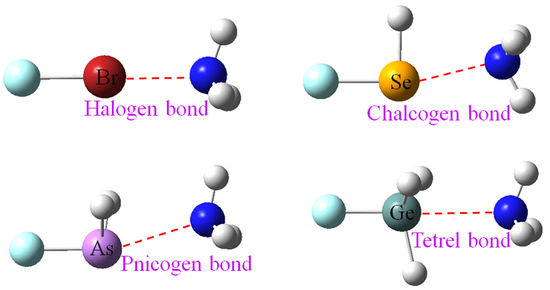
Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors
Abstract
:1. Introduction
2. Systems and Theoretical Methods
3. Results
3.1. Energies and Geometries
3.2. Analysis of the Wave Functions
3.3. 2nd Row Atoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lommerse, J.P.M.; Stone, A.J.; Taylor, R.; Allen, F.H. The nature and geometry of intermolecular interactions between halogens and oxygen or nitrogen. J. Am. Chem. Soc. 1996, 118, 3108–3116. [Google Scholar] [CrossRef]
- Alkorta, I.; Rozas, S.; Elguero, J. Charge-transfer complexes between dihalogen compounds and electron donors. J. Phys. Chem. A 1998, 102, 9278–9285. [Google Scholar] [CrossRef]
- Farina, A.; Meille, S.V.; Messina, M.T.; Metrangolo, P.; Resnati, G.; Vecchio, G. Resolution of racemic 1,2-dibromohexafluoropropane through halogen-bonded supramolecular helices. Angew. Chem. Int. Ed. Engl. 1999, 38, 2433–2436. [Google Scholar] [CrossRef]
- Wash, P.L.; Ma, S.; Obst, U.; Rebek, J. Nitrogen-halogen intermolecular forces in solution. J. Am. Chem. Soc. 1999, 121, 7973–7974. [Google Scholar] [CrossRef]
- Legon, A.C. Prereactive complexes of dihalogens XY with Lewis bases B in the gas phase: A systematic case for the halogen analogue B···XY of the hydrogen bond B···HX. Angew. Chem. Int. Ed. Engl. 1999, 38, 2686–2714. [Google Scholar] [CrossRef]
- Caronna, T.; Liantonio, R.; Logothetis, T.A.; Metrangolo, P.; Pilati, T.; Resnati, G. Halogen bonding and π···π stacking control reactivity in the solid state. J. Am. Chem. Soc. 2004, 126, 4500–4501. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Chen, N.; Wu, H.; Knotts, N.; Kaupp, M. 13C NMR study of halogen bonding of haloarenes: Measurements of solvent effects and theoretical analysis. J. Am. Chem. Soc. 2004, 126, 4412–4419. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J.; Bilewicz, E. Cooperativity halogen bonding effect—Ab initio calculations on H2CO···(ClF)n complexes. Chem. Phys. Lett. 2006, 427, 51–55. [Google Scholar] [CrossRef]
- Riley, K.E.; Merz, K.M. Insights into the strength and origin of halogen bonding: The halobenzene-formaldehyde dimer. J. Phys. Chem. A 2007, 111, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.E.; Parthasarathy, R.; Dunitz, J.D. Directional preferences of nonbonded atomic contacts with divalent sulfur. 1. Electrophiles and nucleophiles. J. Am. Chem. Soc. 1977, 99, 4860–4862. [Google Scholar] [CrossRef]
- Row, T.N.G.; Parthasarathy, R. Directional preferences of nonbonded atomic contacts with divalent sulfur in terms of its orbital orientations. 2. Sulfur···sulfur interactions and nonspherical shape of sulfur in crystals. J. Am. Chem. Soc. 1981, 103, 477–479. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Nalini, V. Database analysis of crystal-structure-determininginteractions involving sulphur: Implications for the design of organic metals. J. Mater. Chem. 1991, 1, 201–203. [Google Scholar] [CrossRef]
- Burling, F.T.; Goldstein, B.M. Computational studies of nonbonded sulfur-oxygen and selenium-oxygen interactions in the thiazole and selenazole nucleosides. J. Am. Chem. Soc. 1992, 114, 2313–2320. [Google Scholar] [CrossRef]
- Iwaoka, M.; Tomoda, S. Nature of the intramolecular Se···N nonbonded interaction of 2-selenobenzylamine derivatives. An experimental evaluation by 1H, 77Se, and 15N NMR spectroscopy. J. Am. Chem. Soc. 1996, 118, 8077–8084. [Google Scholar] [CrossRef]
- Werz, D.B.; Gleiter, R.; Rominger, F. Nanotube formation favored by chalcogen-chalcogen interactions. J. Am. Chem. Soc. 2002, 124, 10638–10639. [Google Scholar] [CrossRef] [PubMed]
- Sanz, P.; Mó, O.; Yáñez, M. Characterization of intramolecular hydrogen bonds and competitive chalcogen–chalcogen interactions on the basis of the topology of the charge density. Phys. Chem. Chem. Phys. 2003, 5, 2942–2947. [Google Scholar] [CrossRef]
- Bleiholder, C.; Werz, D.B.; Koppel, H.; Gleiter, R. Theoretical investigations on chalcogen-chalcogen interactions: What makes these nonbonded interactions bonding? J. Am. Chem. Soc. 2006, 128, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Nziko, V.d.P.N.; Scheiner, S. Chalcogen bonding between tetravalent SF4 and amines. J. Phys. Chem. A 2014, 118, 10849–10856. [Google Scholar] [CrossRef] [PubMed]
- Klinkhammer, K.W.; Pyykko, P. Ab initio interpretation of the closed-shell intermolecular E···E attraction in dipnicogen (H2E-EH2)2 and (HE-EH)2 hydride model dimers. Inorg. Chem. 1995, 34, 4134–4138. [Google Scholar] [CrossRef]
- Deiters, J.A.; Holmes, R.R. Ab initio treatment of a phosphorus coordinate, trigonal bipyramidal to pentafluoride-pyridine reaction square pyramidal to octahedral. Phosphorus Sulfur Sillicon Relat. Elem. 1997, 123, 329–340. [Google Scholar] [CrossRef]
- Scheiner, S. The pnicogen bond: Its relation to hydrogen, halogen, and other noncovalent bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.; Levason, W.; Reid, G. Arsenic (III) halide complexes with phosphine and arsine co-ligands: Synthesis, spectroscopic and structural properties. J. Chem. Soc. Dalton Trans. 2002, 1188–1192. [Google Scholar] [CrossRef]
- Scheiner, S. Effects of multiple substitution upon the P···N noncovalent interaction. Chem. Phys. 2011, 387, 79–84. [Google Scholar] [CrossRef]
- Scheiner, S. Can two trivalent N atoms engage in a direct N···N noncovalent interaction? Chem. Phys. Lett. 2011, 514, 32–35. [Google Scholar] [CrossRef]
- Rossi, A.R.; Jasinski, J.M. Theoretical studies of neutral silane-ammonia adducts. Chem. Phys. Lett. 1990, 169, 399–404. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Emilsson, T.; Jaman, A.I.; Germann, T.C.; Gutowsky, H.S. Rotational spectra, dipole moment, and structure of the SiF4–NH3dimer. J. Chem. Phys. 1992, 96, 3441–3446. [Google Scholar] [CrossRef]
- Schoeller, W.W.; Rozhenko, A. Pentacoordination at fluoro-substituted silanes by weak Lewis donor addition. Eur. J. Inorg. Chem 2000, 2000, 375–381. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Lane, P.; Concha, M.C. Electrostatically driven complexes of SiF4 with amines. Int. J. Quantum Chem. 2009, 109, 3773–3780. [Google Scholar] [CrossRef]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel bonding interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Marín-Luna, M.; Alkorta, I.; Elguero, J. A theoretical study of the HnF4−nSi:N-base (n = 1–4) tetrel-bonded complexes. Theor. Chem. Acc. 2017, 136, 41. [Google Scholar] [CrossRef]
- Geboes, Y.; De Proft, F.; Herrebout, W.A. Effect of fluorination on the competition of halogen bonding and hydrogen bonding: Complexes of fluoroiodomethane with dimethyl ether and trimethylamine. J. Phys. Chem. A 2017, 121, 4180–4188. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yang, X.; Xiao, B.; Cheng, J.; Li, Q. Comparison of hydrogen and halogen bonds between dimethyl sulfoxide and hypohalous acid: Competition and Cooperativity. Mol. Phys. 2017, 115, 1614–1623. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Sokalski, W.A. Are various σ-hole bonds steered by the same mechanisms? ChemPhysChem 2017, 18, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Karpfen, A. Theoretical characterization of the trends in halogen bonding. In Halogen Bonding. Fundamentals and Applications; Metrangolo, P., Resnati, G., Eds.; Springer: Berlin, Germany, 2008; pp. 1–15. [Google Scholar]
- Moilanen, J.; Ganesamoorthy, C.; Balakrishna, M.S.; Tuononen, H.M. Weak interactions between trivalent pnictogen centers: Computational analysis of bonding in dimers X3E···EX3 (E = Pnictogen, X = Halogen). Inorg. Chem. 2009, 48, 6740–6747. [Google Scholar] [CrossRef] [PubMed]
- Legon, A.C. The halogen bond: An interim perspective. Phys. Chem. Chem. Phys. 2010, 12, 7736–7747. [Google Scholar] [CrossRef] [PubMed]
- Zahn, S.; Frank, R.; Hey-Hawkins, E.; Kirchner, B. Pnicogen bonds: A new molecular linker? Chem. Eur. J. 2011, 17, 6034–6038. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Scheiner, S. Comparison of P···D (D = P, N) with other noncovalent bonds in molecular aggregates. J. Chem. Phys. 2011, 135, 184306. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Intermolecular weak interactions in HTeXH dimers (X = O, S, Se, Te): Hydrogen bonds, chalcogen–chalcogen contacts and chiral discrimination. ChemPhysChem 2012, 13, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Scheiner, S. Effects of carbon chain substituent on the P···N noncovalent bond. Chem. Phys. Lett. 2012, 536, 30–33. [Google Scholar] [CrossRef]
- Sánchez-Sanz, G.; Trujillo, C.; Solimannejad, M.; Alkorta, I.; Elguero, J. Orthogonal interactions between nitryl derivatives and electron donors: Pnictogen bonds. Phys. Chem. Chem. Phys. 2013, 15, 14310–14318. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Hobza, P. The relative roles of electrostatics and dispersion in the stabilization of halogen bonds. Phys. Chem. Chem. Phys. 2013, 15, 17742–17751. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, R.; Stasyuk, O.A.; Fonseca Guerra, C.; Řezáč, J.; Růžička, A.; Hobza, P. New insight into the nature of bonding in the dimers of Lappert’s stannylene and its Ge analogs: A quantum mechanical study. J. Chem. Theory Comput. 2016, 12, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. σ-Hole opposite to a lone pair: Unconventional pnicogen bonding interactions between ZF3 (Z = N, P, As, and Sb) compounds and several donors. ChemPhysChem 2016, 17, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Chopra, D. Understanding the effect of substitution on the formation of S···F chalcogen bond. J. Chem. Sci. 2016, 128, 1589–1596. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Kiani, H.; Mohammadian-Sabet, F. Tuning of carbon bonds by substituent effects: An ab initio study. Mol. Phys. 2016, 114, 3658–3668. [Google Scholar] [CrossRef]
- Legon, A.C. Tetrel, pnictogen and chalcogen bonds identified in the gas phase before they had names: Asystematic look at non-covalent interactions. Phys. Chem. Chem. Phys. 2017, 19, 14884–14896. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.C.; Wright, J.S.; Carrington, E.J.; Perutz, R.N.; Hunter, C.A.; Brammer, L. Hydrogen bonding vs. halogen bonding: The solvent decides. Chem. Sci. 2017, 8, 5392–5398. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-X.; Li, Q.-Z.; Scheiner, S. Comparison of tetrel bonds in neutraland protonated complexes of pyridineTF3 and furanTF3 (T = C, Si, and Ge) with NH3. Phys. Chem. Chem. Phys. 2017, 19, 5550–5559. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen bonds, and σ-hole and π-hole bonds—Mechanisms protecting doublet and octet electron structures. Phys. Chem. Chem. Phys. 2017, 19, 29742–29759. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Detailed comparison of the pnicogen bond with chalcogen, halogen and hydrogen bonds. Int. J. Quantum Chem. 2013, 113, 1609–1620. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Fasulo, M.; Schultheiss, N.; Desper, J.; Moore, C. Structural competition between hydrogen bonds and halogen bonds. J. Am. Chem. Soc. 2007, 129, 13772–13773. [Google Scholar] [CrossRef] [PubMed]
- Solimannejad, M.; Malekani, M.; Alkorta, I. Cooperativity between the hydrogen bonding and halogen bonding in F3CX···NCH(CNH)···NCH(CNH) complexes (X = Cl, Br). Mol. Phys. 2011, 109, 1641–1648. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Alkorta, I.; Sanchez-Sanz, G.; Elguero, J. 31P–31P Spin–spin coupling constants for pnicogen homodimers. Chem. Phys. Lett. 2011, 512, 184–187. [Google Scholar] [CrossRef]
- Grabowski, S.J. QTAIM characteristics of halogen bond and related interactions. J. Phys. Chem. A 2012, 116, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen and halogen bonds are ruled by the same mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 7249–7259. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Sensitivity of noncovalent bonds to intermolecular separation: Hydrogen, halogen, chalcogen, and pnicogen bonds. CrystEngComm 2013, 15, 3119–3124. [Google Scholar] [CrossRef]
- Riley, K.E.; Rezác, J.; Hobza, P. Competition between halogen, dihalogen and hydrogen bonds in bromo- and iodomethanol dimers. J. Mol. Model. 2013, 19, 2879–2883. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, Q. Interplay between tetrel bonding and hydrogen bonding interactions in complexes involving F2XO (X = C and Si) and HCN. Comput. Theor. Chem. 2014, 1050, 51–57. [Google Scholar] [CrossRef]
- McDowell, S.A.C.; Holder, Z.L. Computational study of non-covalent interactions in oxirane···XF complexes (X = H, F, Cl, Br, Li) and their F-/Li-substituted analogues. Mol. Phys. 2015, 113, 3757–3766. [Google Scholar] [CrossRef]
- Scheiner, S. Comparison of CH···O, SH···O, chalcogen, and tetrel bonds formed by neutral and cationic sulfur-containing compounds. J. Phys. Chem. A 2015, 119, 9189–9199. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Del Bene, J.; Elguero, J. H2XP:OH2 Complexes: Hydrogen vs. pnicogen bonds. Crystals 2016, 6, 19. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Holub, J.; Růžičková, Z.; Řezáč, J.; Lane, P.D.; Wann, D.A.; Hnyk, D.; Růžička, A.; Hobza, P. Competition between halogen, hydrogen and dihydrogen bonding in brominated carboranes. ChemPhysChem 2016, 17, 3373–3376. [Google Scholar] [CrossRef] [PubMed]
- Azofra, L.M.; Scheiner, S. Tetrel, chalcogen, and CH···O hydrogen bonds in complexes pairing carbonyl-containing molecules with 1, 2, and 3 molecules of CO2. J. Chem. Phys. 2015, 142, 034307. [Google Scholar] [CrossRef] [PubMed]
- Domagała, M.; Lutyńska, A.; Palusiak, M. Halogen bond versus hydrogen bond: The many-body interactions approach. Int. J. Quantum Chem. 2017, 117, e25348. [Google Scholar] [CrossRef]
- Sánchez–Sanz, G.; Alkorta, I.; Elguero, J. Theoretical study of intramolecular interactions in peri-substituted naphthalenes: Chalcogen and hydrogen bonds. Molecules 2017, 22, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lu, Y.; Zhu, Z.; Liu, H. Theoretical exploration of halogen bonding interactions in the complexes of novel nitroxide radical probes and comparison with hydrogen bonds. J. Phys. Chem. A 2018, 122, 5058–5068. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H.J. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. V. Core-valence basis sets for boron through neon. J. Chem. Phys. 1995, 103, 4572–4585. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Latajka, Z.; Scheiner, S. Primary and secondary basis set superposition error at the SCF and MP2 levels: H3N–Li+ and H2O–Li+. J. Chem. Phys. 1987, 87, 1194–1204. [Google Scholar] [CrossRef]
- Bulat, F.A.; Toro-Labbé, A.; Brinck, T.; Murray, J.S.; Politzer, P. Quantitative analysis of molecular surfaces: Areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 2010, 16, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules, A Quantum Theory; Clarendon Press: Oxford, UK, 1990; p. 438. [Google Scholar]
- Bader, R.F.W. AIM2000; 2.0; McMaster University: Hamilton, ON, Canada, 2000. [Google Scholar]
- Su, P.; Li, H. Energy decomposition analysis of covalent bonds and intermolecular interactions. J. Chem. Phys. 2009, 131, 014102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Bauzá, A.; Quiñonero, D.; Deyà, P.M.; Frontera, A. Halogen bonding versus chalcogen and pnicogen bonding: Acombined Cambridge structural database and theoretical study. CrystEngComm 2013, 15, 3137–3144. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, J.; Li, Q.; Li, W. Competition of hydrogen, halogen, and pnicogen bonds in the complexes of HArF with XH2P (X = F, Cl, and Br). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 101, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Chopra, D. “Pnicogen bonds” or “chalcogen bonds”: Exploiting the effect of substitution on the formation of P···Se noncovalent bonds. Phys. Chem. Chem. Phys. 2016, 18, 13820–13829. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; An, X.; Li, Q. Se···N Chalcogen bond and Se···X halogen bond involving F2C=Se: Influence of hybridization, substitution, and cooperativity. J. Phys. Chem. A 2015, 119, 3518–3527. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Akhgarpour, H. An ab initio study on competition between pnicogen and chalcogen bond interactions in binary XHS:PH2X complexes (X = F, Cl, CCH, COH, CH3, OH, OCH3 and NH2). Mol. Phys. 2016, 114, 1847–1855. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadirad, N. Characterization of σ-hole interactions in 1:1 and 1:2 complexes of YOF2X (X = F, Cl, Br, I; Y = P, As) with ammonia: Competition between halogen and pnicogen bonds. Struct. Chem. 2016, 27, 939–946. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mohammadian-Sabet, F. σ-Hole bond tunability in YO2X2:NH3 and YO2X2:H2O complexes (X = F, Cl, Br; Y = S, Se): Trends and theoretical aspects. Struct. Chem. 2016, 27, 617–625. [Google Scholar] [CrossRef]
- Jiao, Y.; Liu, Y.; Zhao, W.; Wang, Z.; Ding, X.; Liu, H.; Lu, T. Theoretical study on the interactions of halogen-bonds and pnicogen-bonds in phosphine derivatives with Br2, BrCl, and BrF. Int. J. Quantum Chem. 2017, 117, e25443. [Google Scholar] [CrossRef]
- Benz, S.; Poblador-Bahamonde, A.I.; Low-Ders, N.; Matile, S. Catalysis with pnictogen, chalcogen, and halogen bonds. Angew. Chem. Int. Ed. 2018, 57, 5408–5412. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. On the properties of X···N noncovalent interactions for first-, second- and third-row X atoms. J. Chem. Phys. 2011, 134, 164313. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Scheiner, S. The S···N noncovalent interaction: Comparison with hydrogen and halogen bonds. Chem. Phys. Lett. 2011, 514, 36–39. [Google Scholar] [CrossRef]
- Nepal, B.; Scheiner, S. Long-range behavior of noncovalent bonds. Neutral and charged H-bonds, pnicogen, chalcogen, and halogen bonds. Chem. Phys. 2015, 456, 34–40. [Google Scholar] [CrossRef]
- Adhikari, U.; Scheiner, S. Sensitivity of pnicogen, chalcogen, halogen and H-bonds to angular distortions. Chem. Phys. Lett. 2012, 532, 31–35. [Google Scholar] [CrossRef]
- Adhikari, U.; Scheiner, S. Substituent effects on Cl···N, S···N, and P···N noncovalent bonds. J. Phys. Chem. A 2012, 116, 3487–3497. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Assembly of effective halide receptors from components. Comparing hydrogen, halogen, and tetrel bonds. J. Phys. Chem. A 2017, 121, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Highly selective halide receptors based on chalcogen, pnicogen, and tetrel bonds. Chem. Eur. J. 2016, 22, 18850–18858. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Comparison of halide receptors based on H, halogen, chalcogen, pnicogen, and tetrel bonds. Faraday Discuss. Chem. Soc. 2017, 203, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.; Lu, J. Halogen, chalcogen, and pnicogen bonding involving hypervalent atoms. Chem. Eur. J. 2018, 24, 8167–8177. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Lewis Acid | Eint,MP2 | Eint,CCSD(T) | Eb,MP2 | R | θ(R-A···N) |
|---|---|---|---|---|---|
| H-HnA | |||||
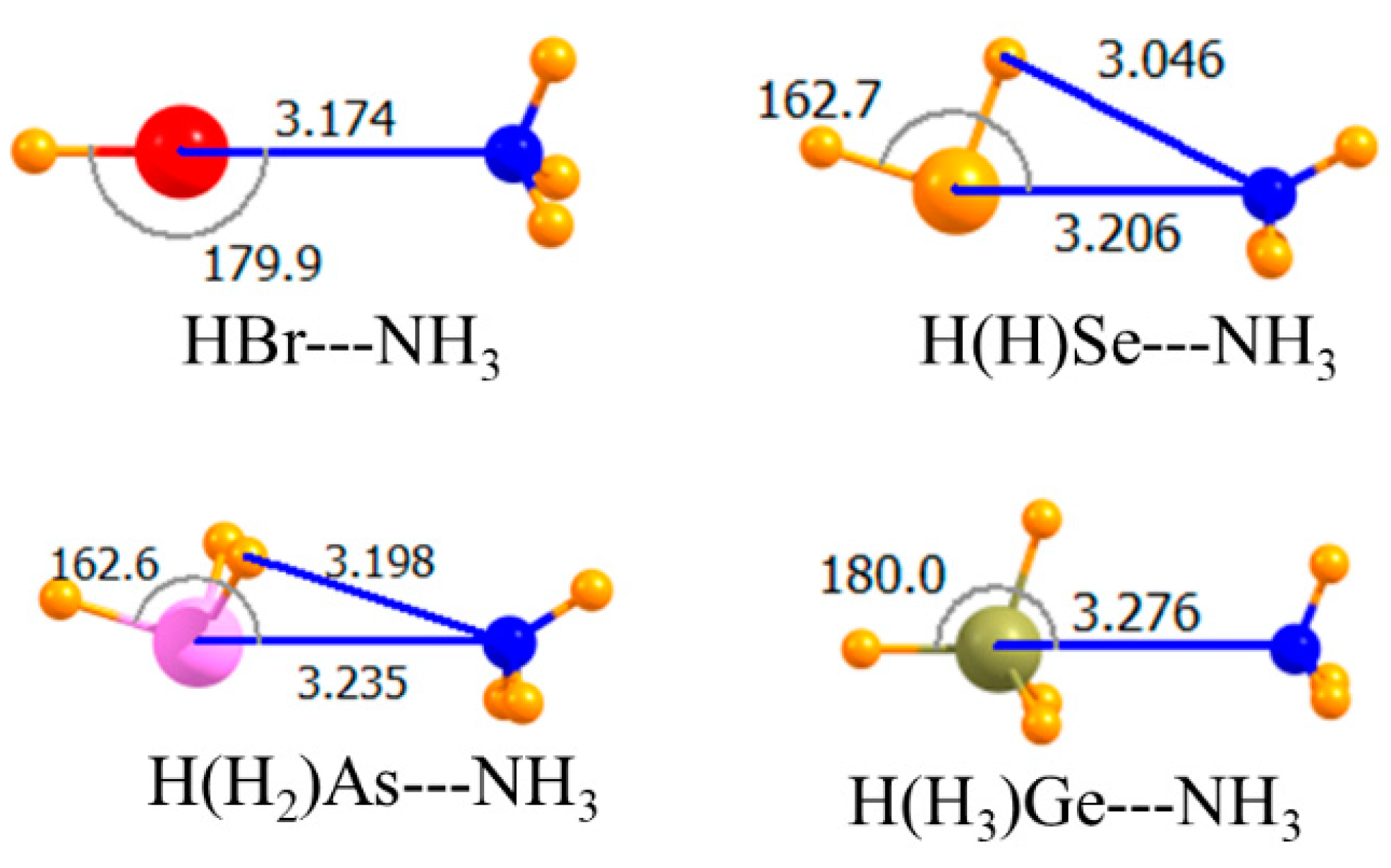
| HBr | −7.79 | −7.57 | −7.77 | 3.174 | 179.9 |
| H(H)Se | −9.08 | −8.68 | −8.98 | 3.206 | 162.7 |
| H(H2)As | −7.10 | −6.81 | −7.02 | 3.235 | 162.6 |
| H(H3)Ge | −6.83 | −7.07 | −6.42 | 3.276 | 180.0 |
| Me-HnA | |||||
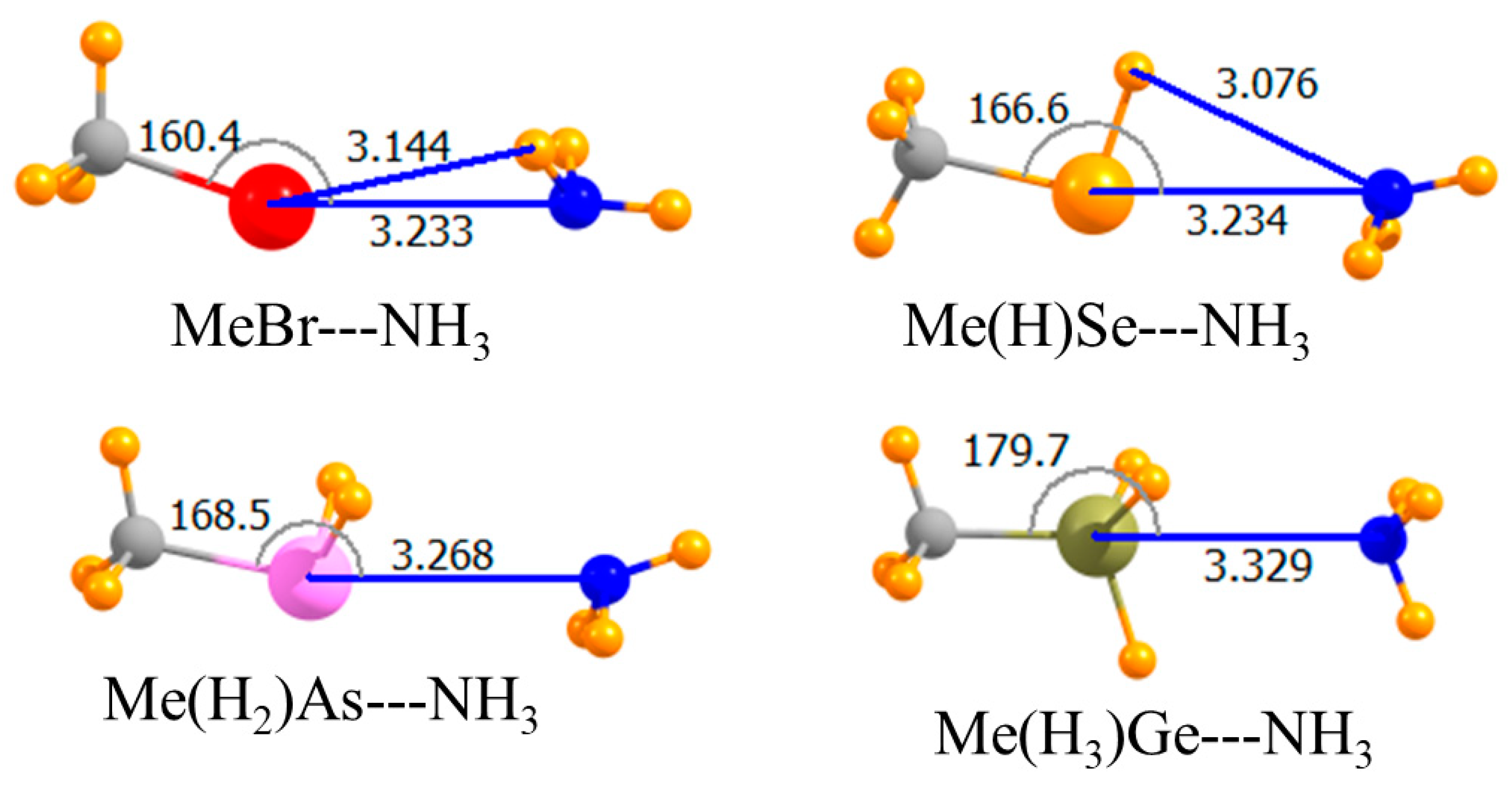
| MeBr | −5.01 | −4.57 | −4.99 | 3.233 | 160.4 |
| Me(H)Se | −7.90 | −7.50 | −7.82 | 3.234 | 166.6 |
| Me(H2)As | −6.27 | −6.09 | −6.19 | 3.268 | 168.5 |
| Me(H3)Ge | −5.29 | −5.71 | −4.90 | 3.329 | 179.7 |
| H-FnA | |||||
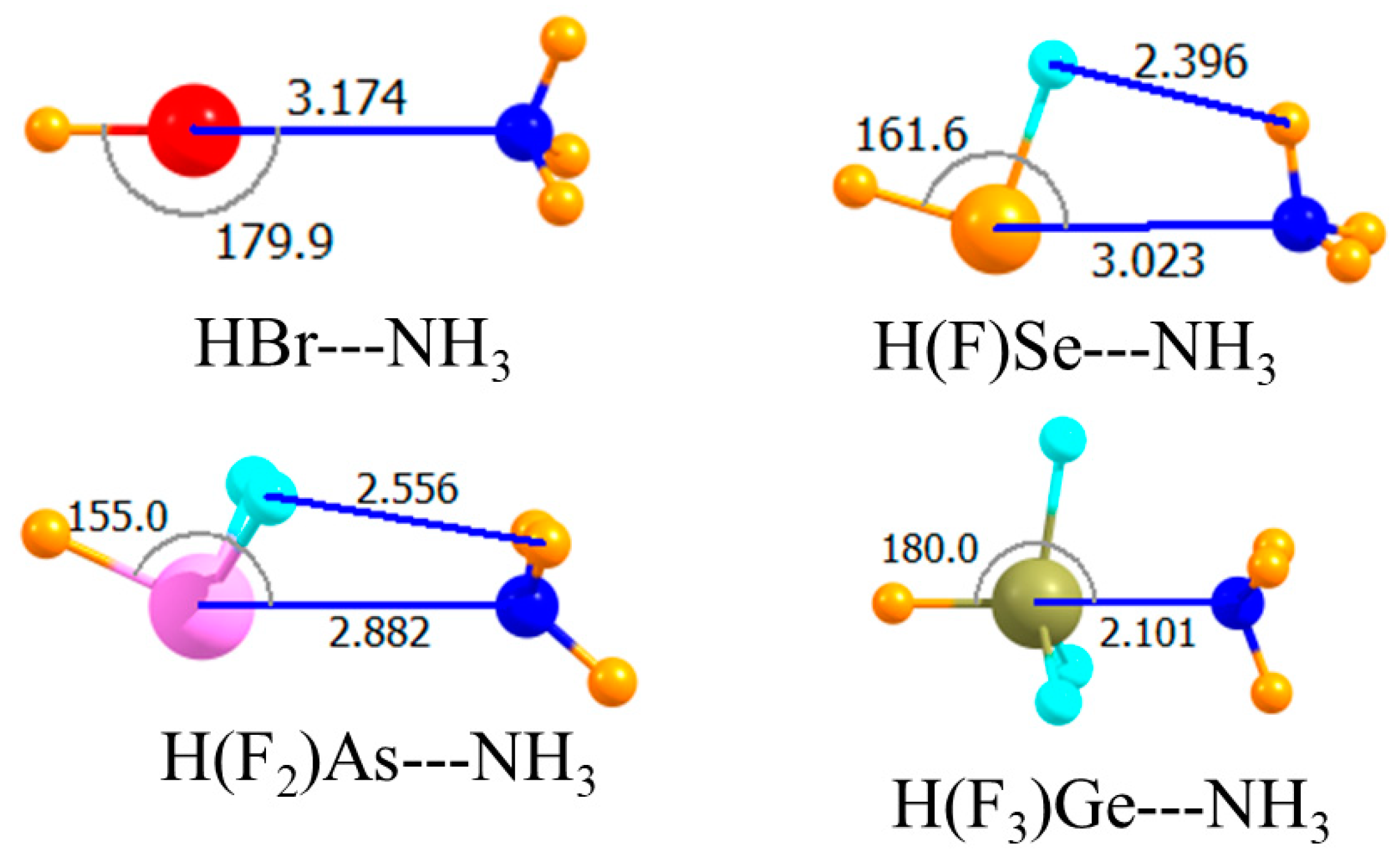
| H(F)Se | −14.43 | −14.08 | −13.89 | 3.023 | 161.6 |
| H(F2)As | −18.35 | −18.36 | −17.11 | 2.882 | 155.0 |
| H(F3)Ge | −120.73 | −122.35 | −35.04 | 2.101 | 180.0 |
| Me-FnA | |||||
| Me(F)Se | −11.86 | −11.66 | −11.46 | 3.114 | 162.7 |
| Me(F2)As | −14.44 | −14.73 | −13.64 | 3.021 | 155.3 |
| Me(F3)Ge | −111.65 | −111.27 | −25.77 | 2.115 | 180.0 |
| F-HnA | |||||
| FBr | −67.87 | −59.18 | −61.71 | 2.293 | 180.0 |
| F(H)Se | −49.25 | −44.35 | −45.97 | 2.423 | 169.6 |
| F(H2)As | −34.57 | −32.30 | −32.76 | 2.597 | 165.3 |
| F(H3)Ge | −30.93 | −30.59 | −25.28 | 2.640 | 179.9 |
| Lewis Acid | Vs,max au | CT me | E(2) a kcal/mol | E(2) b kcal/mol | Δr(A-R) Å | ∆ν(A-R) cm−1 |
|---|---|---|---|---|---|---|
| H-HnA | ||||||
| HBr | 0.027 | 2 | 11.37 | - | 0.004 | −26.8 |
| H(H)Se | 0.030 | 4 | 8.99 | 0.54 | 0.003 | −107.6 |
| H(H2)As | 0.028 | 7 | 10.70 | 2.51 | 0.006 | −5.4 |
| H(H3)Ge | 0.032 | 9 | 12.37 | 6.65 | 0.008 | +1.2 |
| Me-HnA | ||||||
| MeBr | 0.030 | 1 | 3.26 | - | 0.001 | −1.3 |
| Me(H)Se | 0.018 | 2 | 5.10 | 0.25 | 0.001 | −0.7 |
| Me(H2)As | 0.019 | 4 | 7.77 | 1.67 | 0.003 | −2.2 |
| Me(H3)Ge | 0.024 | 8 | 11.87 | 5.02 | 0.007 | −10.1 |
| H-FnA | ||||||
| H(F)Se | 0.036 | 8 | 14.00 | 4.26 | 0.007 | −38.1 |
| H(F2)As | 0.046 | 27 | 15.34 | 14.46 | 0.009 | −44.8 |
| H(F3)Ge | 0.069 | 175 | 86.19 | 416.95 | 0.017 | −2.2 |
| Me-FnA | ||||||
| Me(F)Se | 0.022 | 5 | 8.95 | 2.63 | 0.005 | −6.7 |
| Me(F2)As | 0.033 | 7 | 8.03 | 7.65 | 0.009 | −35.7 |
| Me(F3)Ge | 0.055 | 172 | 80.47 | 417.54 | 0.021 | −101.6 |
| F-HnA | ||||||
| FBr | 0.093 | 143 | 255.40 | - | 0.070 | −114.9 |
| F(H)Se· | 0.089 | 90 | 152.82 | 11.03 | 0.049 | −107.6 |
| F(H2)As | 0.079 | 53 | 82.81 | 14.96 | 0.033 | −60.2 |
| F(H3)Ge | 0.077 | 46 | 59.11 | 39.12 | 0.027 | −63.3 |
| Lewis Acid | ρ | ∇²ρ | H |
|---|---|---|---|
| H-HnA | |||
| HBr | 0.010 | 0.038 | 0.002 |
| H(H)Se | 0.009 | 0.034 | 0.001 |
| H(H2)As | 0.009 | 0.029 | 0.001 |
| H(H3)Ge | 0.008 | 0.025 | 0.001 |
| Me-HnA | |||
| MeBr | 0.008 | 0.034 | 0.002 |
| Me(H)Se | 0.008 | 0.032 | 0.002 |
| Me(H2)As | 0.008 | 0.027 | 0.001 |
| Me(H3)Ge | 0.007 | 0.023 | 0.001 |
| H-FnA | |||
| H(F)Se | 0.014 | 0.046 | 0.001 |
| H(F2)As | 0.018 | 0.046 | 0.000 |
| H(F3)Ge | 0.077 | 0.216 | −0.030 |
| Me-FnA | |||
| Me(F)Se | 0.011 | 0.039 | 0.002 |
| Me(F2)As | 0.013 | 0.037 | 0.001 |
| Me(F3)Ge | 0.075 | 0.209 | −0.028 |
| F-HnA | |||
| FBr | 0.061 | 0.132 | −0.015 |
| F(H)Se· | 0.044 | 0.105 | −0.007 |
| F(H2)As | 0.029 | 0.074 | −0.002 |
| F(H3)Ge | 0.023 | 0.072 | 0.000 |
| Lewis Acid | Eele | Eex | Erep | Epol | Edisp | Eint |
|---|---|---|---|---|---|---|
| H-HnA | ||||||
| HBr | −16.30 | −10.29 | 49.53 | −4.81 | −7.27 | −7.90 |
| H(H)Se | −16.85 | −10.29 | 48.61 | −4.14 | −7.69 | −9.11 |
| H(H2)As | −16.18 | −32.02 | 52.88 | −4.43 | −7.40 | −7.15 |
| H(H3)Ge | −16.80 | −32.65 | 53.63 | −4.64 | −6.40 | −6.86 |
| Me-HnA | ||||||
| MeBr | −8.15 | −7.53 | 35.57 | −2.34 | −8.78 | −5.02 |
| Me(H)Se | −12.79 | −9.04 | 42.39 | −3.18 | −8.74 | −7.86 |
| Me(H2)As | −13.00 | −28.72 | 47.23 | −3.55 | −8.23 | −6.31 |
| Me(H3)Ge | −13.29 | −29.59 | 48.03 | −4.14 | −6.40 | −5.35 |
| H-FnA | ||||||
| H(F)Se | −34.19 | −19.00 | 93.92 | −9.91 | −10.66 | −14.50 |
| H(F2)As | −55.05 | −83.77 | 149.44 | −17.56 | −11.58 | −18.52 |
| H(F3)Ge | −363.74 | −371.98 | 782.50 | −165.65 | −2.17 | −121.05 |
| Me-FnA | ||||||
| Me(F)Se | −25.67 | −15.70 | 76.37 | −7.44 | −10.87 | −11.95 |
| Me(F2)As | −36.91 | −59.31 | 103.54 | −10.41 | −11.50 | −14.59 |
| Me(F3)Ge | −352.88 | −369.72 | 773.13 | −158.21 | −4.31 | −111.94 |
| F-HnA | ||||||
| FBr | −197.84 | −97.95 | 554.14 | −120.05 | −27.67 | −68.09 |
| F(H)Se | −146.89 | −75.42 | 407.59 | −74.03 | −23.12 | −49.49 |
| F(H2)As | −98.61 | −147.64 | 269.90 | −40.67 | −17.85 | −34.86 |
| F(H3)Ge | −89.20 | −125.82 | 228.60 | −31.27 | −13.42 | −31.10 |
| Lewis Acid | Eint,MP2 | Eint,CCSD(T) | Eb,MP2 | R | θ(R-A···N) |
|---|---|---|---|---|---|
| H-HnA | |||||
| HCl | −3.77 | −3.73 | −3.77 | 3.254 | 156.5 |
| H(H)S a | --- | --- | --- | --- | --- |
| H(H2)P | −7.01 | −6.47 | −6.94 | 3.281 | 166.3 |
| H(H3)Si | −8.75 | −8.87 | −8.05 | 3.187 | 180.0 |
| Me-HnA | |||||
| MeCl | −4.01 | −3.81 | −3.98 | 3.409 | 145.2 |
| Me(H)S a | --- | --- | --- | --- | --- |
| Me(H2)P | −6.57 | −6.42 | −6.50 | 3.294 | 171.2 |
| Me(H3)Si | −6.38 | −6.74 | −5.77 | 3.257 | 180.0 |
| H-FnA | |||||
| H(F)S | −11.12 | −9.11 | −8.82 | 3.176 | 164.0 |
| H(F2)P | −11.41 | −11.79 | −10.93 | 3.051 | 159.0 |
| H(F3)Si | −106.23 | −108.75 | −17.19 | 2.099 | 180.0 |
| Me-FnA | |||||
| Me(F)S | −7.09 | −7.29 | −6.92 | 3.315 | 164.2 |
| Me(F2)P | −8.25 | −8.84 | −7.95 | 3.247 | 157.0 |
| Me(F3)Si | −12.06 | −13.51 | −7.57 | 3.086 | 179.8 |
| F-HnA | |||||
| FCl | −53.34 | −43.90 | −45.78 | 2.231 | 180.0 |
| F(H)S | −37.36 | −33.14 | −34.98 | 2.435 | 171.0 |
| F(H2)P | −28.34 | −26.42 | −27.12 | 2.604 | 167.7 |
| F(H3)Si | −35.26 | −34.76 | −25.01 | 2.489 | 180.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Li, Q.; Scheiner, S. Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors. Molecules 2018, 23, 1681. https://doi.org/10.3390/molecules23071681
Dong W, Li Q, Scheiner S. Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors. Molecules. 2018; 23(7):1681. https://doi.org/10.3390/molecules23071681
Chicago/Turabian StyleDong, Wenbo, Qingzhong Li, and Steve Scheiner. 2018. "Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors" Molecules 23, no. 7: 1681. https://doi.org/10.3390/molecules23071681