Simultaneous Determination of the Traditional Herbal Formula Ukgansan and the In Vitro Antioxidant Activity of Ferulic Acid as an Active Compound
Abstract
:1. Introduction
2. Results
2.1. Optimization of HPLC Separation
2.2. Regression Equation, Linearity, and Limits of Detection (LOD) and Quantification (LOQ)
2.3. Determination of the Seven Marker Compounds of the UGS Extract
2.4. Precision, Accuracy, and Recovery
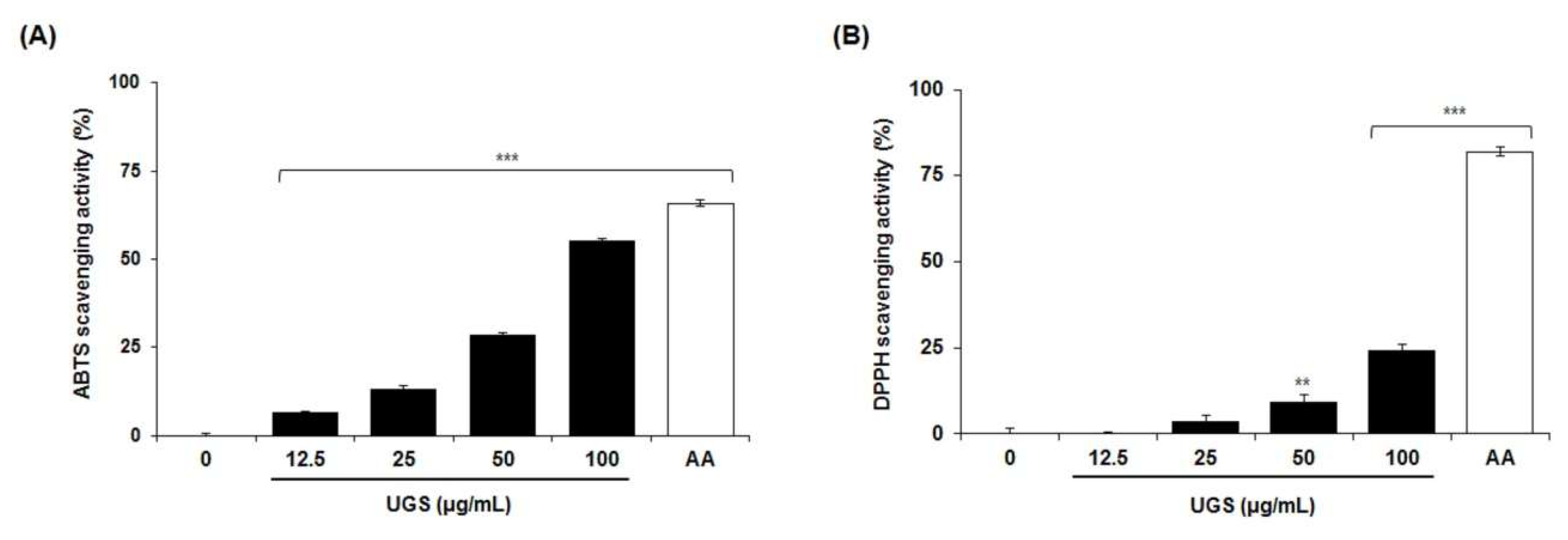
2.5. Antioxidant Activity of the Marker Compounds of UGS
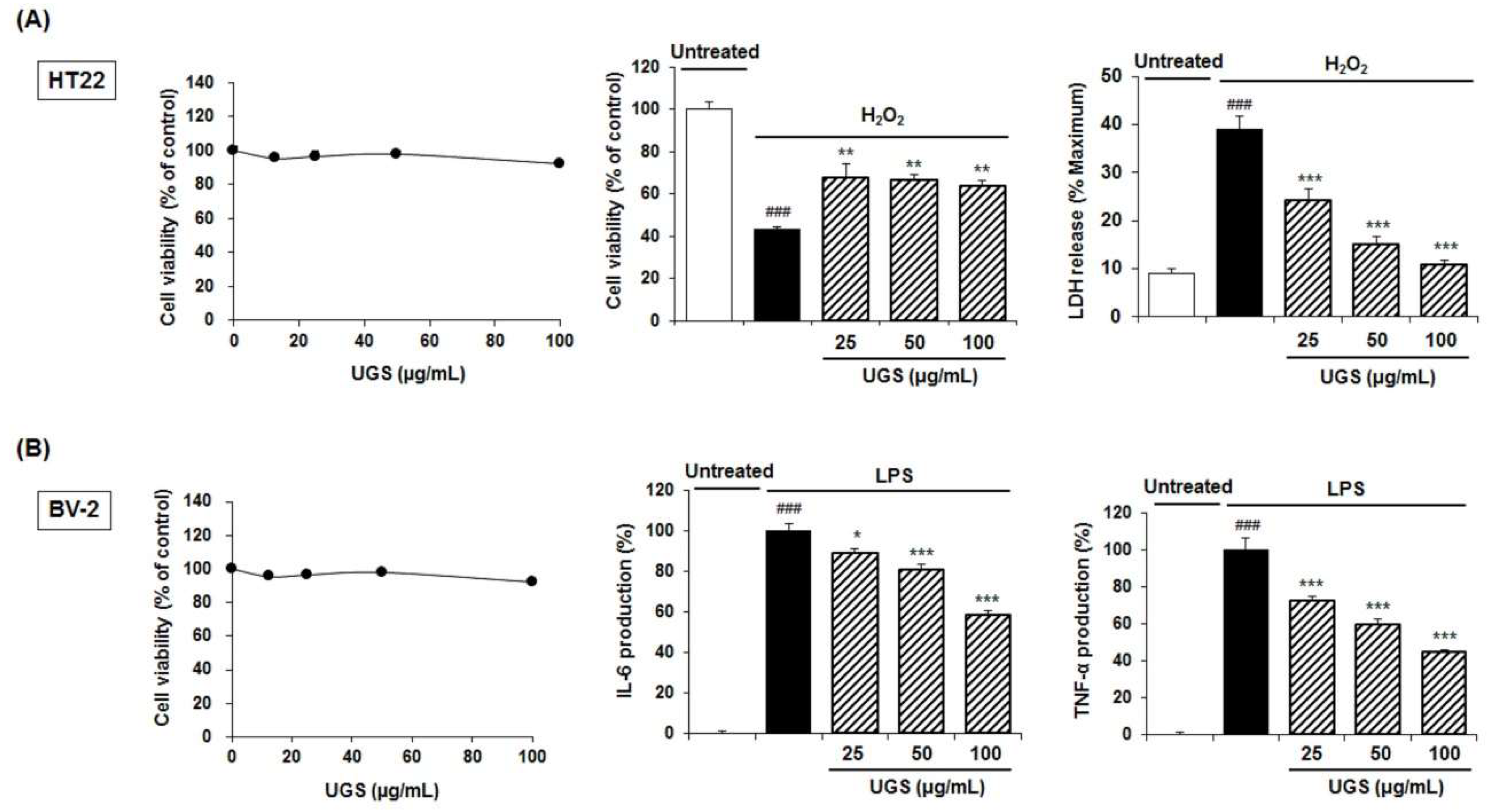
2.6. Biological Activitis of the UGS Extract in Neuronal Cell Lines
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Chemicals and Reagents
4.3. Apparatus and Chromatographic Conditions
4.4. Preparation of Standard Solutions
4.5. Preparation of the UGS 70% Ethanol Extract and Sample Solutions
4.6. Calibration Curve, LOD, and LOQ
4.7. Precision, Accuracy, and Recovery
4.8. ABTS-Scavenging Activity
4.9. DPPH-Scavenging Activity
4.10. Cell Lines and Culture
4.11. Cell Counting Kit (CCK) Assay
4.12. LDH Release Assay
4.13. Enzyme-Linked Immunosorbent Assays (ELISAs) for Cytokine Production
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogawa, Y.; Fujii, Y.; Sugiyama, R.; Konishi, T. The role of the seven crude drug components in the sleep-promoting effect of Yokukansan. J. Ethnopharmacol. 2016, 177, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ozone, M.; Yagi, T.; Chiba, S.; Aoki, K.; Kuroda, A.; Mitsui, K.; Ithoh, H.; Sasaki, M. Effect of yokukansan on psychophysiological insomnia evaluated using cyclic alternating pattern as an objective marker of sleep instability. Sleep. Biol. Rhythms 2012, 10, 157–160. [Google Scholar] [CrossRef]
- Horiguchi, J. Clinical usage of Yi-gan san-schizophrenia, borderline personality disorder, dyskinesia etc-. Psychiatria et neurologia Japonica 2012, 114, 708–718. [Google Scholar] [PubMed]
- Miyaoka, T.; Furuya, M.; Yasuda, H.; Hayashida, M.; Nishida, A.; Inagaki, T.; Horiguchi, J. Yi-gan san for the treatment of neuroleptic-induced tardive dyskinesia: An open-label study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.H.; Park, J.Y.; Choi, W.W.; Min, I.K.; Park, S.U.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H.; et al. Herbal medicine treatment for drug-induced parkinsonism. J. Altern. Complement. Med. 2015, 21, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yang, T.J.; Jeong, S.J.; Wei, T.S. Effects of Ukgansan Pharmacopuncture at GB20 on Cognitive Impariment Induced by Focal Brain Injury in Rats. J. Acupunct Res. 2016, 33, 101–116. [Google Scholar] [CrossRef]
- Nagata, K.; Yokoyama, E.; Yamazaki, T.; Takano, D.; Maeda, T.; Takahashi, S.; Terayama, Y. Effects of yokukansan on behavioral and psychological symptoms of vascular dementia: An open-label trial. Phytomedicine 2012, 19, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, H.; Mantani, A.; Nishiyama, S.; Fujiwaki, S.; Ohta, S.; Masuda, Y.; Tomita, Y.; Tarumoto, N.; Yamawaki, S. Yokukansan treatment of chronic renal failure patients receiving hemodialysis, with behavioral and psychological symptoms of dementia: An open-label study. Am. J. Geriatr. Psychiatry 2013, 21, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, S.; Kishi, T.; Iwata, N. Yokukansan in the Treatment of Behavioral and Psychological Symptoms of Dementia: An Updated Meta-Analysis of Randomized Controlled Trials. J. Alzheimers Dis. 2016, 54, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Ramalingayya, G.V.; Sonawane, V.; Cheruku, S.P.; Kishore, A.; Nayak, P.G.; Kumar, N.; Shenoy, R.S.; Nandakumar, K. Insulin Protects against Brain Oxidative Stress with an Apparent Effect on Episodic Memory in Doxorubicin-Induced Cognitive Dysfunction in Wistar Rats. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Jang, J.S.; Son, D.J.; Im, H.S.; Kim, J.Y.; Park, J.E.; Choi, W.R.; Han, S.B.; Hong, J.T. Antarctic Krill Oil Diet Protects against Lipopolysaccharide-Induced Oxidative Stress, Neuroinflammation and Cognitive Impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef] [PubMed]
- Piri, H.; Haghdoost-Yazdi, H.; Fraidouni, N.; Dargahi, T.; Yaghoubidoust, M.; Azadmehr, A. The Anti-Parkinsonism Effects of KATP Channel Blockade in the 6-Hydroxydopamine-Induced Animal Model: The Role of Oxidative Stress. Basic. Clin. Neurosci. 2017, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seo, C.S.; Kim, S.S.; Shin, H.K. Quality Assessment of Ojeok-San, a Traditional Herbal Formula, Using High-Performance Liquid Chromatography Combined with Chemometric Analysis. J. Anal. Methods Chem. 2015, 2015, 607252. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Chen, H.; Wu, C.; Feng, F.; Wang, Y.; Liu, W.; Wang, S. Analysis of the traditional medicine YiGan San by the fragmentation patterns of cadambine indole alkaloids using HPLC coupled with high-resolution MS. J. Sep. Sci. 2013, 36, 3723–3732. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shi, Y.-Y.; Wei, M.-L.; Liu, W.-Y.; Feng, F. Chemical profile of the active fraction of Yi-Gan San by HPLC-DAD-Q-TOF-MS and its neuroprotective effect against glutamate-induced cytotoxicity. Chin. J. Nat. Med. 2014, 12, 869–880. [Google Scholar] [CrossRef]
- Machado, K.N.; Freitas, A.A.; Cunha, L.H.; Faraco, A.A.G.; Pádua, R.M.; Braga, F.C.; Vianna-Soares, C.D.; Castilho, R.O. A rapid simultaneous determination of methylxanthines and proanthocyanidins in Brazilian guaraná (Paullinia cupana Kunth.). Food Chem. 2018, 239, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.-S.; Shin, H.-K. Simultaneous Quantification of Eight Marker Compounds in Yongdamsagan-Tang Using a High-Performance Liquid Chromatography Equipped with Photodiode Array Detector. J. Chromatogr. Sci. 2017, 55, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Kim, O.S.; Kim, B.Y.; Jeong, S.J. Apigetrin from Scutellaria baicalensis Georgi Inhibits Neuroinflammation in BV-2 Microglia and Exerts Neuroprotective Effect in HT22 Hippocampal Cells. J. Med. Food 2016, 19, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, H.S.; Lee, J.; Jeong, S.J. Quantitative Analysis of Psoralea corylifolia Linne and its Neuroprotective and Anti-Neuroinflammatory Effects in HT22 Hippocampal Cells and BV-2 Microglia. Molecules 2016, 21, 1076. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Kim, B.Y.; Kim, Y.J.; Jeong, S.J. Phytochemical allylguaiacol exerts a neuroprotective effect on hippocampal cells and ameliorates scopolamine-induced memory impairment in mice. Behav. Brain Res. 2018, 339, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Kushida, H.; Fukutake, M.; Tabuchi, M.; Katsuhara, T.; Nishimura, H.; Ikarashi, Y.; Kanitani, M.; Kase, Y. Simultaneous quantitative analyses of indole and oxindole alkaloids of Uncaria Hook in rat plasma and brain after oral administration of the traditional Japanese medicine Yokukansan using high-performance liquid chromatography with tandem mass spectrometry. Biomed. Chromatogr. 2013, 27, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and Antinociceptive Constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Hou, C.C.; Chang, C.L.; Yang, W.C. Anti-Hyperglycemic Properties of Crude Extract and Triterpenes from Poria cocos. Evid. Based. Complement. Alternat. Med. 2011, 2011, 128402. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Dong, X.; Yin, X.; Wang, W.; You, L.; Ni, J. Radix Bupleuri: A Review of Traditional Uses, Botany, Phytochemistry, Pharmacology, and Toxicology. Biomed Res. Int. 2017, 2017, 7597596. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kim, H.M.; Lee, K.H.; Kim, K.Y.; Huang, D.S.; Kim, J.H.; Seong, R.S. Quantitative analysis of marker compounds in Angelica gigas, Angelica sinensis, and Angelica acutiloba by HPLC/DAD. Chem. Pharm. Bull. 2015, 63, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Yoo, H.H.; Sung, C.K.; Dong, M.S.; Park, Y.I.; Jin, C.; Kim, D.H. Simultaneous determination of phenolic acids and phthalide compounds by liquid chromatography for quality assessment of Rhizoma cnidii. J. AOAC Int. 2009, 92, 375–381. [Google Scholar] [PubMed]
- Lee, B.; Weon, J.B.; Yun, B.R.; Lee, J.; Eom, M.R.; Ma, C.J. Simultaneous determination of 11 major components in Palmul-tang by HPLC-DAD and LC-MS-MS. J. Chromatogr. Sci. 2014, 52, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A. 2009, 1216, 1954–1969. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Gumula, I.; Alao, J.P.; Ndiege, I.O.; Sunnerhagen, P.; Yenesew, A.; Erdélyi, M. Flemingins G–O, Cytotoxic and Antioxidant Constituents of the Leaves of Flemingia grahamiana. J. Nat. Prod. 2014, 77, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; You, C.Y.; Dong, K.; You, H.S.; Xing, J.F. Anti-inflammatory, analgesic and antioxidant activities of 3,4-oxo-isopropylidene-shikimic acid. Pharm. Biol. 2016, 54, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Xu, G.; Jiang, S.; Li, H.; Yuan, G. In Vitro Antioxidant activities and anti-diabetic effect of a polysaccharide from Schisandra sphenanthera in rats with type 2 diabetes. Int. J. Biol. Macromol. 2017, 94, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.K.; Dobhal, M.P.; Sharma, M.C. In Vitro Antioxidant Activity of Selected Medicinal Plants Reported in Ancient Ayurveda Traditions. Curr. Tradit. Med. 2017, 3, 190–194. [Google Scholar] [CrossRef]
- Jeong, S.J.; Yoo, S.R.; Kim, O.S.; Seo, C.S.; Shin, H.K. Antioxidant and antiadipogenic activities of galkeun-tang, a traditional korean herbal formula. Evid. Based Complement. Alternat. Med. 2014, 2014, 763494. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.R.; Seo, C.S.; Kim, O.S.; Shin, H.K.; Jeong, S.J. Anti-adipogenic and antioxidant effects of the traditional Korean herbal formula Samchulgeonbi-tang: An in vitro study. Int. J. Clin. Exp. Med. 2015, 8, 8698–8708. [Google Scholar] [PubMed]
- Liang, C.P.; Chang, C.H.; Liang, C.C.; Hung, K.Y.; Hsieh, C.W. In vitro antioxidant activities, free radical scavenging capacity, and tyrosinase inhibitory of flavonoid compounds and ferulic acid from Spiranthes sinensis (Pers.) Ames. Molecules 2014, 19, 4681–4694. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds liquiritin apioside, liquiritin, ferulic acid, glycyrrhizin, decursin, decursinol angelate, and atractylenolide I are commercially available. |



| Latin Name | Scientific Name | Amount (g) | Origin |
|---|---|---|---|
| Uncariae Ramulus et Uncus | Uncaria sinensis | 6 | China |
| Atractylodis Rhizoma Alba | Atractylodes japonica | 8 | China |
| Poria Sclerotium | Poria cocos | 8 | China |
| Bupleuri Radix | Bupleurum falcatum | 4 | China |
| Angelicae Gigantis Radix | Angelica gigas | 6 | Bonghwa, Korea |
| Cnidii Rhizoma | Cnidium officinale | 6 | China |
| Glycyrrhizae Radix et Rhizoma | Glycyrrhiza uralensis | 3 | China |
| Total amount | 41 |
| Compound. | Linear Range (μg/mL) | Regression Equation (y = ax + b) a) | r2 | LOD b) (μg/mL) | LOQ c) (μg/mL) | |
|---|---|---|---|---|---|---|
| Slope (a) | Intercept (b) | |||||
| Liquiritin apioside | 3.125–50 | 15290 | 4536.9 | 1.0000 | 0.177 | 0.537 |
| Liquiritin | 1.5625–25 | 18759 | 2614.9 | 0.9999 | 0.052 | 0.157 |
| Ferulic acid | 0.78125–25 | 56995 | 1865.6 | 1.0000 | 0.039 | 0.118 |
| Glycyrrhizin | 6.25–200 | 4882.5 | 1533 | 1.0000 | 0.619 | 1.876 |
| Decursin | 12.5–400 | 30409 | 77457 | 0.9998 | 0.925 | 2.804 |
| Decursinol angelate | 6.25–200 | 35125 | 38848 | 0.9998 | 0.232 | 0.705 |
| Atractylenolide I | 0.78125–12.5 | 62615 | 1322.9 | 1.0000 | 0.015 | 0.046 |
| Compound | Content (mg/g) |
|---|---|
| Liquiritin apioside | 1.671 ± 0.004 |
| Liquiritin | 2.014 ± 0.004 |
| Ferulic acid | 0.605 ± 0.002 |
| Glycyrrhizin | 10.267 ± 0.05 |
| Decursin | 16.431 ± 0.04 |
| Decursinol angelate | 7.606 ± 0.002 |
| Atractylenolide I | 0.190 ± 0.001 |
| Compound | Fortified Conc. (μg/mL) | Intra-Day (n = 5) | Inter-Day (n = 5) | ||||
|---|---|---|---|---|---|---|---|
| Observed Conc. (μg/mL) | Precision a) (%) | Accuracy b) (%) | Observed Conc. (μg/mL) | Precision (%) | Accuracy (%) | ||
| Liquiritin apioside | 5 | 4.84 | 2.11 | 96.88 | 5.01 | 1.25 | 100.18 |
| 10 | 10.14 | 1.49 | 101.36 | 10.19 | 0.96 | 101.87 | |
| 20 | 20.10 | 0.96 | 100.50 | 20.15 | 0.61 | 100.76 | |
| Liquiritin | 5 | 4.99 | 2.52 | 99.78 | 5.09 | 0.74 | 101.84 |
| 10 | 10.50 | 1.65 | 105.04 | 10.36 | 1.14 | 103.63 | |
| 20 | 20.49 | 1.13 | 102.46 | 20.44 | 0.73 | 102.18 | |
| Ferulic acid | 1.5 | 1.46 | 3.36 | 97.63 | 1.47 | 2.73 | 97.81 |
| 3 | 3.15 | 1.36 | 105.12 | 2.97 | 2.98 | 98.93 | |
| 6 | 6.28 | 0.84 | 104.63 | 5.85 | 2.90 | 97.42 | |
| Glycyrrhizin | 12.5 | 12.26 | 0.97 | 98.08 | 12.25 | 0.96 | 97.98 |
| 25 | 24.98 | 2.00 | 99.92 | 24.47 | 0.86 | 97.90 | |
| 50 | 49.44 | 0.38 | 98.88 | 49.51 | 0.42 | 99.02 | |
| Decursin | 20 | 19.74 | 1.42 | 98.71 | 19.68 | 1.48 | 98.42 |
| 40 | 41.51 | 0.97 | 103.78 | 40.47 | 1.22 | 101.17 | |
| 80 | 81.37 | 0.72 | 101.71 | 81.34 | 0.53 | 101.67 | |
| Decursinol angelate | 10 | 9.58 | 0.93 | 95.76 | 9.51 | 2.61 | 95.12 |
| 20 | 19.55 | 0.73 | 97.73 | 19.50 | 0.45 | 97.51 | |
| 40 | 39.13 | 0.43 | 97.83 | 39.11 | 0.46 | 97.78 | |
| Atractylenolide I | 1 | 0.98 | 2.53 | 98.40 | 1.00 | 1.14 | 100.07 |
| 2 | 2.05 | 1.48 | 102.60 | 2.02 | 0.95 | 101.23 | |
| 4 | 4.02 | 0.98 | 100.50 | 4.01 | 0.69 | 100.37 | |
| Compound | Retention Time (min) | Peak Area (AU) | ||
|---|---|---|---|---|
| Mean ± SD | RSD (%) | Mean ± SD | RSD (%) | |
| Liquiritin apioside | 12.48 ± 0.05 | 0.43 | 234009.83 ± 1419.74 | 0.61 |
| Liquiritin | 13.00 ± 0.06 | 0.49 | 143858.00 ± 903.60 | 0.63 |
| Ferulic acid | 14.09 ± 0.08 | 0.55 | 212067.33 ± 1604.31 | 0.76 |
| Glycyrrhizin | 33.46 ± 0.18 | 0.52 | 144019.50 ± 726.13 | 0.50 |
| Decursin | 42.93 ± 0.07 | 0.16 | 1905525.83 ± 12044.19 | 0.63 |
| Decursinol angelate | 43.45 ± 0.07 | 0.16 | 1094712.83 ± 4431.44 | 0.40 |
| Atractylenolide I | 50.27 ± 0.12 | 0.24 | 234499.67 ± 1056.72 | 0.45 |
| Compound | Original Conc. (μg/mL) | Spiked Conc. (μg/mL) | Found Conc. (μg/mL) | Recovery a) ± SD (%) | RSD (%) |
|---|---|---|---|---|---|
| Liquiritin apioside | 17.75 | 4 | 21.59 | 95.99 ± 0.75 | 0.78 |
| 10 | 27.86 | 101.14 ± 1.34 | 1.33 | ||
| 20 | 37.56 | 99.07 ± 1.28 | 1.29 | ||
| Liquiritin | 21.27 | 4 | 25.46 | 104.94 ± 0.84 | 0.80 |
| 10 | 31.61 | 103.49 ± 1.72 | 1.66 | ||
| 20 | 41.42 | 100.80 ± 1.41 | 1.40 | ||
| Ferulic acid | 6.63 | 1.5 | 8.16 | 101.49 ± 1.55 | 1.53 |
| 3 | 9.73 | 103.14 ± 0.76 | 0.74 | ||
| 6 | 12.79 | 102.70 ± 0.61 | 0.60 | ||
| Glycyrrhizin | 9.21 | 2.5 | 11.63 | 97.04 ± 1.32 | 1.36 |
| 5 | 14.08 | 97.45 ± 1.21 | 1.24 | ||
| 10 | 18.90 | 96.94 ± 1.40 | 1.44 | ||
| Decursin | 14.81 | 4 | 18.84 | 100.68 ± 3.23 | 3.21 |
| 8 | 22.56 | 96.77 ± 1.05 | 1.08 | ||
| 16 | 30.48 | 97.91 ± 0.75 | 0.77 | ||
| Decursinol angelate | 6.90 | 2 | 8.85 | 97.26 ± 1.16 | 1.20 |
| 4 | 10.76 | 96.53 ± 0.47 | 0.48 | ||
| 8 | 15.22 | 104.00 ± 0.35 | 0.33 | ||
| Atractylenolide I | 2.08 | 1 | 3.11 | 102.93 ± 1.30 | 1.26 |
| 2 | 4.14 | 102.68 ± 1.49 | 1.46 | ||
| 4 | 5.97 | 97.24 ± 0.42 | 0.43 |
| μM | 1 | 2 | 3 | 4 | 5 | 6 | 7 | nM | L-ascorbicAcid * |
|---|---|---|---|---|---|---|---|---|---|
| ABTS | |||||||||
| 0 | 0.0 ± 0.9 | 0.0 ± 0.9 | 0.0 ± 0.9 | 0.0 ± 0.9 | 0.0 ± 0.9 | 0.0 ± 0.9 | 0.0 ± 0.9 | 0 | 0.0 ± 0.9 |
| 1.5625 | 2.3 ± 0.6 | 1.9 ± 0.3 | 20.8 ± 0.7 | 0.7 ± 0.9 | 0.1 ± 0.3 | –0.2 ± 0.3 | –1.1 ± 0.6 | 0.78125 | –0.9 ± 0.9 |
| 3.125 | 3.2 ± 0.6 | 3.5 ± 0.5 | 36.7 ± 0.4 | 0.0 ± 0.8 | 0.3 ± 0.2 | –1.1 ± 0.2 | 2.2 ± 1.3 | 1.5625 | 0.8 ± 0.6 |
| 6.25 | 7.0 ± 0.6 | 5.5 ± 0.4 | 38.0 ± 0.8 | –0.1 ± 0.7 | 0.7 ± 0.6 | 0.0 ± 0.2 | –0.4 ± 1.1 | 3.125 | -0.2 ± 0.5 |
| 12.5 | 10.1 ± 0.3 | 10.5 ± 0.1 | 64.4 ± 0.2 | –0.8 ± 0.9 | –1.5 ± 0.7 | –0.3 ± 0.2 | 0.4 ± 0.6 | 6.25 | 2.7 ± 0.8 |
| 25 | 18.8 ± 0.8 | 19.7 ± 0.8 | 99.7 ± 0.1 | 1.3 ± 0.1 | 0.1 ± 0.4 | –0.4 ± 0.6 | 1.0 ± 1.1 | 12.5 | 7.4 ± 0.5 |
| 50 | 28.3 ± 0.4 | 29.0 ± 0.6 | 99.8 ± 0.0 | 2.7 ± 1.0 | 0.5 ± 0.9 | 1.5 ± 0.2 | 2.0 ± 0.5 | 25 | 22.4 ± 0.6 |
| 100 | 43.6 ± 0.0 | 43.1 ± 0.7 | 100.0 ± 0.1 | 3.6 ± 0.2 | 0.0 ± 0.1 | 2.6 ± 0.3 | –0.7 ± 0.3 | 50 | 65.9 ± 1.0 |
| DPPH | |||||||||
| 0 | 0.0 ± 2.5 | 0.0 ± 2.5 | 0.0 ± 2.5 | 0.0 ± 2.5 | 0.0 ± 2.5 | 0.0 ± 2.5 | 0.0 ± 2.5 | 0 | 0.0 ± 2.5 |
| 1.5625 | 0.9 ± 0.2 | 0.9 ± 0.2 | 4.8 ± 0.5 | –0.1 ± 0.7 | 0.9 ± 0.7 | –0.2 ± 1.0 | 2.7 ± 0.4 | 0.78125 | 5.7 ± 1.1 |
| 3.125 | –0.2 ± 0.3 | –0.2 ± 0.3 | 8.6 ± 0.5 | –0.7 ± 0.7 | 0.1 ± 1.4 | 3.7 ± 1.0 | –0.5 ± 0.5 | 1.5625 | 9.1 ± 3.0 |
| 6.25 | 2.5 ± 1.0 | 2.5 ± 1.0 | 16.9 ± 0.1 | –1.7 ± 1.2 | 0.3 ± 1.3 | 2.3 ± 0.5 | 0.4 ± 0.8 | 3.125 | 12.4 ± 1.0 |
| 12.5 | 2.8 ± 0.5 | 2.8 ± 0.5 | 34.6 ± 2.0 | –0.9 ± 0.6 | 0.6 ± 0.8 | 3.6 ± 1.4 | 1.0 ± 0.7 | 6.25 | 21.3 ± 1.0 |
| 25 | 1.7 ± 0.7 | 1.7 ± 0.7 | 55.1 ± 1.7 | 0.5 ± 1.3 | 0.5 ± 0.3 | 5.5 ± 1.4 | 1.3 ± 1.0 | 12.5 | 41.5 ± 3.6 |
| 50 | 4.1 ± 1.2 | 4.1 ± 1.2 | 75.5 ± 0.9 | 1.9 ± 0.9 | 0.4 ± 0.2 | 4.1 ± 1.2 | 3.1 ± 0.5 | 25 | 61.8 ± 1.4 |
| 100 | 2.2 ± 1.3 | 2.2 ± 1.3 | 87.8 ± 0.5 | 4.1 ± 0.4 | 0.2 ± 1.6 | 0.9 ± 1.6 | –1.8 ± 0.6 | 50 | 82.0 ± 1.2 |
| Latin Name | Scientific Name | Amount (g) | Origin |
|---|---|---|---|
| Uncariae Ramulus et Uncus | Uncaria sinensis | 6 | China |
| Atractylodis Rhizoma Alba | Atractylodes japonica | 8 | China |
| Poria Sclerotium | Poria cocos | 8 | China |
| Bupleuri Radix | Bupleurum falcatum | 4 | China |
| Angelicae Gigantis Radix | Angelica gigas | 6 | Bonghwa, Korea |
| Cnidii Rhizoma | Cnidium officinale | 6 | China |
| Glycyrrhizae Radix et Rhizoma | Glycyrrhiza uralensis | 3 | China |
| Total amount | 41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.J.; Jeong, S.-J.; Seo, C.-S.; Lim, H.-S.; Sohn, E.; Yun, J.; Kim, B.-Y. Simultaneous Determination of the Traditional Herbal Formula Ukgansan and the In Vitro Antioxidant Activity of Ferulic Acid as an Active Compound. Molecules 2018, 23, 1659. https://doi.org/10.3390/molecules23071659
Kim YJ, Jeong S-J, Seo C-S, Lim H-S, Sohn E, Yun J, Kim B-Y. Simultaneous Determination of the Traditional Herbal Formula Ukgansan and the In Vitro Antioxidant Activity of Ferulic Acid as an Active Compound. Molecules. 2018; 23(7):1659. https://doi.org/10.3390/molecules23071659
Chicago/Turabian StyleKim, Yu Jin, Soo-Jin Jeong, Chang-Seob Seo, Hye-Sun Lim, Eunjin Sohn, Jiyeon Yun, and Bu-Yeo Kim. 2018. "Simultaneous Determination of the Traditional Herbal Formula Ukgansan and the In Vitro Antioxidant Activity of Ferulic Acid as an Active Compound" Molecules 23, no. 7: 1659. https://doi.org/10.3390/molecules23071659





