1. Introduction
The meat industry annually produces tons of by-products, which results in a huge volume of waste and causes environmental issues [
1]. Among these products, livestock bones account for a large proportion. They represent a highly nutritious resource both for food and medicine, which contain a variety of proteins, oils, chondroitin, phosphorproteins, mucopolysaccharides, amino acids, vitamins, calcium, and phosphorus as well as minerals such as iron, zinc, copper, strontium and other health beneficial factors [
2,
3]. Bone protein, which has numerous biological functions including antioxidant [
4], antimicrobial [
5], immuno-modulatory [
6], antitumor [
7] and hypoglycemic [
8] activities, is the most important component of animal bone.
Bone is comprised of a protein and calcium network structure, with tubes filled with bone marrow, which is extremely rich in nutrients [
9,
10]. Previous studies on animal bone have mainly used bone directly for the purpose of manufacturing related products, so there had been no need for the separation of bone and bone marrow, and it consequently resulted in the reduction of the nutritional value of the products on a large scale [
11]. Therefore, it is urgent to develop technologies and scientific methods in the field of basic research for the comprehensive utilization of animal bones.
Usually, bone marrow studies are more concerned in bone-related diseases and transplants [
12,
13]. There is a lack of reports regarding the composition and biological activities of compounds from animal bones, in addition, according to our estimations there have been no reports on the molecular mass and composition of bone marrow. In traditional Chinese medicine, the bones of wildlife species such as tiger, bear, rhinoceros, and sika deer have been the main source of ethnic medicines for the treatment of arthritis [
14]. Though more limited in number, ethnic medicine records relating to the bones of domestic animals are increasingly becoming a prime source for modern scientific research and medical applications [
15,
16]. Yet, research attempts focusing on the properties of bone proteins and peptides as well as their related pharmacological compounds are still missing. Therefore, this study will provide a broad assessment on the discovery and effective utilization of new bioactive monomer peptide resources.
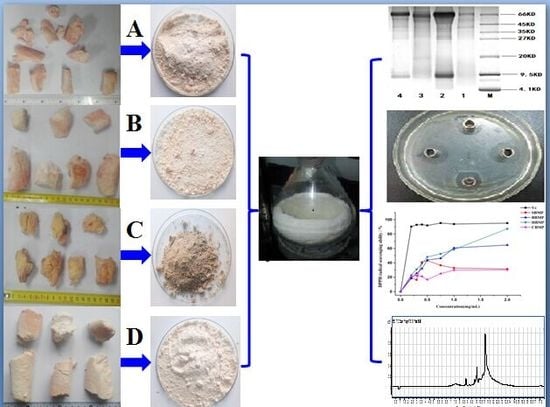
Herein, we intended to provide a technical and theoretical foundation for the development of modern peptide health foods or drugs. Instead of wildlife, our research firstly focused on the isolation and evaluation of bioactive proteins and peptides from the bone marrow of domestic animals. Thus, we narrowed our focus to the bone marrows from sheep (SBMP), bovines (BBMP), horses (HBMP) and camels (CMBP), which were separated from the bone, extracted with water and Tris-HCl buffer, and then fractionated with an ammonium sulfate gradient (30%, 50%, 70%) in order to identify new bioactive proteins, and evaluate the best bone marrow recourses. The isolated fractions were further characterized by SDS-PAGE, FT-IR, SEM and LC/MS. Also, their antimicrobial, and antioxidant activities were assessed in vitro.
3. Materials and Methods
3.1. Materials
Fresh raw materials were obtained from a slaughter house in Urumqi (Xinjiang, China). All of the marrow was removed from the front and rear leg bones, and immediately frozen after rinsing with cold water and warm water three times in order to remove crushed bones and blood, then was crushed using nitrogen (1:6, w/v) into a powder, which was stored at −20 °C until use. This article does not contain any studies with human participants or animals.
DPPH, bovine serum albumin (BSA), Trizma base, aspartic acid, arginine, glutamic acid, etc. and 18 kinds of amino acid standards were purchased from Sigma Corporation (Cookstown, NJ, USA). The electrophoresis reagentsf, such as 10% SDS, 0.1 mol/L Tris-HCl (pH = 6.8, 8.8) were purchased from Biosharp Corporation (Beijing, china). Running buffer, Bis-Tris gels, and Coomassie Brilliant Blue G 250 were obtained from Invitrogen (Carlsbad, CA, USA). All other chemical reagents used in this study were purchased from local suppliers.
3.2. Extraction and Isolation of Bone Marrow Protein (BMP)
For each sample 100 g of bone marrow powder was taken. Firstly extraction was performed with water under the following conditions: solid-liquid ratio 1:5 (w/v), extraction time 3 h, temperature 45 °C, times 3. The extracts were centrifuged at 10,000 rpm for 10 min and the supernatants were concentrated on a rotary evaporator (BUCHI R300, Flawil, Switzerland), and dialyzed (cut-off 1000 Da) for 48 h against distilled water and lyophilized (FDU-2100, EYELA, Tokyo, Japan). Secondly, samples were extracted with Tris-HCl buffer, and further fractionated with different concentrations of ammonium sulfate (30%, 50%, 70%) for 24 h at 4 °C, then centrifuged at 12,000 rpm for 10 min. The precipitated were washed with deionized water, redissolved, and dialyzed for 48 h. Finally, the solution was lyophilized to obtain a crude BMP powder.
3.3. Determination of Protein Concentration and Extraction Yield
The protein concentration of sample was measured by using a Pierce
® BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA), and expressed as mg/g [
28]. Yield of the protein extraction was estimated as follows:
where M
b represents the weight of bone marrow protein (g); M
a represents as the weight of bone marrow defatted powder (g) and Ca represents as the protein content (mg/mL).
3.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Electrophoretic analyses of proteins and peptides were carried out according to the reported procedures with slight modifications [
29,
30] using 12% separating gel and 4% stacking gel. The electrophoresis was run at 75 V for 1 h in the stacking gel and 150 V in the separating gel until the tracking dye reached the bottom of the gel. The gel stored at fixed solution for 1 h and stained with Comassie Brilliant Blue G 250 for 1 h, then held in a decoloring solution for 1.5 h.
3.5. Amino Acid Composition of BMP
The amino acid composition was analyzed according to the reported procedures with slight modifications [
31]. Four kinds of BMP powder (30 mg) were taken and hydrolyzed with 6 mol/L HCl (10 mL) at 110 °C for 24 h. The hydrolysates were diluted to 50 mL, then 1 mL of solution was evaporated to dryness at 25 °C and diluted to 5 mL with 0.02 mol/L HCl. The phenylisothiocyanate (PITC) method was used to derivatize the samples. The hydrolysates were determined using an amino acid analyzer (Hitachi L-8900, Shimadzu Seisakusho Co., Ltd., Kyoto, Japan).
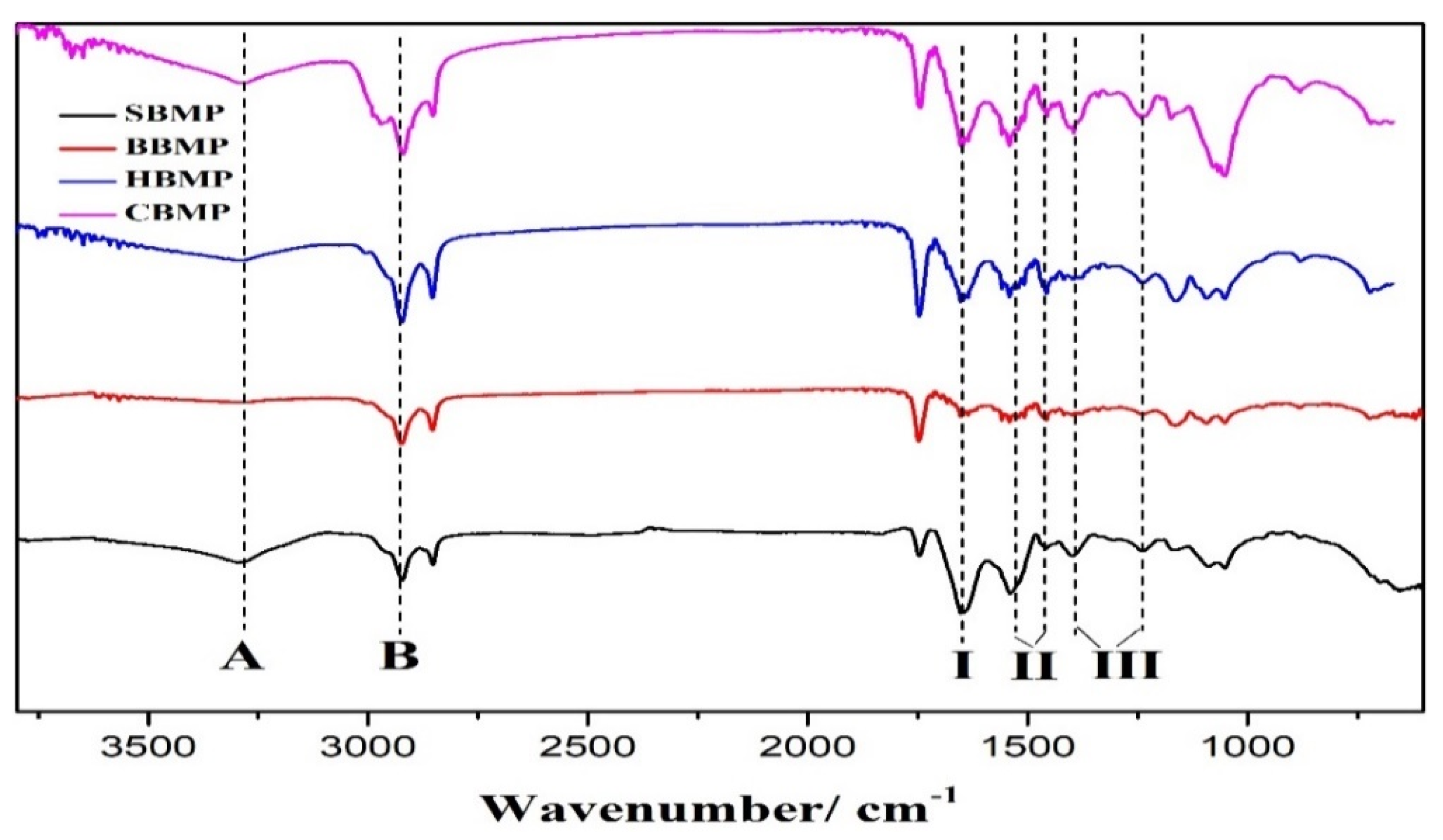
3.6. Fourier-Transform Infrared Spectroscopy Analysis (FT-IR)
The IR spectra were recorde using a Fourier transform infrared spectrophotometer (NICOLET 6700, Thermo fisher, Madison, WI, USA). Samples were pressed into pellets, and then subjected to FT-IR spectrophotometry in the range of 400–4000 cm−1.
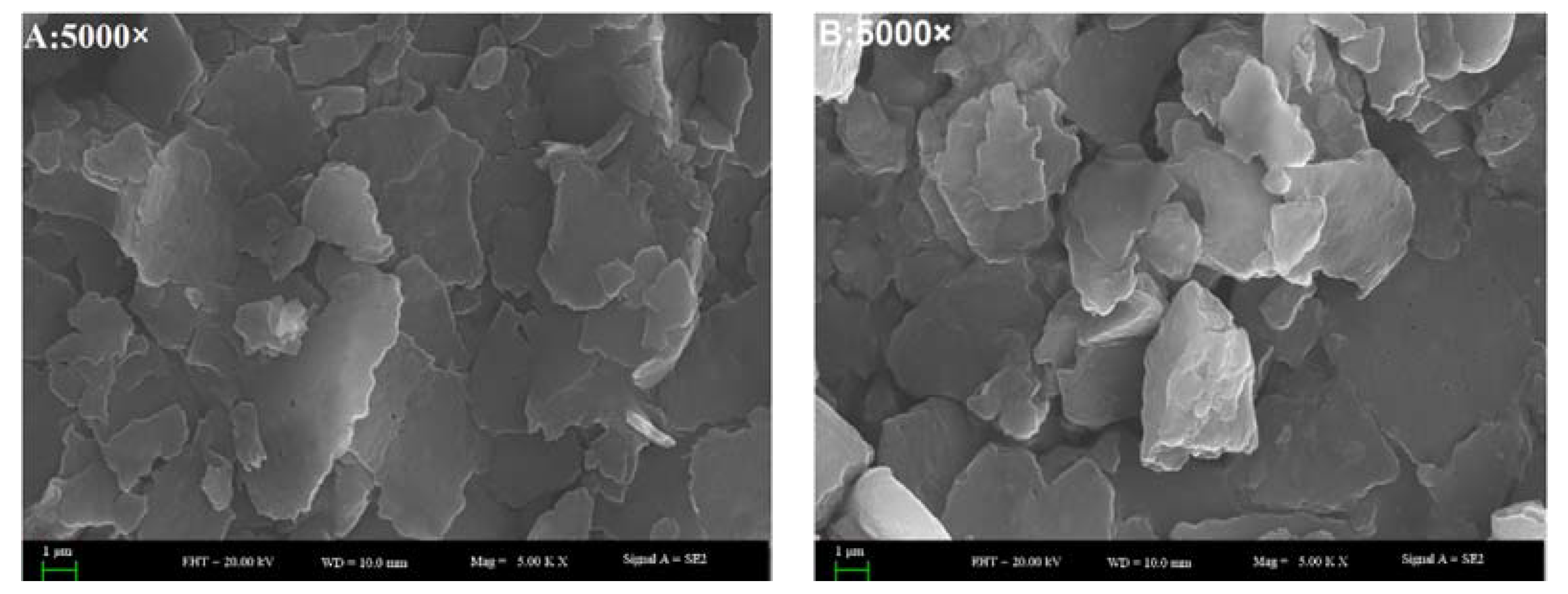
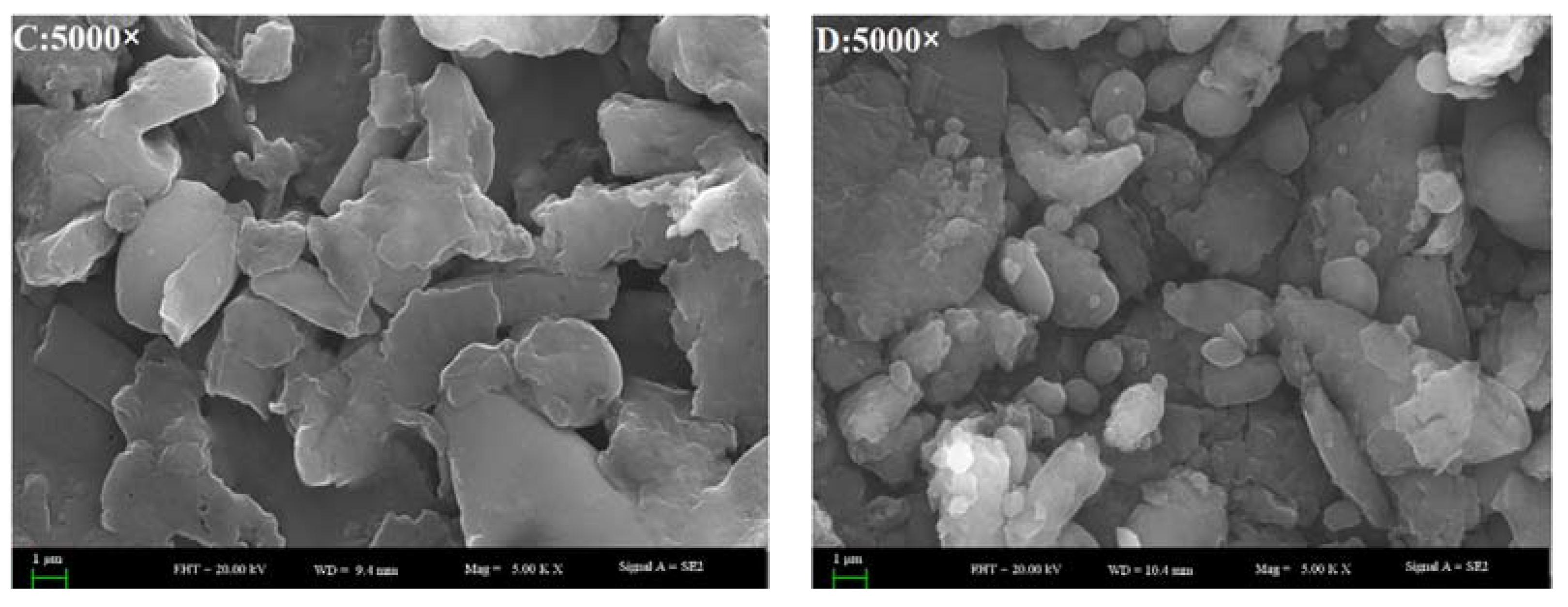
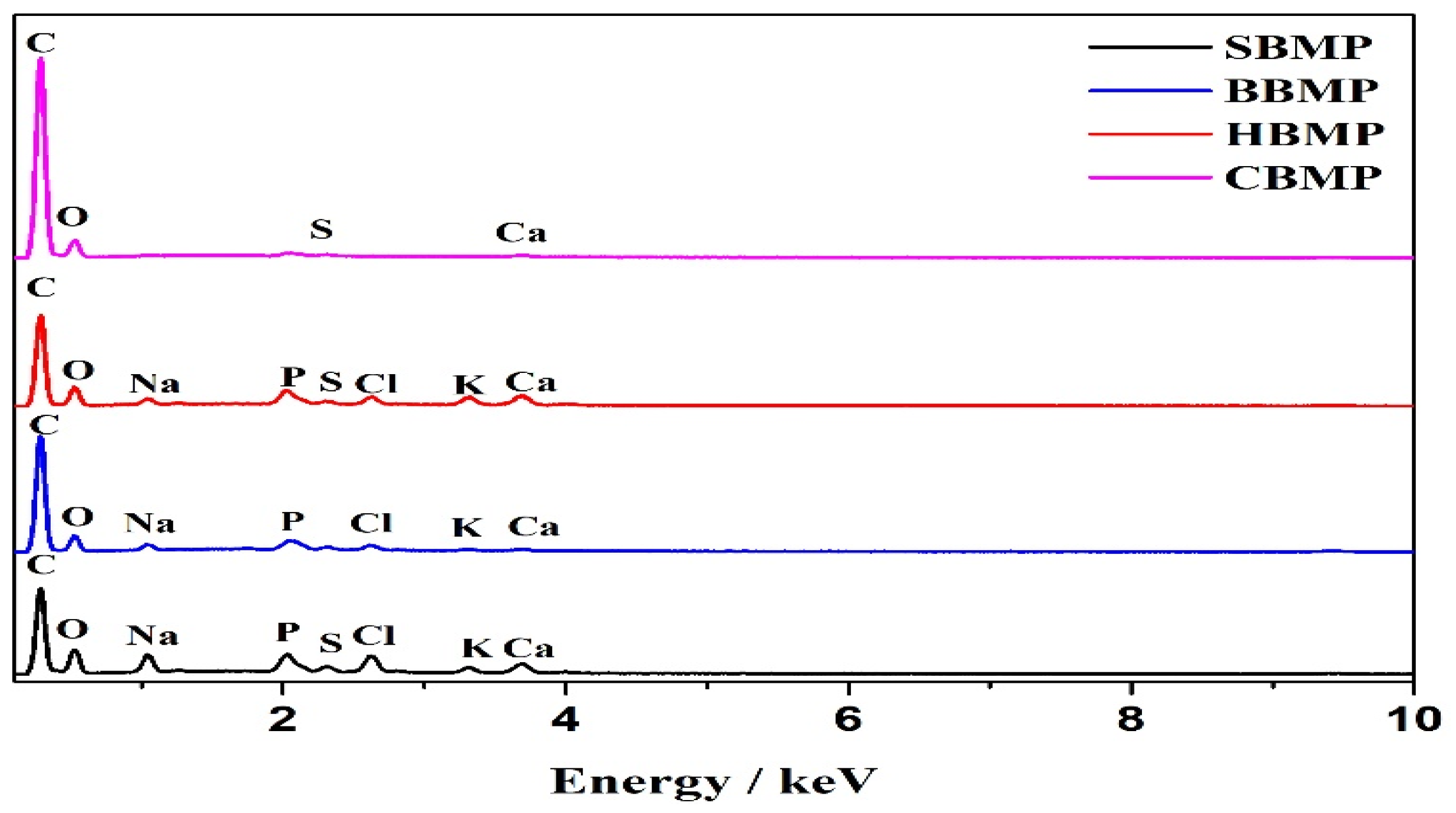
3.7. Scanning Electron Microscope-Energy Dispersive X-ray (SEM-EDX) Analysis
SEM-EDX analysis of the fractions were performed to observe the surface characteristics and elemental analysis. Dried samples of the purified fractions were fixed on a silicon wafer, coated using ion beam sputtering deposition, and the images were collected at a voltage of 20.0 kV with magnification at 2000–10,000× under high vacuum. To determine the elemental composition of the four kinds of extract, an EX-250 energy dispersive X-ray spectrometer (HORIBA Ltd., Kyoto, Japan) equipped with a cold field emission was utilized operating at 5 kV with an analysis time of 50 live seconds.
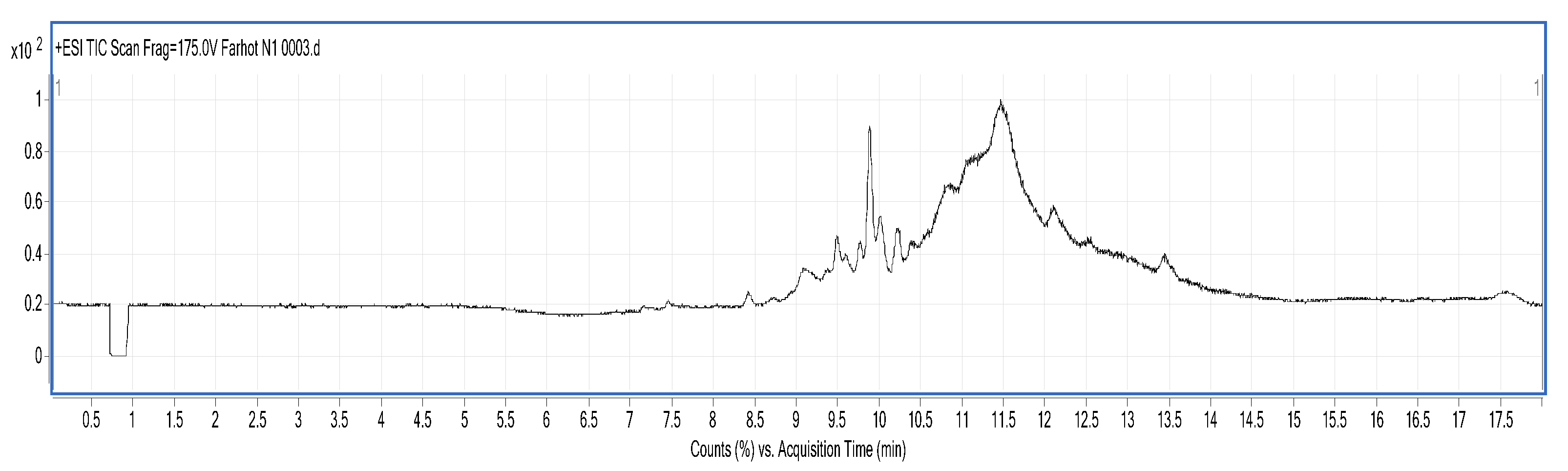
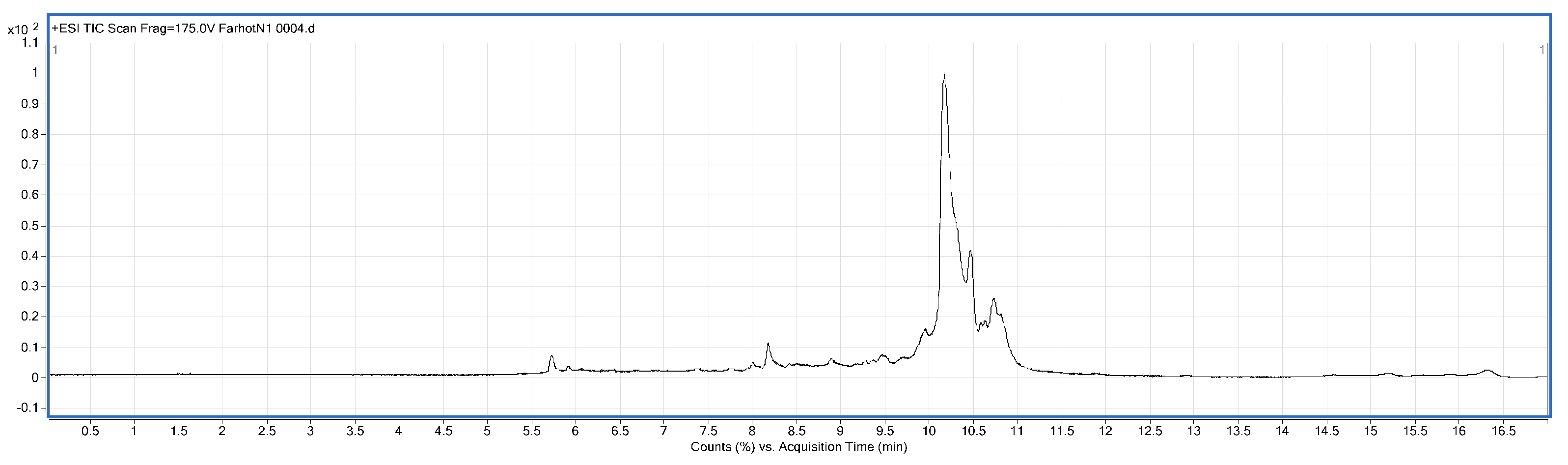
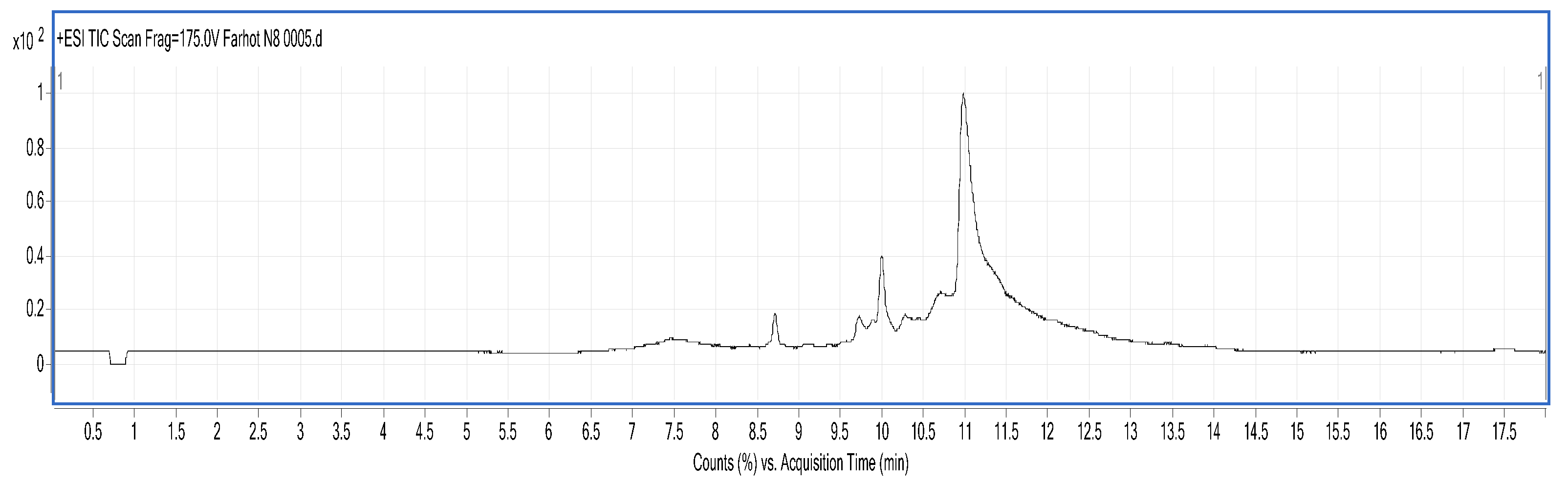
3.8. Identification of BMP by Liquid Chromatography-Mass Spectrometry (LC/MS)
LC/MS analysis was performed using a Series 6520B CHIP-Q-TOF LC-MS instrument (Agilent Technologies, Foster, CA, USA) according to the reported method. The ionization source was ESI+, skimmer cone potential 65 V, drying gas temperature 350 °C, fragmentation at 175 V. Mass range in MS mode 300–3000
m/
z. Positive ionization mode was used. An Agilent Zorbax 300 SB-C18, C18 column (150 mm × 7.5 mm, 5 μm), mobile phases A (0.1% formic acid) and B (MeCN + 0.1% formic acid), gradient procedure: 0–5%/0–3 min, 5–80%/3–13 min, 80%/13–15 min, 5–80%/15–17 min [
32,
33] were used.
3.9. Biological Activity
3.9.1. Antimicrobial Activity
Sample solution (20 μL) was taken from the concentrated 50 mg/mL samples and placed in the incubator at 37 °C for about 30–60 min. A Vernier caliper was used to measure and record the diameter of bacteriostatic halos after 16–18 h. The samples were considers as ineffective when the inhibition zone diameter ≥7 mm. Antimicrobial activity was determined using following microorganisms: CA:
Candida albicans (ATCC10231), EC:
Escherichia coli (ATCC11229) [
34].
3.9.2. Antioxidant Activity
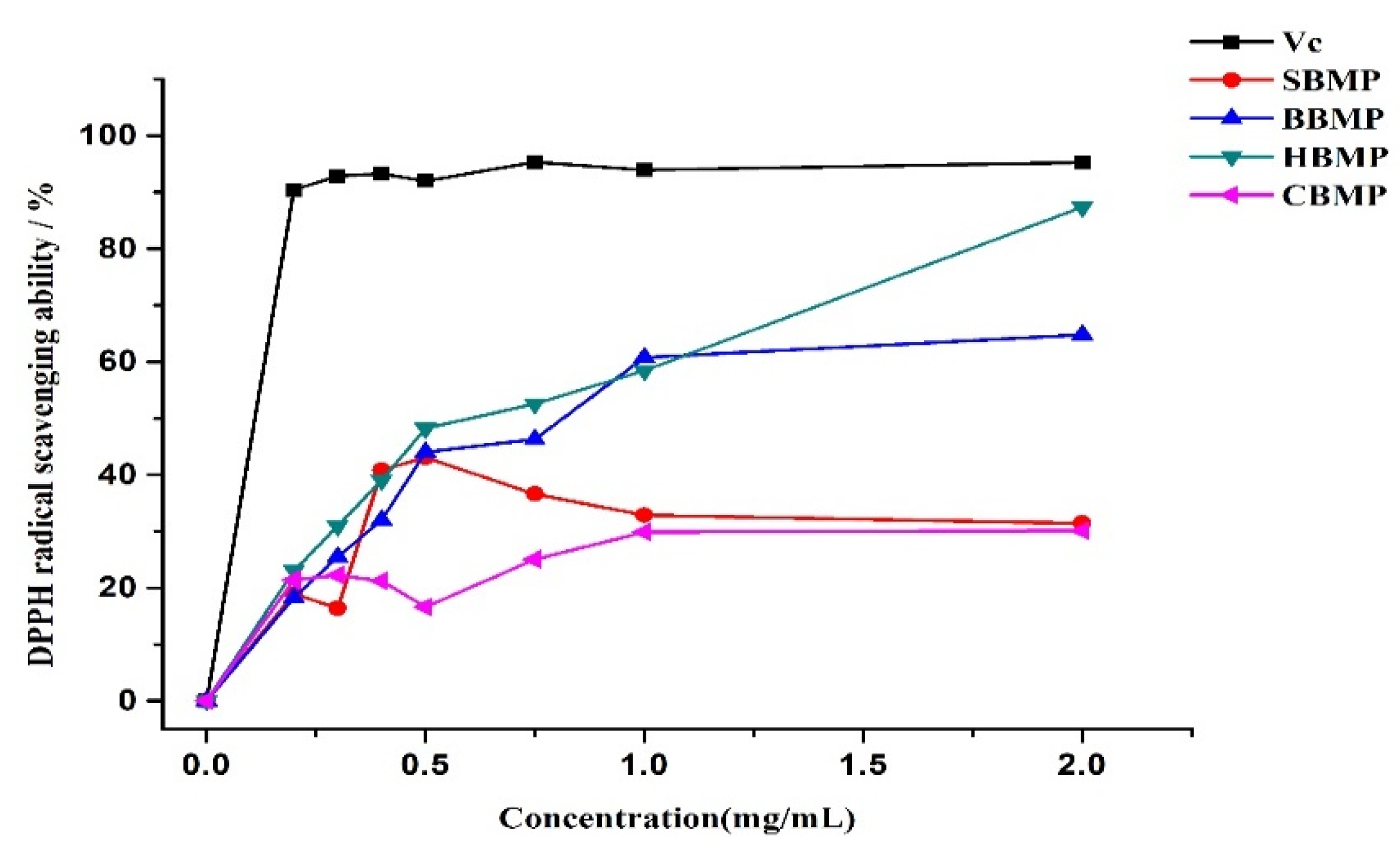
The DPPH radical scavenging activity of the water extracted proteins was determined. Water extracted protein samples (20 mg) prepared to different concentration (0.025 mg/mL, 0.125 mg/mL, 0.25 mg/mL, 0.5 mg/mL, 0.75 mg/mL, 1 mg/mL, 2 mg/mL) were used. Tests were conducted according to the reported method with some modifications [
35]. The DPPH radical scavenging activity was calculated as follows:
where, A
0 is the absorbance of the DPPH solution with distilled water; A
i is the absorbance of the sample mixed with DPPH solution; A
j is the absorbance of the sample with methanol.
4. Conclusions
In short, the structural characteristics of four kinds of proteins extracted with water and Tris-HCl buffer were investigated by SDS-PAGE, FT-IR, SEM and LC/MS analysis. Nutritional values were evaluated by determination of the amino acid composition. Biological activities were evaluated by antimicrobial and antioxidant activity in vitro. The properties of SBMP, BBMP, HBMP and CBMP were considerably different, including Mw, morphological structures, amino acid contents and biological activities. The corresponding marrow proteins exhibited significant antibacterial and antioxidant activities, which reveal the potential application of bone marrow protein in functional foods and nutraceuticals. To the best of our knowledge, this is the first report of the in vitro antibacterial and antioxidant activity of peptides derived from sheep, bovine, horse and camel bone marrow proteins.
This research initially verified the potential value of bone marrow proteins as a promising natural bioactive resource. Differences in amino acid sequences between bone marrows will produce different biological effects. Therefore, extensive studies are required for further identification of the peptide sequences responsible for the various biological activities. We insist that animal waste recovery is an important research subject; this work will provide further assistance for the subsequent research on domestic animal bone processing.