0.1 ± 0.01 | ||||||
| 978 | 1118 | β-Pinene | 0.7 ± 0.02 | 0.6 ± 0.02 | 0.7 ± 0.03 | 0.7 ± 0.02 | 0.7 ± 0.01 | |
| 987 | p-3-menthene | 0.4 ± 0.01 | 0.6 ± 0.01 | 2.7 ± 0.09 | 3.9 ± 0.06 | 0.6 ± 0.03 | 1.1 ± 0.06 | |
| 993 | 1174 | Myrcene | 0.1 ± 0.01 | 0.1 ± 0.01 | ||||
| 1029 | 1218 | β-Phellandrene | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.4 ± 0.01 | ||
| 1030 | 1203 | Limonene | 1.7 ± 0.03 | 1.4 ± 0.04 | 2.3 ± 0.07 | 1.5 ± 0.05 | 2.2 ± 0.04 | |
| Oxygenated monoterpenes | 92.0 | 94.8 | 90.6 | 88.3 | 92.5 | 90.3 | ||
| 1034 | 1213 | 1,8-Cineole | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.01 | 3.7 ± 0.09 | 1.8 ± 0.01 | |
| 1063 | 1550 | (Z)-Sabinene hydrate | 0.4 ± 0.01 | |||||
| 1150 | 1475 | Menthone | 14.5 ± 0.09 | 7.6 ± 0.08 | 11.9 ± 0.13 | 13.0 ± 0.09 | 15.9 ± 0.06 | 9.5 ± 0.04 |
| 1164 | 1460 | Menthofuran | 7.8 ± 0.12 | 33.9 ± 0.14 | 12.9 ± 0.18 | 35.0 ± 0.13 | 25.4 ± 0.08 | 34.0 ± 0.19 |
| 1165 | 1570 | Neomenthol | 1.6 ± 0.07 | 6.8 ± 0.08 | 1.3 ± 0.05 | |||
| 1173 | 1626 | Menthol | 56.6 ± 0.34 | 38.5 ± 0.21 | 27.5 ± 0.16 | 24.3 ± 0.16 | 12.2 ± 0.05 | 5.6 ± 0.07 |
| 1234 | 1662 | Pulegone | 5.6 ± 0.16 | 7.8 ± 0.19 | 10.9 ± 0.11 | 14.7 ± 0.09 | 24.3 ± 0.12 | 28.1v0.11 |
| 1291 | 1541 | Menthyl acetate | 2.1 ± 0.11 | 4.5 ± 0.09 | 15.4 ± 0.14 | 4.8 ± 0.07 | 6.1 ± 0.03 | |
| 1343 | 1748 | Piperitone | 0.5 ± 0.03 | 0.2 ± 0.01 | ||||
| 1366 | 1983 | Piperitenone oxide | 1.9 ± 0.04 | 1.7 ± 0.03 | 3.7 ± 0.07 | 4.3 ± 0.03 | 3.9 ± 0.02 | |
| 1579 | 2008 | Isomenthone | 1.1 ± 0.01 | 0.3 ± 0.01 | 1.2 ± 0.02 | 1.5 ± 0.02 | 1.3 ± 0.01 | |
| Sesquiterpene hydrocarbons | 0.6 | 0.6 | 0.5 | 6.4 | 1.6 | 2.2 | ||
| 1418 | 1612 | β-Caryophyllene | 0.3 ± 0.01 | 0.4 ± 0.02 | 0.5±0.02 | 0.7±0.03 | 0.9 ± 0.04 | 1.2 ± 0.04 |
| 1458 | t-β-farnesene | 0.1 ± 0.01 | 0.1 ± 0.01 | 0.2 ± 0.01 | ||||
| 1463 | 1667 | Alloaromadendrene | 0.2 ± 0.01 | 2.6 ± 0.09 | 0.4 ± 0.01 | 0.3 ± 0.01 | ||
| 1477 | 1726 | Germacrene D | - | 0.1±0.01 | 3.1 ± 0.08 | 0.3 ± 0.01 | 0.5 ± 0.01 | |
| Oxygenated sesquiterpenes | 0.2 | 0.3 | 0.3 | 0.3 | ||||
| 1580 | 2150 | Caryophyllene oxide | 0.2 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.3 ± 0.02 | ||
| Others | 2.3 | 0.8 | 1.8 | 1.8 | 2.0 | |||
| 1195 | 1655 | Methylchavicol | 0.5 ± 0.01 | 0.2 ± 0.01 | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.2 ± 0.01 | |
| 1446 | 2345 | Mint-furanone I | 1.8 ± 0.05 | 0.6 ± 0.02 | 1.4 ± 0.04 | 1.4 ± 0.03 | 1.8 ± 0.02 | |
| Total | 99.4 | 99.8 | 99.9 | 98.6 | 99.8 | 99.8 |
| MIC (mg/mL) * | ||||||
|---|---|---|---|---|---|---|
| Bacterial Strains | MpH1 | MpH2 | MpH3 | MaH1 | MaH2 | MaH3 |
| S. epidermidis | 2 | 8 | 18.5 | 10 | 10 | 10 |
| S. aureus | 10 | 12 | 20 | 10 | 10 | 10 |
| B. subtilis | 0.1 | 2.5 | 0.06 | 10 | 10 | 0.1 |
| B. cereus | 0.08 | 8 | 10 | 10 | 15 | 2 |
| T3 | T2 | T1 | Light | Hours in a Day |
|---|---|---|---|---|
| 31 | 28 | 18 | dark | 24–4 |
| 27 | 26 | 14 | dark | 4–8 |
| 37 | 33 | 24 | light | 8–13 |
| 45 | 40 | 32 | light | 13–19 |
| 38 | 33 | 25 | light | 19–24 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydari, M.; Zanfardino, A.; Taleei, A.; Shahnejat Bushehri, A.A.; Hadian, J.; Maresca, V.; Sorbo, S.; Di Napoli, M.; Varcamonti, M.; Basile, A.; et al. Effect of Heat Stress on Yield, Monoterpene Content and Antibacterial Activity of Essential Oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens. Molecules 2018, 23, 1903. https://doi.org/10.3390/molecules23081903
Heydari M, Zanfardino A, Taleei A, Shahnejat Bushehri AA, Hadian J, Maresca V, Sorbo S, Di Napoli M, Varcamonti M, Basile A, et al. Effect of Heat Stress on Yield, Monoterpene Content and Antibacterial Activity of Essential Oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens. Molecules. 2018; 23(8):1903. https://doi.org/10.3390/molecules23081903
Chicago/Turabian StyleHeydari, Milad, Anna Zanfardino, Alireza Taleei, Ali Akbar Shahnejat Bushehri, Javad Hadian, Viviana Maresca, Sergio Sorbo, Michela Di Napoli, Mario Varcamonti, Adriana Basile, and et al. 2018. "Effect of Heat Stress on Yield, Monoterpene Content and Antibacterial Activity of Essential Oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens" Molecules 23, no. 8: 1903. https://doi.org/10.3390/molecules23081903
APA StyleHeydari, M., Zanfardino, A., Taleei, A., Shahnejat Bushehri, A. A., Hadian, J., Maresca, V., Sorbo, S., Di Napoli, M., Varcamonti, M., Basile, A., & Rigano, D. (2018). Effect of Heat Stress on Yield, Monoterpene Content and Antibacterial Activity of Essential Oils of Mentha x piperita var. Mitcham and Mentha arvensis var. piperascens. Molecules, 23(8), 1903. https://doi.org/10.3390/molecules23081903








