HPLC Separation of 2-Ethyl-5(6)-methylpyrazine and Its Electroantennogram and Alarm Activities on Fire Ants (Solenopsis invicta Buren)
Abstract
1. Introduction
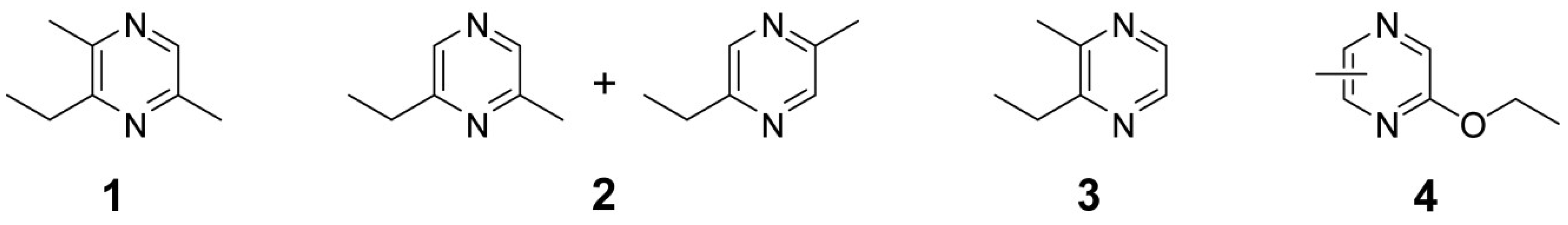
2. Results and Discussion
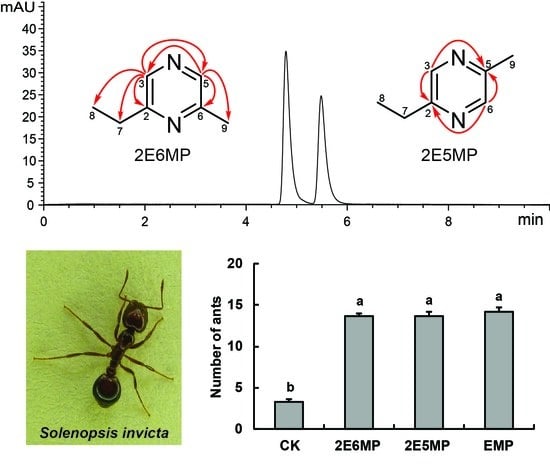
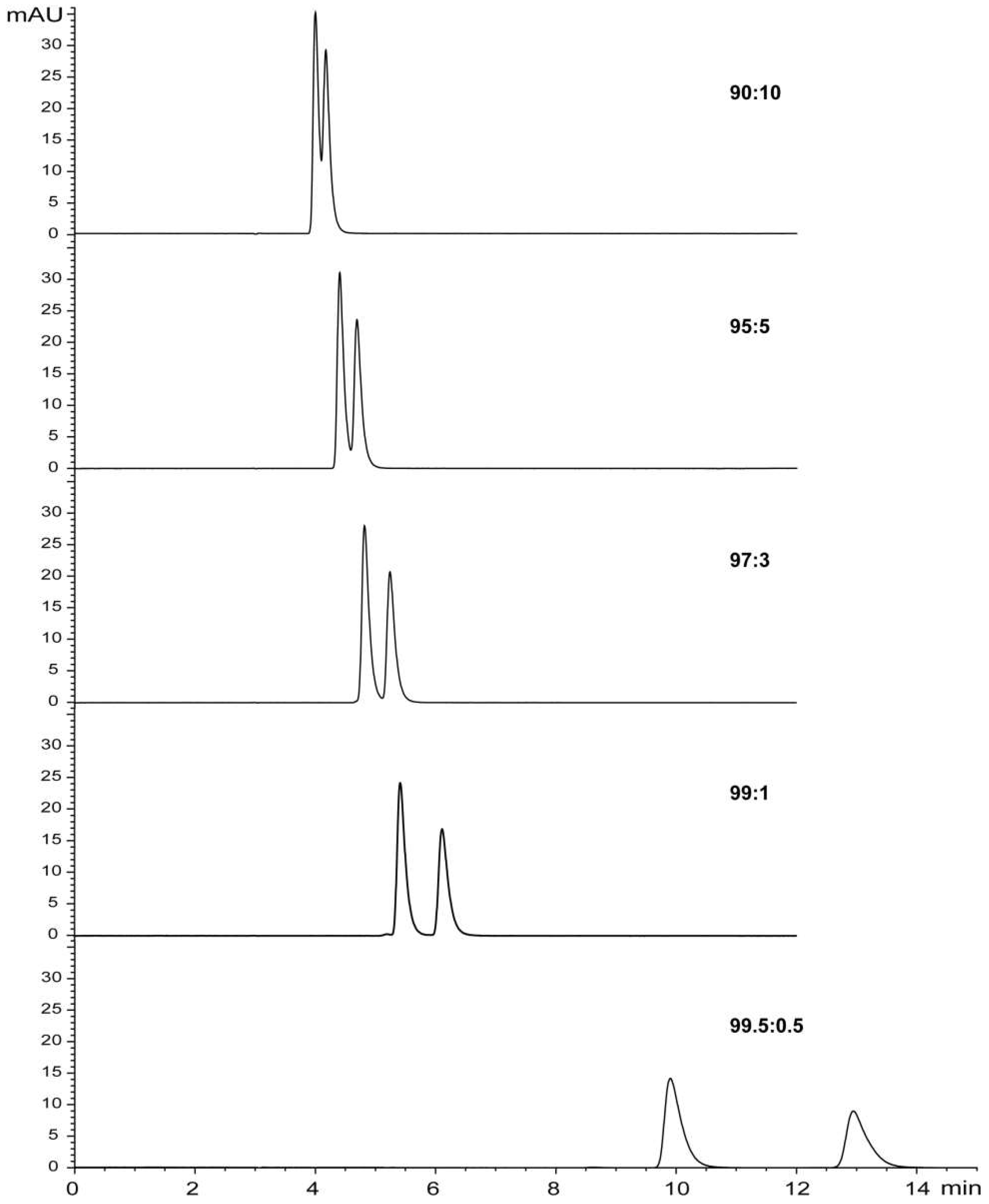
2.1. Preparative HPLC
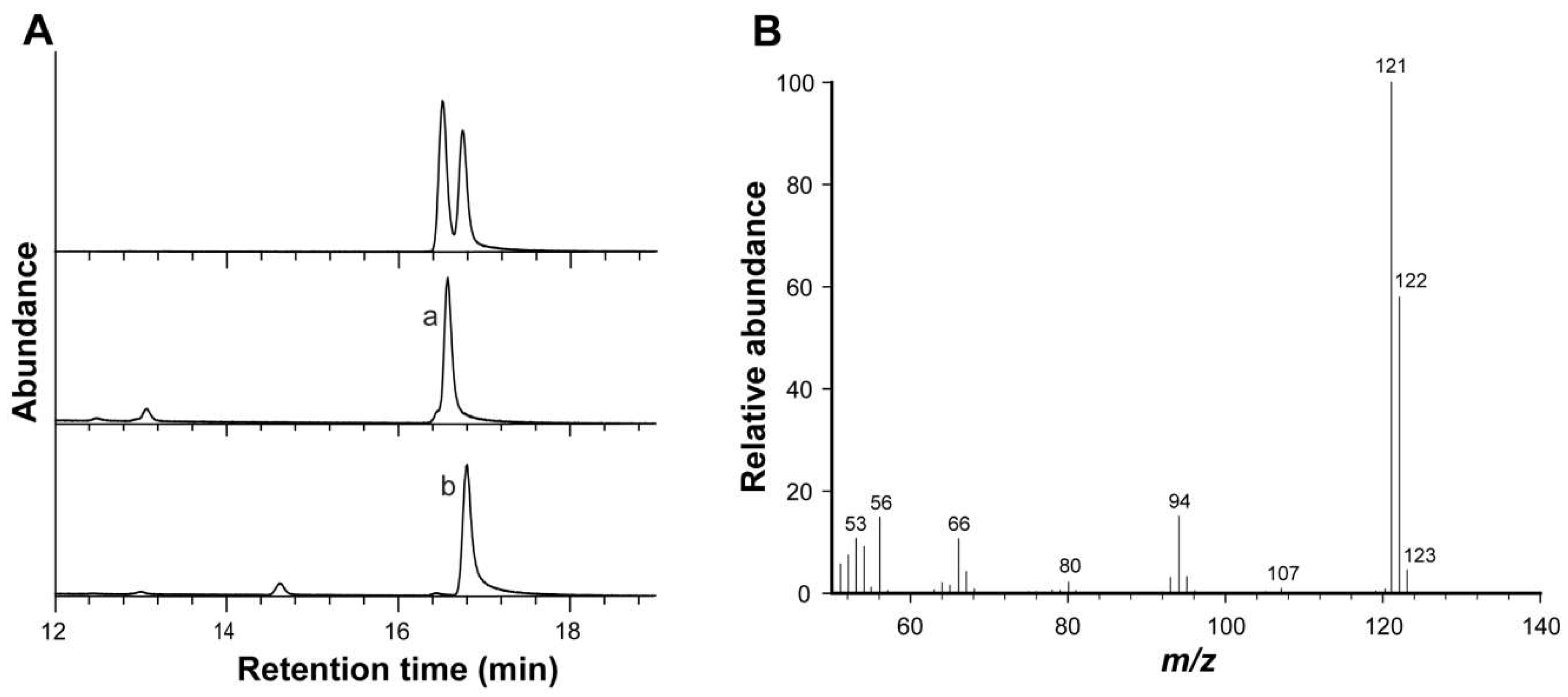
2.2. Gas Chromatography–Mass Spectrometry
2.3. NMR
2.4. HPLC Quantitation
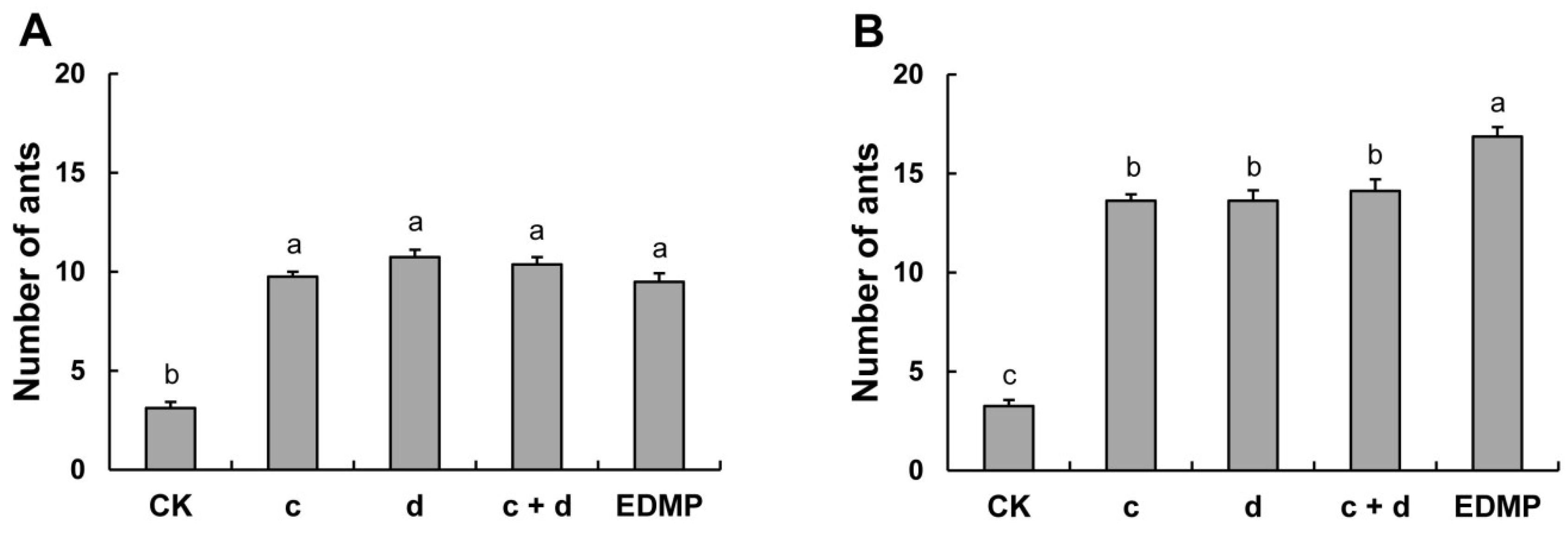
2.5. Bioassay
3. Materials and Methods
3.1. Chemicals and Solvents
3.2. HPLC
3.3. Gas Chromatography–Mass Spectrometry
3.4. NMR Spectroscopy
3.5. HPLC Quantitation
3.6. Insects
3.7. Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maga, J.A. Pyrazines in foods: An update. CRC Crit. Rev. Food Sci. Nutr. 1982, 16, 1–48. [Google Scholar] [CrossRef]
- Maga, J.A.; Sizer, C.E. Pyrazines in foods. Review. J. Agric. Food Chem. 1973, 21, 22–30. [Google Scholar] [CrossRef]
- Gallois, A. Pyrazines in food—A review. Sci. Aliments 1984, 4, 145–166. [Google Scholar]
- Oh, Y.C.; Hartman, T.G.; Ho, C.T. Volatile compounds generated from the Maillard reaction of Pro-Gly, Gly-Pro, and a mixture of glycine and proline with glucose. J. Agric. Food Chem. 1992, 40, 1878–1880. [Google Scholar] [CrossRef]
- Pickard, S.; Becker, I.; Merz, K.-H.; Richling, E. Determination of the alkylpyrazine composition of coffee using stable isotope dilution–gas chromatography–mass spectrometry (SIDA-GC-MS). J. Agric. Food Chem. 2013, 61, 6274–6281. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Czerny, M.; Bielohradsky, J.; Grosch, W. Structure-odour-activity relationships of alkylpyrazines. Eur. Food Res. Technol. 1999, 208, 308–316. [Google Scholar] [CrossRef]
- Mihara, S.; Masuda, H. Structure-odor relationships for disubstituted pyrazines. J. Agric. Food Chem. 1988, 36, 1242–1247. [Google Scholar] [CrossRef]
- Laska, M.; Persson, O.; Hernandez Salazar, L.T. Olfactory sensitivity for alkylpyrazines—A comparative study in CD-1 mice and spider monkeys. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2009, 311A, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, M.; Moore, B.P.; Brown, W.V. Pyrazines as warning odour components in the Monarch butterfly, Danaus plexippus, and in moths of the genera Zygaena and Amata (Lepidoptera). Biol. J. Linn. Soc. 1984, 23, 375–380. [Google Scholar] [CrossRef]
- Cross, J.H.; Byler, R.C.; Ravid, U.; Silverstein, R.M.; Robinson, S.W.; Baker, P.M.; Oliveira, J.S.; Jutsum, A.R.; Cherrett, J.M. The major component of the trail pheromone of the leaf-cutting ant, Atta sexdens rubropilosa Forel. J. Chem. Ecol. 1979, 5, 187–203. [Google Scholar] [CrossRef]
- Evershed, R.P.; Morgan, E.D. The amounts of trail pheromone substances in the venom of workers of four species of attine ants. Insect Biochem. 1983, 13, 469–474. [Google Scholar] [CrossRef]
- Attygalle, A.B.; Lancaster, V.K.; Morgan, E.D. The trial pheromone of the ant Manica rubida (Hymenoptera, Formicidae). Actes des Colloques Insectes Sociaux 1985, 2, 159–166. [Google Scholar]
- Attygalle, A.B.; Cammaerts, M.-C.; Cammaerts, R.; Morgan, E.D.; Ollett, D.G. Chemical and ethological studies of the trail pheromone of the ant Manica rubida (Hymenoptera: Formicidae). Physiol. Entomol. 1986, 11, 125–132. [Google Scholar] [CrossRef]
- Attygalle, A.B.; Morgan, E.D. Ant trail pheromones. Adv. Insect Physiol. 1985, 18, 1–30. [Google Scholar]
- Jackson, B.D.; Morgan, E.D. Insect chemical communication: Pheromones and exocrine glands of ants. Chemoecology 1993, 4, 125–144. [Google Scholar] [CrossRef]
- Evershed, R.P.; Morgan, E.D.; Cammaerts, M.C. 3-Ethyl-2,5-dimethylpyrazine, the trail pheromone from the venom gland of eight species of Myrmica ants. Insect Biochem. 1982, 12, 383–391. [Google Scholar] [CrossRef]
- Morgan, E.D. Trail pheromones of ants. Physiol. Entomol. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Billen, J.; Morgan, E.D. Pheromone communication in social insects: Sources and secretions. In Pheromone Communication in Social Insects: Ants, Wasps, Bees and Termites; Vander Meer, R.K., Breed, M.D., Espelie, K.E., Winston, M.L., Eds.; Westview Press: Boulder, CO, USA, 1998; pp. 3–33. [Google Scholar]
- Attygalle, A.B.; Morgan, E.D. Chemicals from the glands of ants. Chem. Soc. Rev. 1984, 13, 245–278. [Google Scholar] [CrossRef]
- Duffield, R.M.; Blum, M.S.; Wheeler, J.W. Alkylpyrazine alarm pheromones in primitive ants with small colonial units. Comp. Biochem. Physiol. 1976, 54B, 439–440. [Google Scholar] [CrossRef]
- Brophy, J.J.; Nelson, D. 2,5-Dimethyl-3-n-propylpyrazine from the head of the bull ant Myrmecia gulosa (Fabr.). Insect Biochem. 1985, 15, 363–365. [Google Scholar] [CrossRef]
- Showalter, D.; Troyer, E.; Aklu, M.; Jang, E.; Siderhurst, M. Alkylpyrazines: Alarm pheromone components of the little fire ant, Wasmannia auropunctata (Roger) (Hymenoptera, Formicidae). Insectes Soc. 2010, 57, 223–232. [Google Scholar] [CrossRef]
- Longhurst, C.; Baker, R.; Howse, P.E.; Speed, W. Alkylpyrazines in ponerine ants: Their presence in three genera, and caste specific behavioural responses to them in Odontomachus troglodytes. J. Insect Physiol. 1978, 24, 833–837. [Google Scholar] [CrossRef]
- Vander Meer, R.K.; Preston, C.; Choi, M.-Y. Isolation of a pyrazine alarm pheromone component from the fire ant, Solenopsis invicta. J. Chem. Ecol. 2010, 36, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shao, K.-M.; Lu, Y.-Y.; Shi, Q.-H.; Wang, W.-K.; Chen, L. Electrophysiological and alarm behavioral responses of Solenopsis invicta Buren (Hymenoptera: Formicidae) to alkoxypyrazines. J. Asia-Pacif. Entomol. 2017, 20, 541–546. [Google Scholar] [CrossRef]
- Guan, D.; Lu, Y.-Y.; Liao, X.-L.; Wang, L.; Chen, L. Electroantennogram and behavioral responses of the imported fire ant, Solenopsis invicta Buren, to an alarm pheromone component and its analogues. J. Agric. Food Chem. 2014, 62, 11924–11932. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Er, C.; Li, J.; Wang, H.; Zhu, S.; Sun, J. Removal of caffeine from green tea by microwave-enhanced vacuum ice water extraction. Anal. Chim. Acta 2012, 716, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Yogo, K.; Quiming, N.S.; Saito, Y.; Jinno, K. Prediction of chromatographic retention of pyrazine and alkylpyrazines in RP-LC. Chromatographia 2009, 70, 677–684. [Google Scholar] [CrossRef]
- Yogo, K.; Takemura, C.; Saito, Y.; Jinno, K. An abnormal temperature dependence of alkylpyrazines’ retention in reversed-phase liquid chromatography. Anal. Sci. 2011, 27, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Yomota, C.; Furusho, N.; Sato, K.; Yamazaki, T.; Tanamoto, K. Application of liquid chromatography–nuclear magnetic resonance spectroscopy for the identification of ethyldimethylpyrazine, a food flavouring agent. Food Addit. Contam. 2006, 23, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Yamagami, C.; Ogura, T.; Takao, N. Hydrophobicity parameters determined by reversed-phase liquid chromatography: I. Relationship between capacity factors and octanol-water partition coefficients for monosubstituted pyrazines and the related pyridines. J. Chromatogr. 1990, 514, 123–136. [Google Scholar] [CrossRef]
- Kim, K.H. Description of the reversed-phase high-performance liquid chromatography (RP-HPLC) capacity factors and octanol-water partition coefficients of 2-pyrazine and 2-pyridine analogues directly from the three-dimensional structures using comparative molecular field analysis (COMFA) approach. Quant. Struct. Act. Relat. 1995, 14, 8–18. [Google Scholar]
- Yu, A.; Peng, D.; Hu, K.; Cao, A.; Chang, J.; Wu, Y.; Zhang, S. A new 4-ferrocenylbenzoyl chloride-bonded stationary phase for high performance liquid chromatography. J. Chromatogr. 2013, 1283, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-C.; Chen, Q.-H.; Guo, Q.; Ruan, S.; Ruan, H.; He, G.-Q.; Gu, Q. Simultaneous determination of acetoin and tetramethylpyrazine in traditional vinegars by HPLC method. Food Chem. 2010, 122, 1247–1252. [Google Scholar] [CrossRef]
- Caccamese, S.; Principato, G.; Jokela, R.; Tolvanen, A.; Din Belle, D. Chiral HPLC separation and CD spectra of the enantiomers of the alkaloid tacamonine and related compounds. Chirality 2001, 13, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Shiratsuchi, H.; Nakada, Y.; Wu, Y.; Osajima, Y. Identification and sensory characterization of volatile flavor compounds in sesame seed oil. J. Agric. Food Chem. 1996, 44, 3909–3912. [Google Scholar] [CrossRef]
- Jung, M.Y.; Bock, J.Y.; Back, S.O.; Lee, T.K.; Kim, J.H. Pyrazine contents and oxidative stabilities of roasted soybean oils. Food Chem. 1997, 60, 95–102. [Google Scholar] [CrossRef]
- Cho, S.; Kays, S.J. Aroma-active compounds of wild rice (Zizania palustris L.). Food Res. Int. 2013, 54, 1463–1470. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Q.; Liu, Y.; Huang, J.; Wang, X.; Mao, W.; Wang, S. Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil. J. Food Sci. 2011, 76, C404–C412. [Google Scholar] [CrossRef] [PubMed]
- Pallabi, P.; Indrani, M.; Kunal, R. Predictive QSPR modelling for the olfactory threshold of a series of pyrazine derivatives. Flavour Fragr. J. 2013, 28, 102–117. [Google Scholar]
- Sharma, K.; Vander Meer, R.K.; Fadamiro, H.Y. Phorid fly, Pseudacteon tricuspis, response to alkylpyrazine analogs of a fire ant, Solenopsis invicta, alarm pheromone. J. Insect Physiol. 2011, 57, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Ngumbi, E.; Fadamiro, H. Comparative responses of four Pseudacteon phorid fly species to host fire ant alarm pheromone and analogs. Chemoecology 2015, 25, 85–92. [Google Scholar] [CrossRef]
- Qin, P.; Ma, T.; Wu, L.; Shan, F.; Ren, G. Identification of tartary buckwheat tea aroma compounds with gas chromatography-mass spectrometry. J. Food Sci. 2011, 76, S401–S407. [Google Scholar] [CrossRef] [PubMed]
- López-Galilea, I.; Fournier, N.; Cid, C.; Guichard, E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. J. Agric. Food Chem. 2006, 54, 8560–8566. [Google Scholar] [CrossRef] [PubMed]
- Maschwitz, U.W. Alarm substances and alarm behaviour in social Hymenoptera. Nature 1964, 204, 324–327. [Google Scholar] [CrossRef]
- Blum, M.S. Alarm pheromones. Annu. Rev. Entomol. 1969, 14, 57–80. [Google Scholar] [CrossRef]
- Hughes, W.O.H.; Goulson, D. The use of alarm pheromones to enhance bait harvest by grass-cutting ants. Bull. Entomol. Res. 2002, 92, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.O.H.; Howse, P.E.; Vilela, E.F.; Knapp, J.J.; Goulson, D. Field evaluation of potential of alarm pheromone compounds to enhance baits for control of grass-cutting ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2002, 95, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Balusu, R.R.; Zhang, W.-Q.; Ajayi, O.S.; Lu, Y.-Y.; Zeng, R.-S.; Fadamiro, H.Y.; Chen, L. Intra- and inter-specific variation in alarm pheromone produced by Solenopsis fire ants. Bull. Entomol. Res. 2018, 108. in press. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fadamiro, H.Y. Behavioral and electroantennogram responses of phorid fly Pseudacteon tricuspis (Diptera: Phoridae) to red imported fire ant Solenopsis invicta odor and trail pheromone. J. Insect Behav. 2007, 20, 267–287. [Google Scholar] [CrossRef]
- SAS Institute. SAS User Guide; SAS Institute: Cary, NC, USA, 2004. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| EMPa | EMPb | ||||
|---|---|---|---|---|---|
| No. | 𝛿H (mult, J, Hz) | 𝛿C | No. | 𝛿H (mult, J, Hz) | 𝛿C |
| 2 | 157.7 | 2 | 155.4 | ||
| 3 | 8.29 (s) | 140.7 | 3 | 8.39 (s) | 142.7 |
| 5 | 8.29 (s) | 141.7 | 5 | 150.7 | |
| 6 | 152.8 | 6 | 8.41 (s) | 143.5 | |
| 7 | 2.81 (q, 7.62) | 28.8 | 7 | 2.86 (q, 7.60) | 28.1 |
| 8 | 1.34 (t, 7.62) | 13.6 | 8 | 1.39 (m) | 13.4 |
| 9 | 2.56 (s) | 21.4 | 9 | 2.59 (s) | 20.8 |
| Compound | EAG (mV ± SE) | |
|---|---|---|
| Dose = 10 µg | Dose = 100 µg | |
| EMPc | 0.47 ± 0.056 | 1.43 ± 0.110 |
| EMPd | 0.54 ± 0.078 | 1.49 ± 0.112 |
| EMPc + EMPd | 0.50 ± 0.047 | 1.41 ± 0.100 |
| EDMP | 0.57 ± 0.050 | 1.66 ± 0.073 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-Y.; Lu, Y.-Y.; Lu, M.; Wei, H.-Y.; Chen, L. HPLC Separation of 2-Ethyl-5(6)-methylpyrazine and Its Electroantennogram and Alarm Activities on Fire Ants (Solenopsis invicta Buren). Molecules 2018, 23, 1661. https://doi.org/10.3390/molecules23071661
Li Y-Y, Lu Y-Y, Lu M, Wei H-Y, Chen L. HPLC Separation of 2-Ethyl-5(6)-methylpyrazine and Its Electroantennogram and Alarm Activities on Fire Ants (Solenopsis invicta Buren). Molecules. 2018; 23(7):1661. https://doi.org/10.3390/molecules23071661
Chicago/Turabian StyleLi, Ya-Ya, Yong-Yue Lu, Min Lu, Hong-Yi Wei, and Li Chen. 2018. "HPLC Separation of 2-Ethyl-5(6)-methylpyrazine and Its Electroantennogram and Alarm Activities on Fire Ants (Solenopsis invicta Buren)" Molecules 23, no. 7: 1661. https://doi.org/10.3390/molecules23071661
APA StyleLi, Y.-Y., Lu, Y.-Y., Lu, M., Wei, H.-Y., & Chen, L. (2018). HPLC Separation of 2-Ethyl-5(6)-methylpyrazine and Its Electroantennogram and Alarm Activities on Fire Ants (Solenopsis invicta Buren). Molecules, 23(7), 1661. https://doi.org/10.3390/molecules23071661