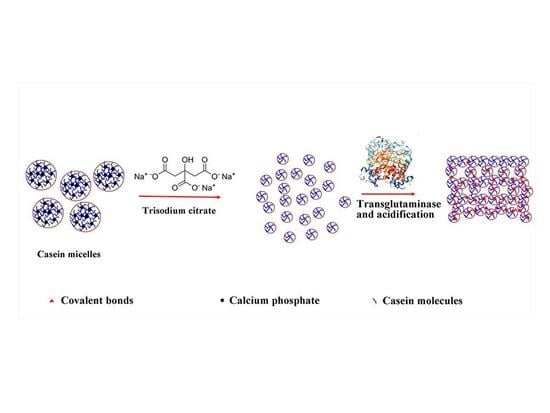
The Use of Trisodium Citrate to Improve the Textural Properties of Acid-Induced, Transglutaminase-Treated Micellar Casein Gels
Abstract
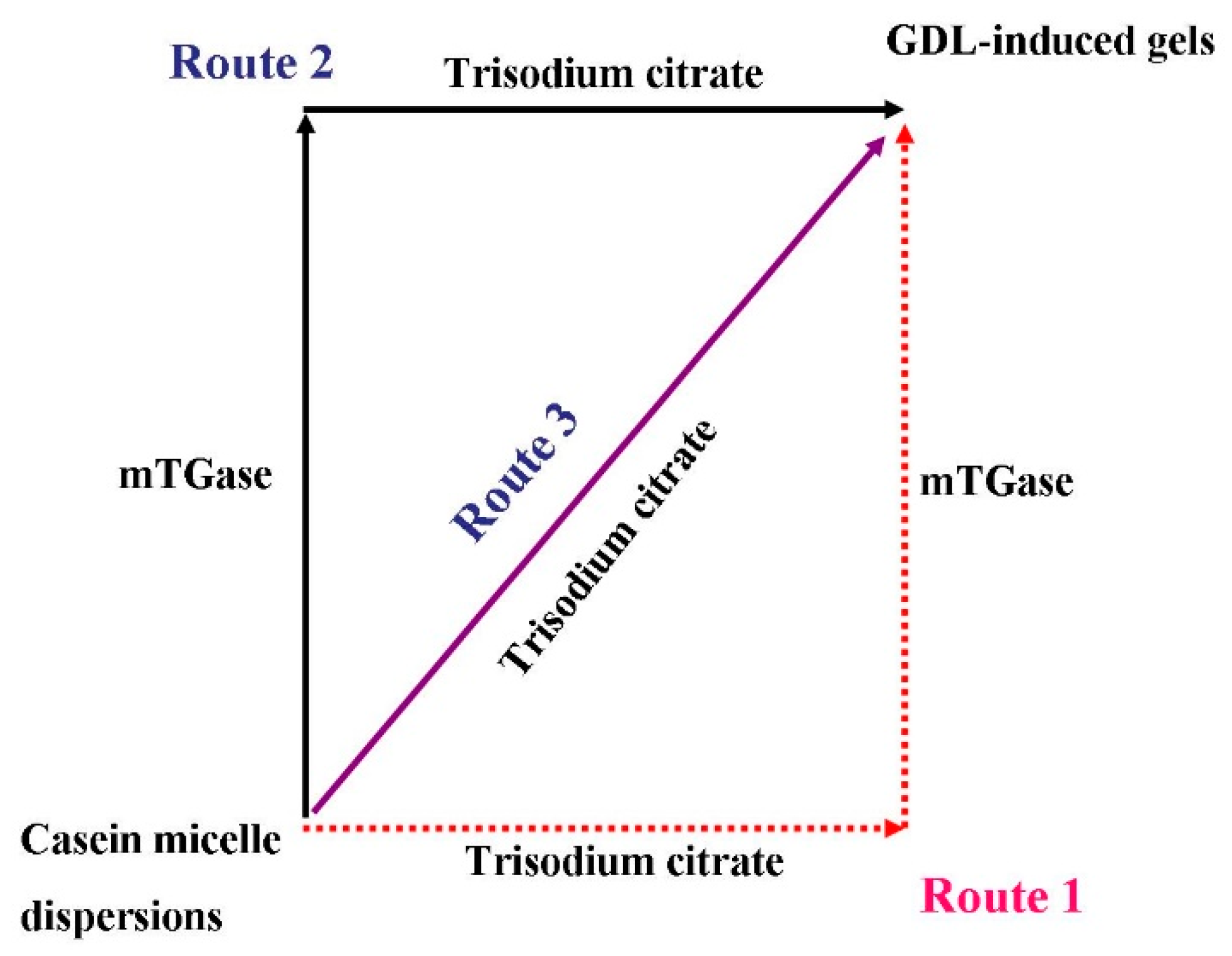
:1. Introduction
2. Results and Discussion
2.1. Particle Size of Casein Micelles and Soluble Calcium Contents
2.2. Gelation Kinetics
2.3. The Firmness of Acid-Induced Casein Gels
2.4. Water Holding Capacity of Acid-Induced Casein Gels
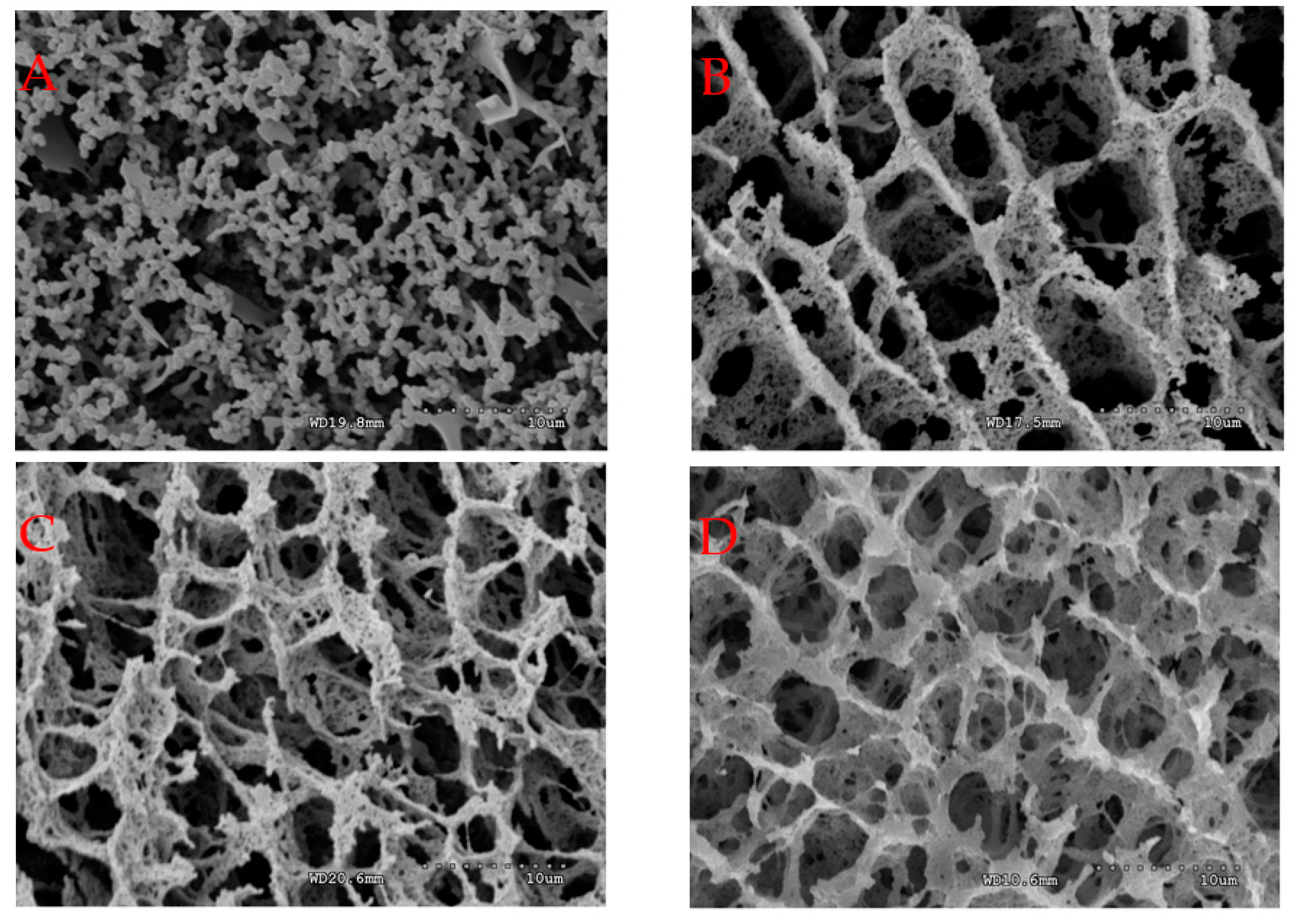
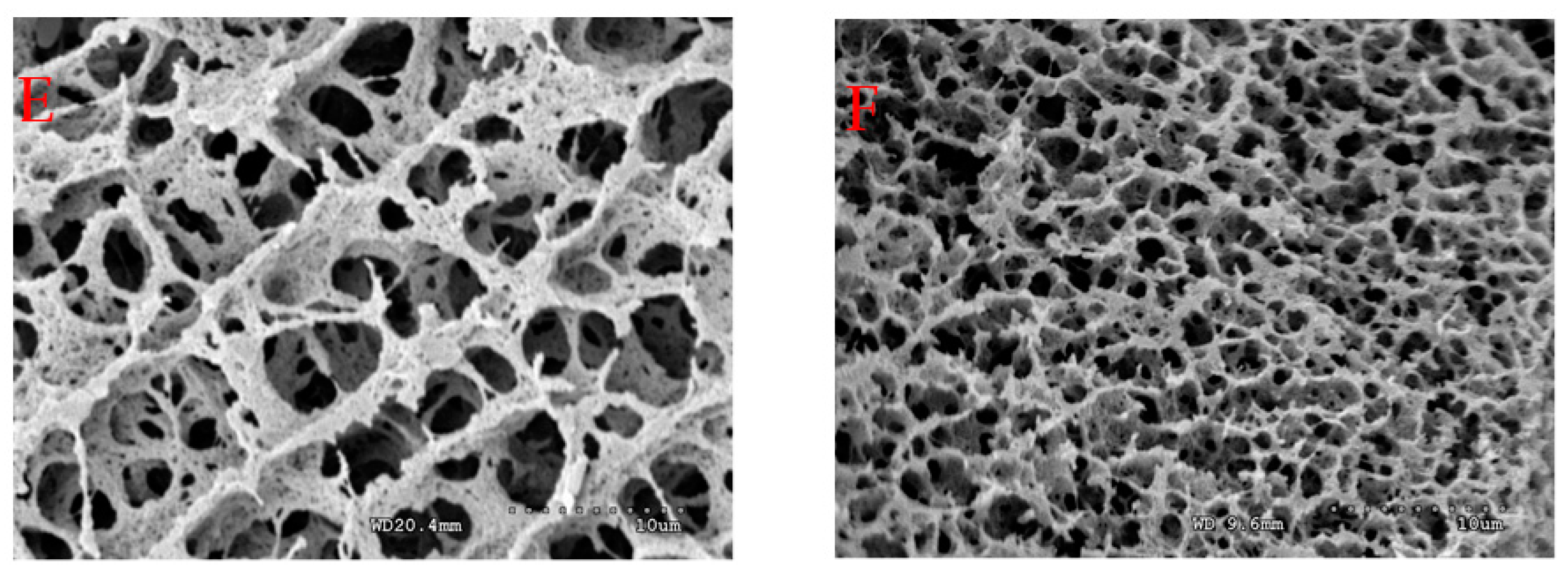
2.5. Microstructure and Degree of Cross-Linking of Acid-Induced Casein Gels
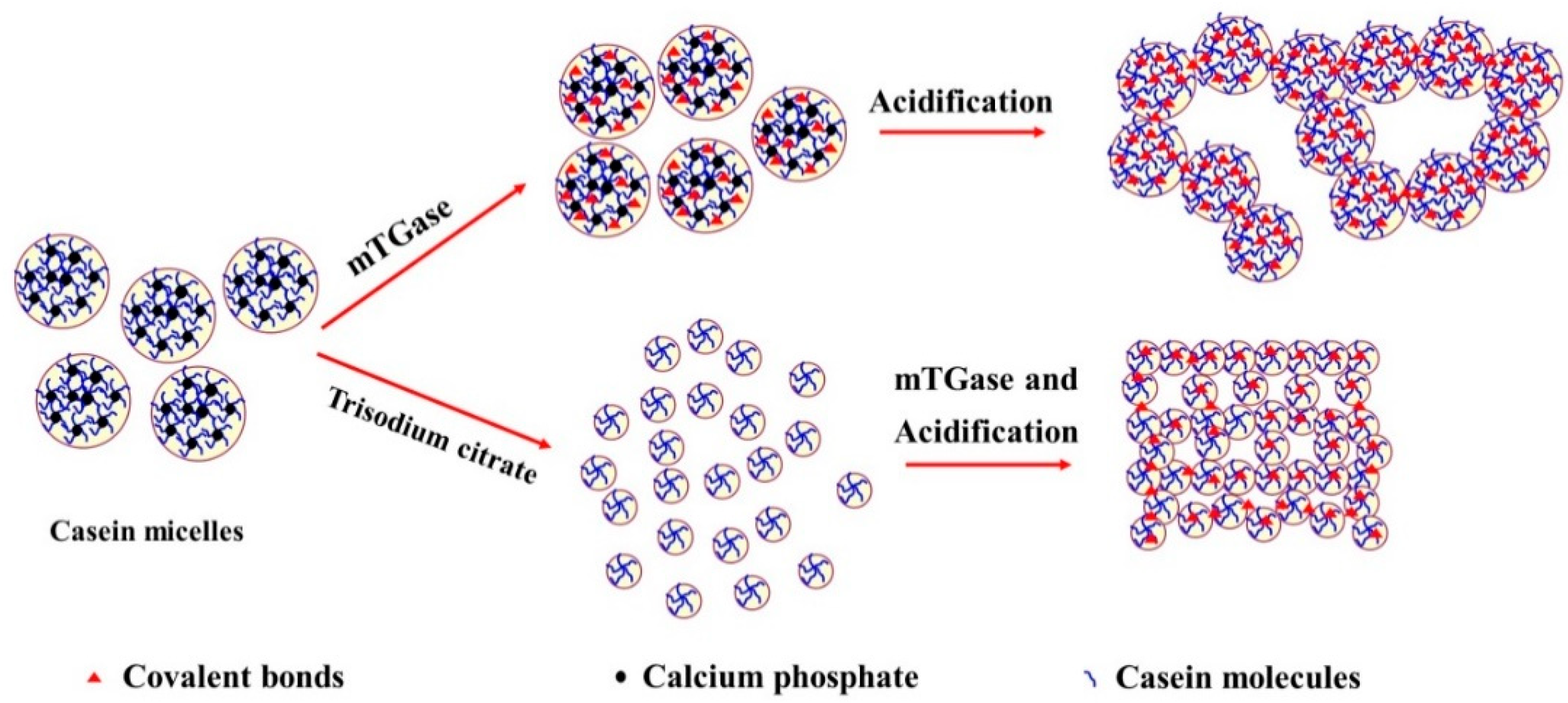
2.6. Proposed Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of Acid-Induced Micellar Casein Gels
3.2.1. Preparation of the Micellar Casein or mTGase Dispersions
3.2.2. Pretreatment of Casein Micelle Dispersions
3.2.3. Preparation of Acid-Induced Casein Gels
3.2.4. Characterization of Casein Micelle Dispersions or Gels
3.2.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of different hydrocolloids on texture, rheology, tribology and sensory perception of texture and mouthfeel of low-fat pot-set yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Faergemand, M.; Qvist, K.B. Transglutaminase: Effect on rheological properties, microstructure and permeability of set style acid skim milk gel. Food Hydrocoll. 1997, 11, 287–292. [Google Scholar] [CrossRef]
- Raak, N.; Rohm, H.; Jaros, D. Enzymatic cross-linking of casein facilitates gel structure weakening induced by overacidification. Food Biophys. 2017, 12, 261–268. [Google Scholar] [CrossRef]
- Raak, N.; Rohm, H.; Jaros, D. Cross-linking with microbial transglutaminase: Isopeptide bonds and polymer size as drivers for acid casein gel stiffness. Int. Dairy J. 2016, 66, 49–55. [Google Scholar] [CrossRef]
- Jaros, D.; Schwarzenbolz, U.; Raak, N.; Lobner, J.; Henle, T.; Rohm, H. Cross-linking with microbial transglutaminase: Relationship between polymerisation degree and stiffness of acid casein gels. Int. Dairy J. 2014, 38, 174–178. [Google Scholar] [CrossRef]
- Schorsch, C.; Carrie, H.; Clark, A.H.; Norton, I.T. Cross-linking casein micelles by a microbial transglutaminase conditions for formation of transglutaminase-induced gels. Int. Dairy J. 2000, 10, 519–528. [Google Scholar] [CrossRef]
- Vasbinder, A.J.; Rollema, H.S.; Bot, A.; de Kruif, C.G. Gelation mechanism of milk as influenced by temperature and pH; studied by the use of transglutaminase cross-linked casein micelles. J. Dairy Sci. 2003, 86, 1556–1563. [Google Scholar] [CrossRef]
- Hinz, K.; Huppertz, T.; Kelly, A.L. Susceptibility of the individual caseins in reconstituted skim milk to cross-linking by transglutaminase: Influence of temperature, pH and mineral equilibria. J. Dairy Res. 2012, 79, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Smiddy, M.A.; Martin, J.; Kelly, A.L.; de Kruif, C.G.; Huppertz, T. Stability of casein micelles cross-linked by transglutaminase. J. Dairy Sci. 2006, 89, 1906–1914. [Google Scholar] [CrossRef]
- Bijl, E.; van Valenberg, H.J.F.; Huppertz, T.; van Hooijdonk, A.C.M. Protein, casein, and micellar salts in milk: Current content and historical perspectives. J. Dairy Sci. 2013, 96, 5455–5464. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T. Chemistry of the Caseins. In Advanced Dairy Chemistry; Springer: Boston, MA, USA, 2013. [Google Scholar]
- De Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jordan, A.; Thomar, P.; Nicolai, T.; Dittmer, J. The effect of pH on the structure and phosphate mobility of casein micelles in aqueous solution. Food Hydrocoll. 2015, 51, 88–94. [Google Scholar] [CrossRef]
- Thorn, D.C.; Ecroyd, H.; Carver, J.A.; Holt, C. Casein structures in the context of unfolded proteins. Int. Dairy J. 2015, 46, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Schorsch, C.; Carrie, H.; Norton, I.T. Cross-linking casein micelles by a microbial transglutaminase: Influence of cross-links in acid-induced gelation. Int. Dairy J. 2000, 10, 529–539. [Google Scholar] [CrossRef]
- Boenisch, M.P.; Lauber, S.; Kulozik, U. Effect of ultra-high temperature treatment on the enzymatic cross-linking of micellar casein and sodium caseinate by transglutaminase. J. Food Sci. 2004, 69, E398–E404. [Google Scholar] [CrossRef]
- Thomar, P.; Nicolai, T. Dissociation of native casein micelles induced by sodium caseinate. Food Hydrocoll. 2015, 49, 224–231. [Google Scholar] [CrossRef]
- Silva, N.F.; Casanova, F.; Gaucheron, F.; de Carvalho Teixeira, A.V.; da Silva, G.M.; Minim, L.A.; de Carvalho, A.F. Combined effect of transglutaminase and sodium citrate on the microstructure and rheological properties of acid milk gel. Food Hydrocoll. 2018, 82, 304–311. [Google Scholar] [CrossRef]
- Sharma, R.; Lorenzen, P.C.; Qvist, K.B. Influence of transglutaminase treatment of skim milk on the formation of ε-(γ-glutamyl)lysine and the susceptibility of individual proteins towards crosslinking. Int. Dairy J. 2001, 11, 785–793. [Google Scholar] [CrossRef]
- Anema, S.G. Effect of whey protein addition and pH on the acid gelation of heated skim milk. Int. Dairy J. 2018, 79, 5–14. [Google Scholar] [CrossRef]
- Morand, M.; Guyomarc’h, F.; Pezennec, S.; Famelart, M.H. On how kappa-casein affects the interactions between the heat-induced whey protein/kappa-casein complexes and the casein micelles during the acid gelation of skim milk. Int. Dairy J. 2011, 21, 670–678. [Google Scholar] [CrossRef]
- Chai, J.; Jiang, P.; Wang, P.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Zhao, L.; Norde, W.; et al. The intelligent delivery systems for bioactive compounds in foods: Physicochemical and physiological conditions, absorption mechanisms, obstacles and responsive strategies. Trends Food Sci. Technol. 2018, 78, 144–154. [Google Scholar] [CrossRef]
- Destribats, M.; Rouvet, M.; Gehin-Delval, C.; Schmitt, C.; Binks, B.P. Emulsions stabilised by whey protein microgel particles: Towards food-grade Pickering emulsions. Soft Matter 2014, 10, 6941–6954. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kanti, F.; Gulotta, A.; Murray, B.S.; Zhang, S. Aqueous lubrication, structure and rheological properties of whey protein microgel particles. Langmuir 2017, 33, 14699–14708. [Google Scholar] [CrossRef] [PubMed]
- Khanji, A.N.; Michaux, F.; Salameh, D.; Rizk, T.; Banon, S.; Jasniewski, J. The study of curcumin interaction with micellar casein and lactic acid bacteria cell envelope. LWT 2018, 91, 293–302. [Google Scholar] [CrossRef]
- Gastaldi, E.; Lagaude, A. Micellar Transition State in Casein Between pH 5.5 and 5.0. J. Food Sci. 1996, 61, 59–64. [Google Scholar] [CrossRef]
- Ozcan-Yilsay, T.; Lee, W.J.; Horne, D.; Lucey, J.A. Effect of trisodium citrate on rheological and physical properties and microstructure of yogurt. J. Dairy Sci. 2007, 90, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Kruif, C.G.D.; Anema, S.G.; Zhu, C.; Havea, P.; Coker, C. Water holding capacity and swelling of casein hydrogels. Food Hydrocoll. 2015, 44, 372–379. [Google Scholar] [CrossRef]
- Zouari, A.; Marchesseau, S.; Chevalier-Lucia, D.; Raffard, G.; Ayadi, M.A.; Picart-Palmade, L. Acid gelation of raw and reconstituted spray-dried dromedary milk: A dynamic approach of gel structuring. Int. Dairy J. 2018, 81, 95–103. [Google Scholar] [CrossRef]
- Pang, Z.; Deeth, H.; Sharma, R.; Bansal, N. Effect of addition of gelatin on the rheological and microstructural properties of acid milk protein gels. Food Hydrocoll. 2015, 43, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Rha, C. Microstructure of soybean protein aggregates and its relation to the physical and textural properties of the curd. J. Food Sci. 2010, 43, 79–84. [Google Scholar] [CrossRef]
- Nieuwland, M.; Bouwman, W.G.; Bennink, M.L.; Silletti, E.; de Jongh, H.H. Characterizing length scales that determine the mechanical behavior of gels from crosslinked casein micelles. Food Biophys. 2015, 10, 416–427. [Google Scholar] [CrossRef]
- Serra, M.; Trujillo, A.J.; Jaramillo, P.D.; Guamis, B.; Ferragut, V. Ultra-high pressure homogenization-induced changes in skim milk: Impact on acid coagulation properties. J. Dairy Res. 2008, 75, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Franzoi, M.; Niero, G.; Penasa, M.; Cassandro, M.; De Marchi, M. Technical note: Development and validation of a new method for the quantification of soluble and micellar calcium, magnesium, and potassium in milk. J. Dairy Sci. 2018, 101, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Dumpler, J.; Kieferle, I.; Wohlschläger, H.; Kulozik, U. Milk ultrafiltrate analysis by ion chromatography and calcium activity for SMUF preparation for different scientific purposes and prediction of its supersaturation. Int. Dairy J. 2017, 68, 60–69. [Google Scholar] [CrossRef]
- Jenness, R. Preparation and properties of a salt solution which simulated milk ultrafiltrate. Neth. Milk Dairy J. 1962, 16, 153–164. [Google Scholar]
- Zang, J.; Chen, H.; Zhao, G.; Wang, F.; Ren, F. Ferritin cage for encapsulation and delivery of bioactive nutrients: From structure, property to applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 3673–3683. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, H.; Wen, P.; Zhang, H.; Guo, H.; Ren, F. The composition, size and hydration of yak casein micelles. Int. Dairy J. 2013, 31, 107–110. [Google Scholar] [CrossRef]
- Wang, P.; Jin, S.; Guo, H.; Zhao, L.; Ren, F. The pressure-induced, lactose-dependent changes in the composition and size of casein micelles. Food Chem. 2015, 173, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Chua, D.; Deeth, H.C.; Oh, H.E.; Bansal, N. Altering the casein to whey protein ratio to enhance structural characteristics and release of major yoghurt volatile aroma compounds of non-fat stirred yoghurts. Int. Dairy J. 2017, 74, 63–73. [Google Scholar] [CrossRef]
- Harbourne, N.; Jacquier, J.C.; O’Riordan, D. Effects of addition of phenolic compounds on the acid gelation of milk. Int. Dairy J. 2011, 21, 185–191. [Google Scholar] [CrossRef]
- Lucey, J.A.; Teo, C.T.; Munro, P.A.; Singh, H. Rheological properties at small (dynamic) and large (yield) deformations of acid gels made from heated milk. J. Dairy Res. 1997, 64, 591–600. [Google Scholar] [CrossRef]
- Mahomud, M.S.; Katsuno, N.; Zhang, L.; Nishizu, T. Physical, rheological, and microstructural properties of whey protein enriched yogurt influenced by heating the milk at different pH values. J. Food Process. Preserv. 2017, 41, e13236. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Biocompatible hydrogel formation of gelatin from cold water fish via enzymatic networking. Polym. J. 2007, 39, 1040–1046. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z.; Zou, R.; Zhang, X. The application of agar oligosaccharides in directly acidified milk drinks. Food Hydrocoll. 2017. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds mTGase and micellar casein powder are available/not available from the authors. |

| Treatment | Trisodium Citrate Concentration (mM) | |||
|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |
| Route 1 | 144.0 ± 6.0 a,A | 88.3 ± 2.9 b,A | 24.7 ± 1.7 c,A | 18.8 ± 1.8 c,A |
| Route 2 | 144.0 ± 6.0 a,A | 129.7 ± 0.8 a,B | 130.3 ± 1.2 a,B | 137.4 ± 2.2 a,B |
| Route 3 (Without mTGase) | 142.2 ± 2.2 a,A | 79.4 ± 9.5 b,A | 20.3 ± 2.9 c,A | 13.6 ± 1.6 c,A |
| Treatment | Trisodium Citrate Concentration (mM) | |||
|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |
| Route 1 | 86.7 ± 6.1 a,A | 114.8 ± 1.3 b,B | 175.2 ± 1.3 c,B | 179.4 ± 8.9 c,C |
| Route 2 | 86.7 ± 6.1 a,A | 68.9 ± 1.6 b,A | 65.0 ± 1.4 b,A | 61.8 ± 1.9 b,A |
| Route 3 (Without mTGase) | 67.8 ± 3.1 a,A | 65.7 ± 5.7 a,A | 71.5 ± 2.5 a,A | 69.7 ± 1.2 a,B |
| Treatment | Trisodium Citrate Concentration (mmol L−1) | |||
|---|---|---|---|---|
| 0 | 10 | 20 | 30 | |
| Route 1 | 65.4 ± 5.2 a,A | 95.4 ± 0.8 b,B | 98.7 ± 0.1 c,B | 97.7 ± 0.2 c,B |
| Route 2 | 65.4 ± 5.2 a,A | 59.4 ± 4.1 a,A | 66.0 ± 2.3 a,A | 64.8 ± 2.2 a,A |
| Route 3 (Without mTGase) | 58.6 ± 1.3 a,A | 63.5 ± 0.7 b,A | 61.4 ± 1.6 a,A | 60.1 ± 1.7 a,A |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Yang, C.; Chen, C.; Ren, F.; Li, Y.; Mu, Z.; Wang, P. The Use of Trisodium Citrate to Improve the Textural Properties of Acid-Induced, Transglutaminase-Treated Micellar Casein Gels. Molecules 2018, 23, 1632. https://doi.org/10.3390/molecules23071632
Li H, Yang C, Chen C, Ren F, Li Y, Mu Z, Wang P. The Use of Trisodium Citrate to Improve the Textural Properties of Acid-Induced, Transglutaminase-Treated Micellar Casein Gels. Molecules. 2018; 23(7):1632. https://doi.org/10.3390/molecules23071632
Chicago/Turabian StyleLi, Hongliang, Chang Yang, Chong Chen, Fazheng Ren, Yuan Li, Zhishen Mu, and Pengjie Wang. 2018. "The Use of Trisodium Citrate to Improve the Textural Properties of Acid-Induced, Transglutaminase-Treated Micellar Casein Gels" Molecules 23, no. 7: 1632. https://doi.org/10.3390/molecules23071632