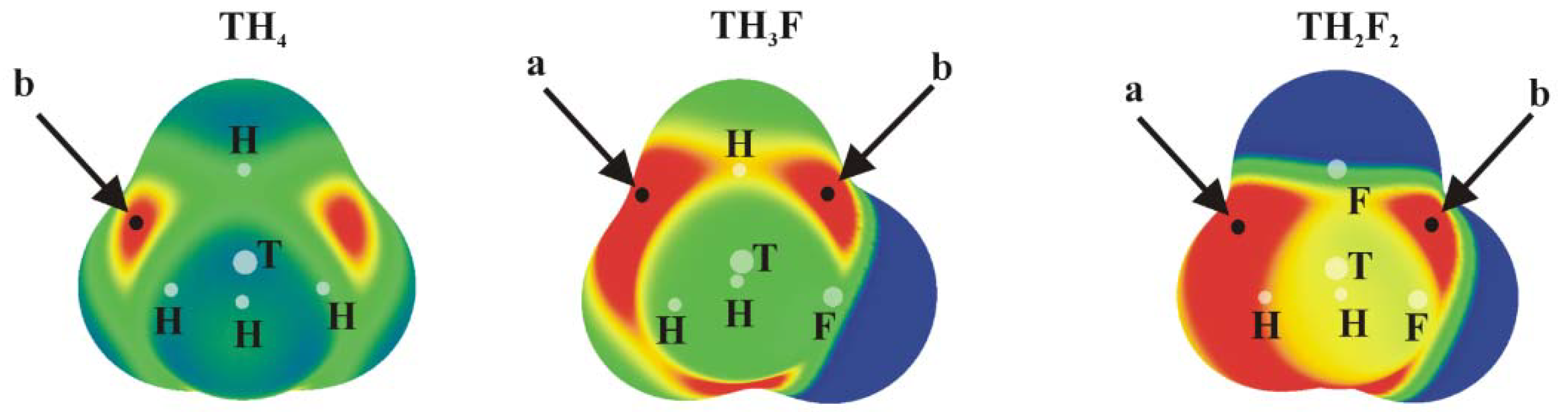
3.1. Electrostatic Potentials of Isolated Molecules
The molecular electrostatic potential (MEP) of the tetravalent, approximately tetrahedral TH
4, TH
3F and TH
2F
2 (T = Si, Ge or Sn) isolated molecules are displayed in
Figure 1; analogous MEPs are shown in
Figure 2 for the trivalent TH
2-nF
n=CH
2 analogues which are roughly planar.
Positive values of the MEP are denoted in red, while blue represents negative regions. Each of the tetrahedral molecules in
Figure 1 contains four σ-holes lying on the extension of each of the four covalent bonds. Due to its symmetry, all four of these MEP maxima are equivalent in TH
4. There are two types of maxima in the fluorosubstituted species: those opposite F are labeled a, and the b designation is applied to those opposite a H atom. The values of these maxima are collected in
Table 1, where it is immediately obvious that a σ-holes opposite F atoms are more intense than their b analogues opposite the H atom. This pattern is consonant with the much greater electronegativity of F; the ratio of a/b values of V
s,max varies between 1.4 and 1.8, and their numerical values are consistent with previous studies [
24]. Another expected pattern evident in
Table 1 is the increase in V
s,max as progressively more F atoms are added to the molecule. One normally expects the hole to intensify as the tetrel atom is enlarged. While Sn certainly corresponds to the largest values of V
s,max, Si and Ge are less distinct from one another.
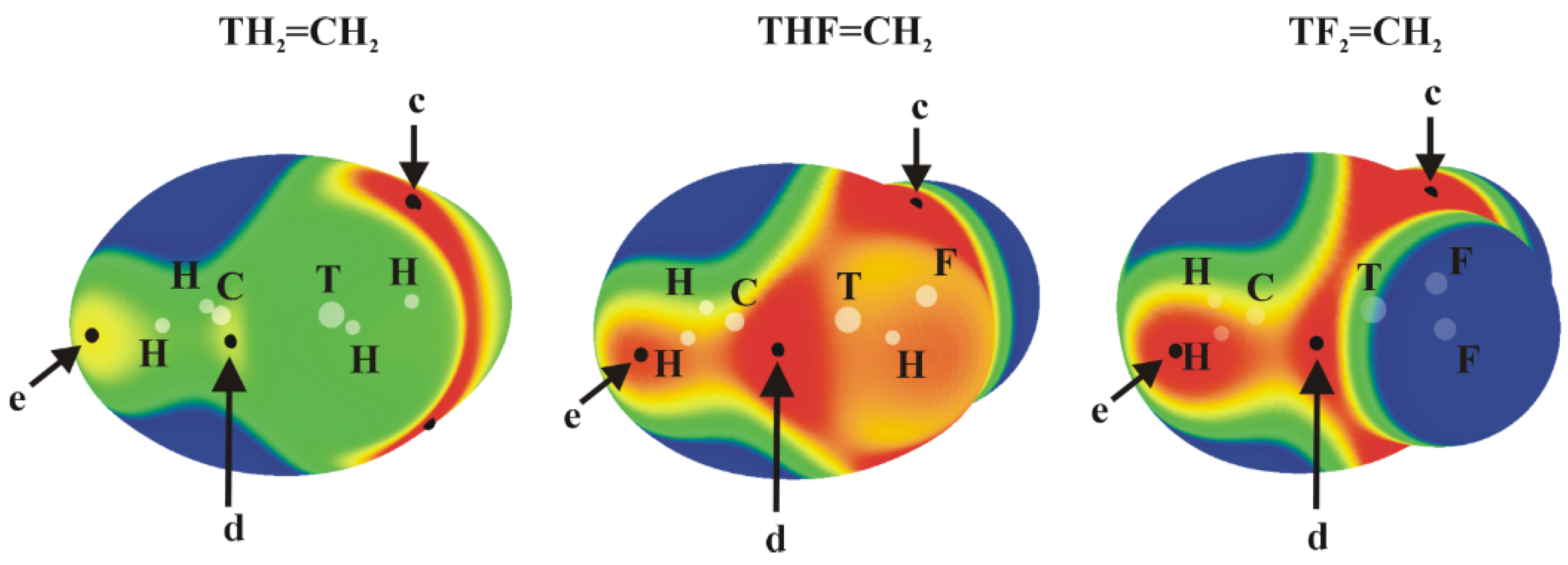
The planar TH
2-nF
n=CH
2 molecules contain three primary types of MEP maximum as shown in
Figure 2 (their values are given in
Table 2). The first, and generally the most intense, is labeled c and occurs roughly above (and below) the T atom, skewed away from the C atom by a certain amount. Maximum d lies in the molecular plane, in a position corresponding roughly to the C=T bond midpoint, approximately on an extension of the T–H or T–F covalent bond.
In most cases, with the sole exceptions of GeHF=CH
2, and SnHF=CH
2, maximum c is considerably more positive than is d (see below for further discussion). The last maximum e is associated with the two CH
2 protons. This position would be pertinent to the formation of any possible CH···N H-bonds with an approaching NH
3 nucleophile. (Several other maxima appear in some of these molecules but are much weaker in intensity.) Focusing on maximum c, the site of the π-hole, one sees a clear intensification as H atoms are replaced by F. On the other hand, the expected trend of growing intensity with tetrel atom size is violated. Although Sn does indeed produce the largest π-holes, Si exceeds its larger Ge congener. The d patterns are more consistent with expectations, with the caveat that the addition of the second F atom reduces V
s,max. This lowering is sensible because the proximity of the very electronegative F atom to the hole would mitigate against its positive value. It might be noted here that several of the molecules in
Figure 2 are not strictly planar. This point will be discussed in greater detail below.
Finally, with respect to the ammonia molecule, the value of Vs, min on the N atom at its lone pair position is −37.7 kcal/mol. Based on the positions and intensities of the various σ-holes, one would anticipate that a nucleophile such as NH3 would be attracted to the a maximum, directly opposite the F atom if one is present, and that the strongest tetrel bonds would occur for T=Sn, followed by Ge and then by Si; TH2F2 ought to engage in a slightly stronger bond than would TH3F.
3.2. σ-Hole Bonded Dimers
The optimized geometries in which NH
3 engages with the σ-holes of the tetravalent TH
4, TH
3F and TH
2F
2 molecules are illustrated in
Figure 3. Consistent with the labeling in
Figure 1a,b designate whether the N is located opposite the F or H atom, respectively. The interaction energies (E
int), corrected for BSSE, are collected in
Table 3, along with the deformation energies (E
def) of the subunits as well as selected intermolecular geometrical parameters.
The presence of a tetrel bond is signaled first by the intermolecular R(N∙∙∙T) distance which is smaller than the sum of the corresponding van der Waals radii. (This sum is equal to 3.85, 3.95 and 4.08 Å for Si, Ge and Sn, respectively.) The N atom lies very nearly directly opposite the F atom of the Lewis acid in the a dimers. The θ(R–T∙∙∙N) angle in the last column of
Table 3 is 180°, with the exception of TH
2F
2. Larger deviations of the θ(HT∙∙∙N) angles from linearity are observed for the b complexes. These nonlinearities are due to attractive forces between the F and H atoms of the Lewis acid and base, respectively.
The interaction energies vary between less than 2 kcal/mol for the TH
4 molecules to as much as 20 kcal/mol for the difluorinated Lewis acids. The patterns match those of V
s, max in
Table 1, although imperfectly. In the first place, a dimers with the base opposite F are more strongly bound than b complexes opposite H, but this trend is reversed for GeH
2F
2 and SnH
2F
2. Whether a or b type, E
int rises in the order Si~Ge < Sn, and also increases as more F atoms are added to the acid.
In order to more fully understand the nature of the tetrel bond, and the effects that factor into it, one must first recognize that the formation of such a bond relies on a certain amount of distortion of the monomer geometry. The crowded nature of the tetravalent bonding surrounding the tetrel atom impedes the approach of a nucleophile. Three of the substituents must be peeled back away from this nucleophile to facilitate its approach, which in turn produces a certain amount of deformation energy within the molecule. The magnitude of this deformation energy is listed in
Table 3 as E
def A for the acid. The NH
3 molecule need undergo only very little internal deformation so E
def B is quite small. E
def A is very small for the unsubstituted TH
4 molecules, not surprising in view of the long intermolecular separations of more than 3 Å. Monofluorination brings the N in much closer, to about 2.6 Å for the a dimers, and the deformation energies are thus larger, nearly 2 kcal/mol. The intermolecular distance is shorter after difluorination and E
def A is correspondingly larger, 4–5 kcal/mol. Note that some of the b dimers have an even closer approach, and thus a correspondingly higher deformation energy.
These energies can be correlated to the geometrical changes within the monomers. Summation of the three θ(R
1TR
2) angles of the R substituents that come into contact with the nucleophile offers a convenient measure of these distortions. On one extreme, in a fully tetrahedral environment, this sum would be equal to three times 109.5° or 328.5°, which would change to 360° if these three substituents peel back to lie in a plane in a bipyramidal arrangement. This measure of the geometry is listed in
Table 4 along with the amount it changes as a result of complexation with NH
3. Note that there is a very strong correlation between the latter change and deformation energy E
def A in
Table 3. In fact, the correlation coefficient is 0.999. In either case, the quantity is larger for b than for the a complex for Ge and Sn.
One would expect that the MEPs of these molecules would likewise be altered by the geometrical distortions accompanying dimerization. The effect of the deformation upon the value of V
s, max is reported in the last three columns of
Table 4 where it may be seen that the partial planarization yields fairly large increases in the MEP maximum, as much as 35 kcal/mol. On a percentage basis, these increases vary from 28% to a near doubling. Note also that the deformation-induced V
s, max increase is especially large for the b dimers of Ge and Sn. And it is in just these complexes that one sees an anomalously large interaction energy. On the other hand, it is not just the b geometries for which V
s, max grows upon deformation.
The MEP maximum rises also in the a structures, albeit by not as much in the Ge and Sn cases. As a net result, V
s,max is larger for a than for b in all of the complexes in
Table 4, so one cannot explain the larger interaction energies for the latter solely in terms of MEP. There are of course other aspects of the interaction besides electrostatic attraction.
Table 5 presents other components based on an EDA analysis, viz. orbital interaction E
oi and dispersion E
disp. E
elec contributes a fairly consistent 52–65% of the total attractive force, differing little between a and b structures. Dispersion makes a smaller contribution, especially in the more strongly bound dimers where it amounts to only about 5%. The orbital interaction term is perhaps more interesting, particularly for the TH
2F
2 systems. Parallel to the full E
def, E
oi is larger for the b dimers than for a for both T=Ge and Sn, but the reverse is true for T=Si. It would thus appear that a large part of this pattern can be traced to orbital interactions.
This supposition is confirmed by NBO analysis of the charge transfer.
Table S1 demonstrates that two measures of charge transfer conform to the trends listed above. The total intermolecular charge transfer CT is computed as the sum of atomic charge on either monomer. ΣE(2) represents the energetic consequence of transfers from particular molecular orbitals, in this case from the N lone pair to the four antibonding σ*(T–R) orbitals. Both of these parameters are larger for the b than for the a dimer for Ge and Sn, but smaller for Si. And furthermore, they are also larger for a than for b for all the monofluorinated TH
3F molecules, as was the case for the full interaction energy.
An alternate means of analyzing the molecular interactions derives from AIM treatment of the topology of the total electron density. Diagrams of the various dimers are provided in
Figure S1 for the illustrative Ge set of dimers where small green dots indicate the position of bond critical points. The density, density Laplacian, and total electron energy at the intermolecular bond critical points are collected in
Table S2. It might first be noted that there are certain anomalies in this data. In addition to the expected T···N bond paths, there are a number of bond paths placed by AIM between N and certain F atoms of the Lewis acid. Such bonds are reported only for the b type dimers, but not in all cases. The presence of a true N···F bond would contribute to the stability of these geometries. In one case, SiH
2F
2(b), a bond path connects N with one of the H atoms of the Lewis acid. Indeed in this case, AIM does not provide evidence of a T···N tetrel bond at all. Dispensing with these anomalies, there are patterns in the AIM data that are consistent with the full energetics. The AIM measures of the Ge···N and Sn···N tetrel bonds in TH
2F
2(b) are larger than those for the a analogue, while the opposite may be said for all three TH
3F dimers.
In summary, the σ-hole directly opposite the F atom is consistently much more positive than one opposite H. Nonetheless, due to a combination of factors, that include deformation-induced intensification, and a greater degree of charge transfer, the latter position becomes competitive with the former as a site for tetrel bonding, and can even surpass the location opposite F as a preferred binding site in certain cases.
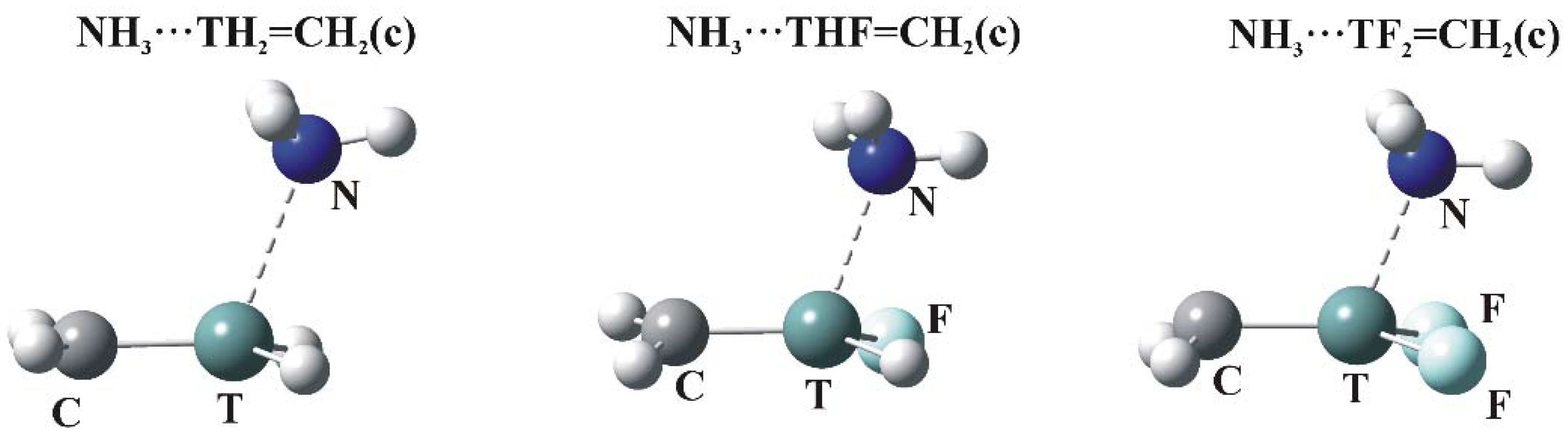
3.3. π-Hole Bonded Complexes
As indicated in
Figure 2, the MEPs of the planar TH
2-nF
n=CH
2 molecules have maxima (c) above the molecular plane, in the plane near the C=T midpoint (d), and (e) associated with the CH
2 protons. The c regions represent the π-hole above the T atom so are the focus of the calculations. The structures of the relevant complexes with NH
3 are illustrated in
Figure 4, and their energetics and geometric details reported in
Table 6.
As in the σ-hole complexes, all T∙∙∙N distances are shorter than the sum of the van der Waals radii of the corresponding atoms. The θ(R–T∙∙∙N) angles are all greater than 90°, reflecting the position of the π-hole maximum. Eint varies from a minimum value of 3.7 kcal/mol all the way up to nearly 30 kcal/mol. Just as in the case of Vs,max for these π-holes, Eint increases steadily as H atoms are replaced by F, with large increments in both quantities associated with each such substitution.
As in the case of the tetravalent σ-hole complexes described above, formation of the π-hole dimers also impose a certain geometric distortion into the monomers. The deformation energies listed in
Table 6 are not insignificant, particularly for the mono and difluorinated species for which E
def A varies between 5 and 8 kcal/mol. In this same vein, the various TH
2-nF
n=CH
2 monomers are not all fully planar and become even less so upon formation of the π-hole dimers. It is a matter of some interest how the interactions might be affected if these molecules were forced to be fully planar within the context of the dimer. Comparison of the second and third columns of
Table 6 reveals that such a restriction would severely diminish the interaction energy. This reduction varies from only 1 kcal/mol for GeH
2=CH
2 and SnH
2=CH
2, but can be as large as 12 kcal/mol for some of the fluorinated species. As a rule of thumb, the various fluorinated Lewis acids lose roughly half of their interaction energy if forced into a planar conformation. But at the same time, it should be stressed that even these reduced interaction energies, in the framework of enforced planarity, still exceed those of the σ-hole dimers in
Table 3. The EDA interaction energy contributions of the π-dimers are listed in
Table 7.
As in the σ-hole dimers, electrostatics contribute roughly 58–64% of the total attractive interaction. Dispersion is considerably smaller in the π complexes, less than 5%. Orbital interactions account for the difference, making up some 32–40%, as compared to roughly 30% for the σ-dimers. Perhaps more revealing are the absolute values of these components. Both the electrostatic and orbital interaction energies are much larger in magnitude for the π-dimers in
Table 7 than for the σ-complexes in
Table 5. For example, E
elec for the three TH
4 complexes vary between 3.5 and 8.1 kcal/mol, whereas the analogous values for the corresponding TH
2=CH
2 systems lie in the 35.2–64.4 kcal/mol range. The monofluorinated σ dimers cover the 21.3–27.9 range, which is greatly exceeded by the 65.9−86.8 kcal/mol range for the corresponding π-dimers. The same sort of enlargement of the π vs σ complexes is observed in the orbital interaction energies. It is only the dispersion component which is quite similar for the two types of complexes. (This similarity may be due to the use of the Grimme empirical correction, which is not sensitive to the variation of the wave function [
61].)
The enlarged contribution from orbital interactions is verified by NBO analysis. As reported in
Table S3, the total charge transfer is quite substantial, varying between 113 and 197 me, larger than the same quantities observed for the σ-hole dimers in
Table S1. The same amplification applies to the sum of E(2) interorbital transfers, which reach up to nearly 80 kcal/mol in some cases. The magnitudes of these quantities do not closely match the interaction energies. For example, the charge transfers are greatest for Si, as compared to Ge and Sn although the dimers involving Si are not the most strongly bound.
Unlike the σ-hole dimers, the AIM molecular diagrams indicate only a single intermolecular bond path, which corresponds to the T∙∙∙N tetrel bond, as illustrated in
Figure S2. The numerical values of the properties of each bond critical point are displayed in
Table S5. Like the interaction energies in
Table 6, each successive replacement of H by F adds an increment. The comparisons between the three tetrel atoms are, however, not as clear. Taking the three THF=CH
2 acids as an example, Ge presents the weakest dimer, whereas it shows the largest ρ
BCP and H. Comparisons show that the AIM indicators of tetrel bond strength are considerably larger for the π than for the σ-hole tetrel bonds, consistent with the energetic data.
It was pointed out above that the tetravalent TR
4 molecules undergo significant distortion upon complexation with NH
3, which in turn enlarges their σ-hole.
Table 8 compiles the same sort of data for the π-bonding TR
2=CH
2 molecules where the deformation from planarity about both the C and the T atoms are measured by the deviation from 360° of the sum of the three bond angles in which they engage. As may be seen from the second column in
Table 8 this nonplanarity only occurs for the difluorinated GeF
2=CH
2 and SnF
2=CH
2 monomers, and is more exaggerated for the C atom. However, all species become significantly nonplanar in the π-bonded dimers. These deformations about the C atom are fairly small, and only occur for fluorinated species, obeying the T = Si < Ge < Sn pattern. Perhaps more to the point of the interaction of NH
3 with the T atom, these nonplanar deformations are fairly small, less than 10°.
Contrary to the C deformations, the T nonplanarities follow the opposite Si > Ge > Sn pattern. (It is interesting that the SnF
2=CH
2 molecule actually becomes more planar about the Sn atom upon complexation.) In summary, the geometrical distortions induced by π-tetrel bonding are less severe than in the σ-bonded cases, where the deformation measures ranged all the way up to nearly 30°. As in the case of the σ-bonded systems, the deformations of the π-bonding TR
2=CH
2 molecules also raise the value of V
s, max, as is evident in
Table 9.
This increase is quite small for TH2=CH2 but grows as F substituents are added. Just as the trivalent molecules undergo larger geometrical perturbations than do their tetravalent sisters, so too are the π-hole enhancements smaller than those observed in the σ-holes.
3.4. Other Geometries
In addition to the c maximum in the MEP of the planar Lewis acids, there is also a d maximum located in the approximate molecular plane, in the vicinity of the T=C midpoint, as detailed in
Figure 2. However, optimization of the dimer geometry does not necessarily lead to a minimum in the potential energy surface with the NH
3 in this position. It is only for the monosubstituted THF=CH
2 molecule that such a configuration represents a minimum. In some sense this structure resembles a σ-hole dimer, with N situated directly opposite the F atom, rather than a π-dimer. The AIM molecular diagram confirms this to be a T∙∙∙N tetrel bond for Ge and Sn although the bond path for the former is much more curved than is usually the case, as illustrated in
Figure S3. But it must be added that this tetrel bond vanishes for the Si system in
Figure S3a, leaving only two weak H∙∙∙N interactions, whose ρ and ∇
2ρ values just barely meet the criteria of hydrogen bonds.
As may be seen in
Table S5, these d dimers are also more weakly bound than the c π-dimers: the former span an E
int range between 2.5 and 8.9 kcal/mol, in comparison to the 14.1–19.4 kcal/mol range of the latter. This comparative weakness is in contrast to the values of V
s,max in
Table 2, for which the d maxima are comparable to, and even exceed the c values. The weaker nature of the d minima extends beyond energetics, encompassing also longer N∙∙∙T distances, and lower E(2) energies, charge transfer, and electronic properties of the BCPs as well, with details contained in
Tables S6 and S7. Given the values of V
s, max in
Table 2, it is perhaps not entirely surprising that it is only the THF=CH
2 unit that engages in this d bonding. More specifically, the c maximum is much larger than d for both TH
2=CH
2 and TF
2=CH
2; it is only the monofluorinated species for which the two maxima have comparable values. The EDA results obtained for d complexes are provided in
Table S8. As in their analogous c complexes, electrostatic energy contributes about 52–64% of the total attractive interaction, while dispersion is considerably larger, from 9 to 27% in SnHF=CH
2 and SiHF=CH
2, respectively. Therefore, the largest contribution of E
disp is for the least stable d complex. Orbital interactions in these complexes account for about 23% (average value) which is smaller than those in their stronger c cousins (average value of 37%).
In addition to the dimer geometries described above there were a number of secondary minima, all quite a bit weaker than those described above, none with E
int larger than 2 kcal/mol. These weak secondary minima are displayed in
Table S9 for the tetrahedral TH
4, TH
3F and TH
2F
2 molecules, along with their calculated properties. Most dimers are held together by weak H-bonds, and none show any evidence of containing any sort of tetrel bond.
Table S10 contains the analogous secondary minima for the planar Lewis acids. Again the primary attractive forces are weak H-bonds and the total interaction energies are rather small.
3.5. Discussion
Although the tetrel bond has not been studied as intensively as some of its cousins, e.g., the H-bond or halogen bond, there are nevertheless some prior data that offer points of comparison and context with our own results. The study of complexes of TH
4 and its mono, tri, and tetrafluorinated derivatives with ammonia (T=Si, Ge, Sn) [
31] led to similar conclusions for this different subset of systems. Comparison between intensities of σ-holes exhibits strong similarities and the same trends as those examined here. This earlier work had shown how incorporating monomer deformation energies into the full energetics can lead to somewhat different patterns than the interactions between pre-deformed subunits. Another recent study [
62] places the same σ-hole donors in complexes with various π-electron systems acting as Lewis bases. The same Si < Ge < Sn pattern was found there as for the weaker b complexes above, somewhat different than for the more strongly bound a complexes. This work also noted that geometry deformation of the Lewis acid can be negligible, but becomes important for the stronger complexes. Decomposition of interaction energies revealed that the complexes are electrostatically driven and dispersion becomes significant only when the complexes are exceptionally weakly bonded. The vital role of the Pauli repulsion which exceeded the absolute value of the electrostatic component was also noticed. Our results are consistent with these observations. One factor driving the small values of dispersion energy may be the small size of the base, including only a single non-hydrogen atom.
There have been a number of prior studies comparing σ- and π-hole bonded systems. Li’s group [
35] paired F
2C=CFTF
3 with three Lewis bases including formaldehyde, water, and ammonia, and found π-hole bonded complexes were generally preferred for T=C but the opposite for Si and Ge. With particular respect to NH
3, the bonding grew in strength as the tetrel atom became larger for both σ- and π-hole complexes, in partial agreement with our results which showed some deviations from this pattern. The interaction energies correlated with the σ-hole intensity of T which was, in turn, strongly associated with the hybridization of C atoms in the order sp
3 < sp
2 < sp. A recent [
26] perspective article indicates the dominating influence of electrostatic and dispersive terms in both weak σ- and π-hole dimers, in complexes whose deformation energies are close to 0, which was confirmed by Xu et al. [
33] based on TH
3F (T=C and Si) complexes with pyrazine and 1,4-dicyanobenzene. As in the current work, the π-complexes were more stable than their σ counterparts in terms of larger interaction energy, also exhibiting shorter binding distance, greater electron density at BCPs, and larger CT. Also consistent with the data reported above was the distribution of attractive and repulsive components of the interaction, and the consistency with the magnitudes of MEP maxima. Distinctions arise on shifting from tetrel to aerogen atoms. In our own earlier study of aerogen bonds formed between AeOF
2 (Ae = Kr, Xe) and diazines [
43], the σ-hole bonded complexes were considerably stronger than their π-hole analogues.
In the context of the replacement of H atoms by the much more electronegative F, it is typically observed that the interaction grows stronger with each such substitution. For example, early work suggested that tetrafluorosilane was bound to ammonia more tightly than unsubstituted silane [
63]. This conclusion was confirmed in later calculations confined to Si [
64,
65] as well as in the other works that extended to complexes containing heavier tetrel atoms [
31,
66], and is consistent with our own findings above.
It has been shown in the literature that there are systems where the intensities of the MEP maxima or minima are not necessarily well correlated with interaction energies [
32,
67,
68]. For instance, in the tetrel-bonded complexes of formamidine with TH
3F (T = C, Si, Ge, and Sn) the interaction energy increases in the order C < Ge < Si < Sn, inconsistent with the magnitude of the σ-hole on the T atom [
68]. A similar pattern was found in our current work for the σ-hole bonded (a) dimers. In a recent work [
32], a series of complexes pairing Lewis acids TF
4 or ZF
5 (T = Si, Ge, Sn and Z = P, As, Sb) with Lewis bases NH
3, pyrazine, and HCN, the tetrel molecules TF
4 have a considerably larger (more than 10 kcal/mol) value of V
s,max than their corresponding pnicogen ZF
5 cousin, but nonetheless smaller interaction energy. Moreover, another inconsistency was observed with respect to V
s,min which is more negative for NCH than for pyrazine, but the latter complexes investigated were more strongly bound. Similar discrepancies arise in halogen bonded complexes involving chlorinated and methylated amines [
67].
The issue of geometrical deformations of the monomers and their impact on tetrel-bonded complexes has been described recently in a few papers [
47,
69]. In our own latest work devoted to implications of monomer deformation upon tetrel and pnicogen bonds [
32] it was shown that complexation can cause monomer deformation which results in a multifold increase in the intensity of V
s,max, which in turn amplifies the magnitude of the interaction energy.