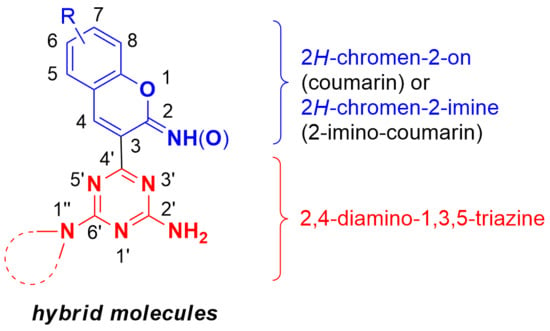
Hybrid Molecules Composed of 2,4-Diamino-1,3,5-triazines and 2-Imino-Coumarins and Coumarins. Synthesis and Cytotoxic Properties
Abstract
:1. Introduction
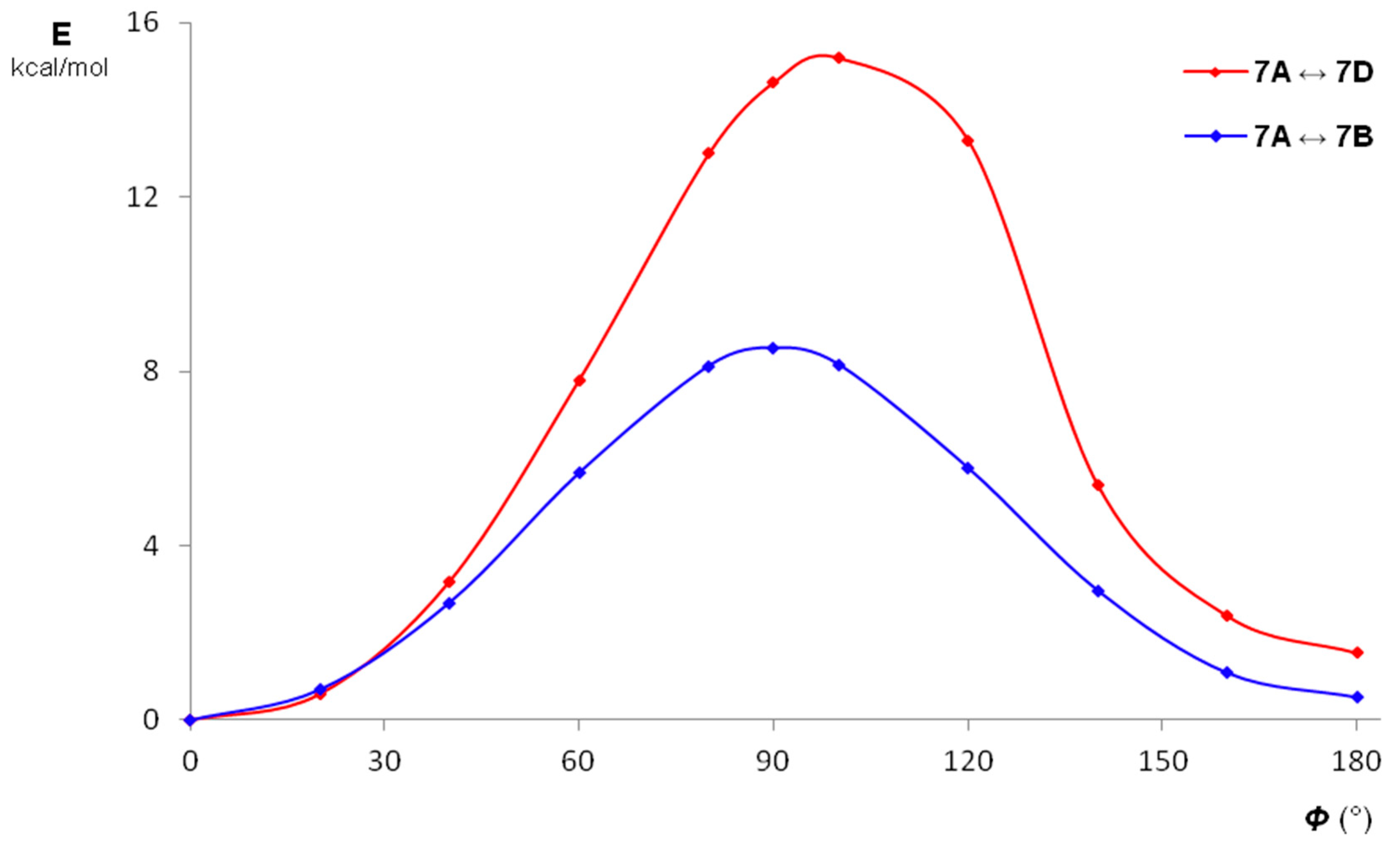
2. Results and Discussion
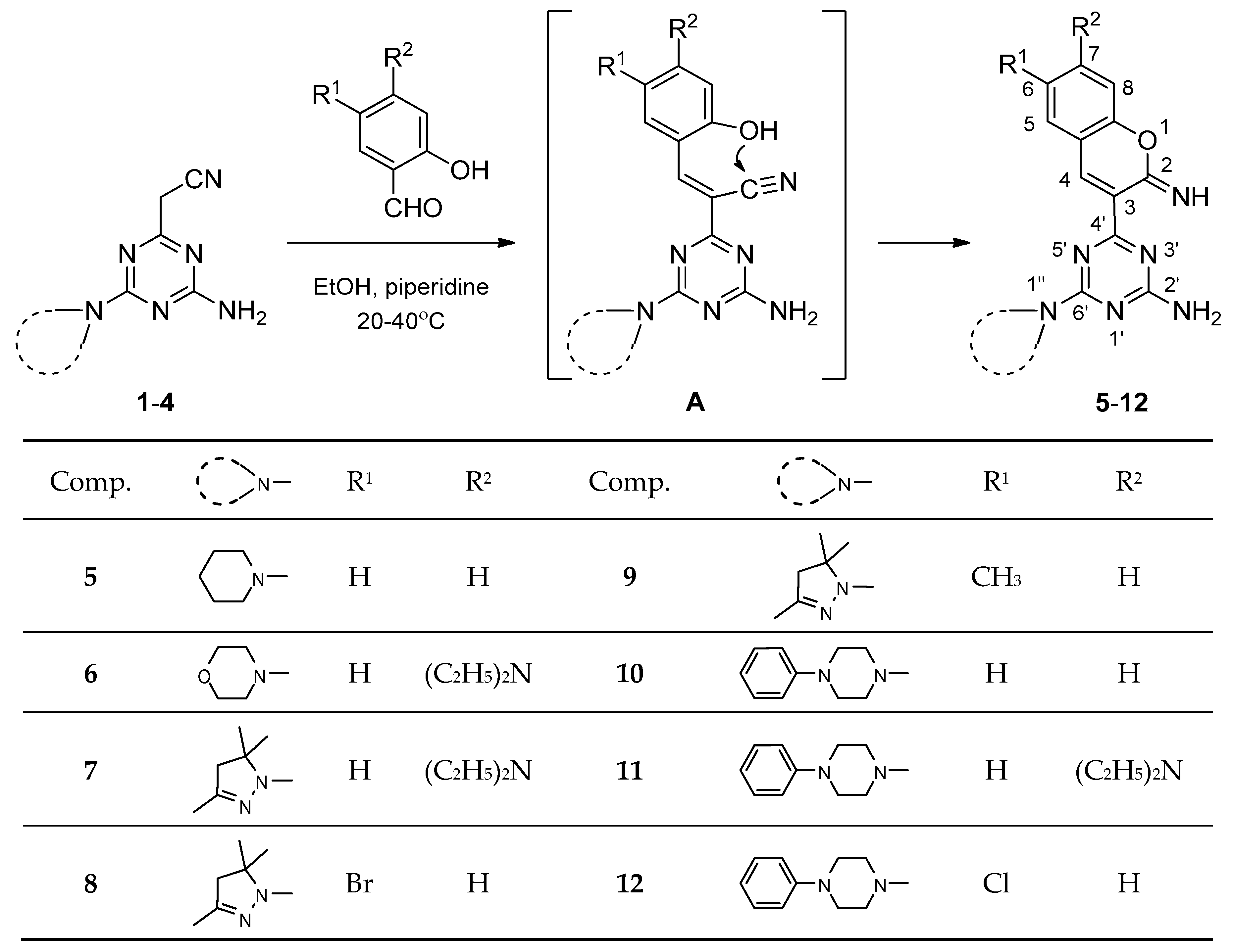
2.1. Synthesis of 2-Imino-2H-chromen-3-yl-1,3,5-triazine Derivatives (Imino-Coumarin Derivatives)
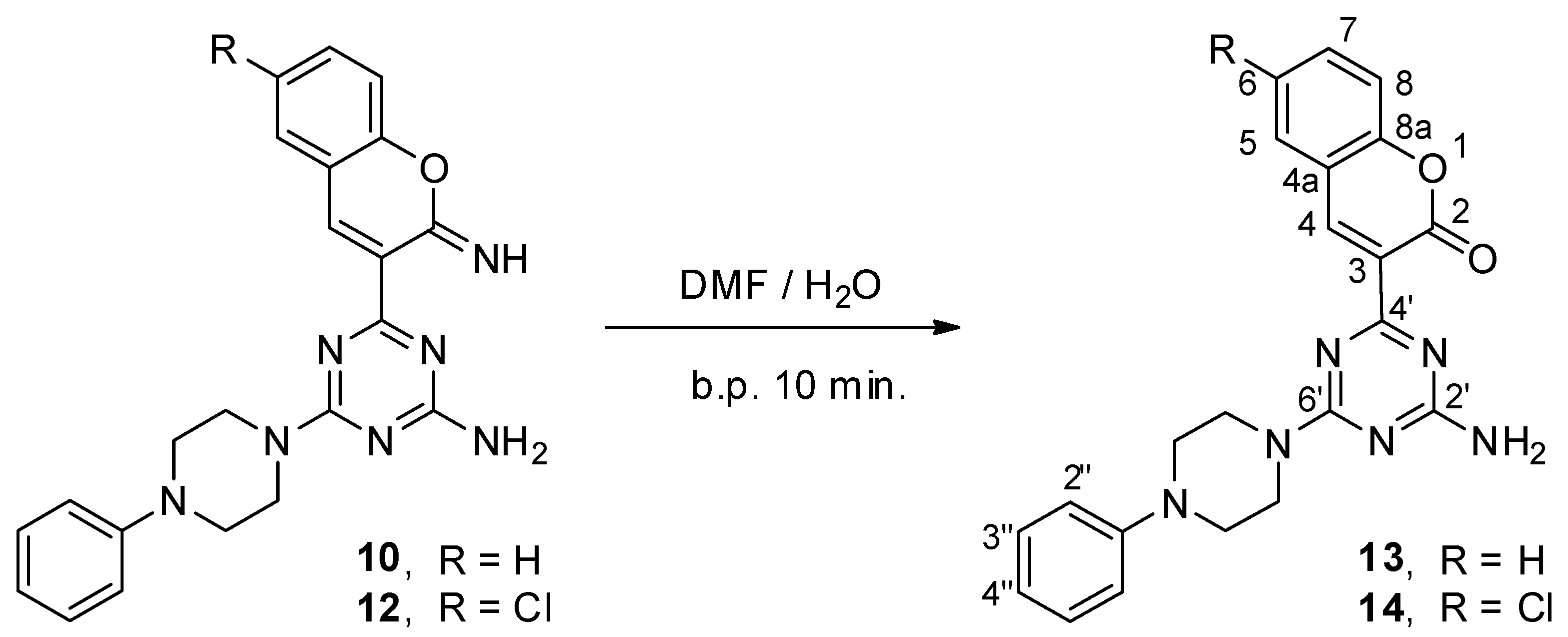
2.2. Synthesis of 2H-Chromen-3-yl-1,3,5-triazine Derivatives (Coumarin Derivatives)
2.3. In Vitro Cytotoxic Activity of 2-Imino-2H-chromen-3-yl-1,3,5-triazines (Imino-Coumarins 5–12) and 2H-Chromen-3-yl-1,3,5-triazines (Coumarins 13, 14)
3. Experimental Section
3.1. General Procedure for Preparation of Biguanide Hydrochlorides (Scheme 1)
3.2. Synthesis of 2-[4,6-Diamine-1,3,5-triazin-2-yl]acetonitrile Derivatives 1, 3 and 4
3.3. Synthesis of 2-(4-amino-6-morpholino-1,3,5-triazin-2-yl)acetonitrile (2)
3.4. Synthesis of 2-Imino-2H-chromen-3-yl-1,3,5-triazine Derivatives 5–12 (General Procedure)
3.5. Synthesis of 2H-chromen-3-yl-1,3,5-triazine Derivatives 13 and 14 (General Procedure)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Decker, M. Hybrid Molecules for Drug Development; Elsevier Ltd.: New York, NY, USA, 2017. [Google Scholar]
- Vance, D.; Shah, M.; Joshi, A.; Kane, R.S. Polyvalency: A promising strategy for drug design. Biotechnol. Bioeng. 2008, 101, 429–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.S.; Singh, P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016, 124, 500–536. [Google Scholar]
- Solomon, V.R.; Hu, C.; Lee, H. Hybrid pharmacophore design and synthesis of isatin-benzothiazole analogs for their anti-breast cancer activity. Bioorg. Med. Chem. 2009, 17, 7585–7592. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Reddy, K.S.; Khan, M.N.A.; Shetti, R.V.C.R.N.C.; Ramaiah, M.J.; Pushpavalli, S.N.C.V.L.; Srinivas, C.; Pal-Bhadra, M.; Chourasia, M.; Sastry, G.N.; et al. Synthesis, DNA-binding ability and anticancer activity of benzothiazole/benzoxazole-pyrrolo[2,1-c][1,4]benzodiazepine conjugates. Bioorg. Med. Chem. 2010, 18, 4747–4761. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.M.; Keely, N.O.; Bright, S.A.; Yassin, B.; Ana, G.; Fayne, D.; Zisterer, D.M.; Meegan, M.J. Novel selective estrogen receptor ligand conjugates incorporating endoxifen-combretastatin and cyclofenil-combretastatin hybrid scaffolds: Synthesis and biochemical evaluation. Molecules 2017, 22, 1440. [Google Scholar] [CrossRef] [PubMed]
- Lödige, M.; Hiersch, L. Design and synthesis of novel hybrid molecules against malaria. Int. J. Med. Chem. 2015, 2015, 458319. [Google Scholar] [CrossRef] [PubMed]
- Spilovska, K.; Korabecny, J.; Sepsova, V.; Jun, D.; Hrabinova, M.; Jost, P.; Muckova, L.; Soukup, O.; Janockova, J.; Kucera, T.; et al. Novel tacrine-scutellarin hybrids as multipotent anti-Alzheimer’s agents: Design, synthesis and biological evaluation. Molecules 2017, 22, 1006. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, C.; Zhang, S.; Hu, Z.; Wu, J.; Dong, C.; Huang, J.; Zhou, H.-B. Novel Bioactive Hybrid Compound Dual Targeting Estrogen Receptor and Histone Deacetylase for the Treatment of Breast Cancer. J. Med. Chem. 2015, 58, 4550–4572. [Google Scholar] [CrossRef] [PubMed]
- Theoduloz, C.; Delporte, C.; Valenzuela-Barra, G.; Silva, X.; Cádiz, S.; Bustamante, F.; Pertino, M.W.; Scheda-Hirschmann, G. Topical anti-inflammatory activity of new hybrid molecules of terpenes and synthetic drugs. Molecules 2015, 20, 11219–11235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Franco, M.I.; Fernández-Bachiller, M.I.; Pérez, C.; Hernández-Ledesma, B.; Bartolomé, B. Novel tacrine-melatonin hybrids as dual-acting drugs for Alzheimer disease, with improved acetylcholinesterase inhibitory and antioxidant properties. J. Med. Chem. 2006, 49, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bachiller, M.I.; Pérez, C.; Campillo, N.E.; Páez, A.; González-Muñoz, G.C.; Usán, P.; García-Palomero, E.; López, M.G.; Villarroya, M.; García, A.G.; et al. Tacrine-melatonin hybrids as multifunctional agents for Alzheimer’s disease, with cholinergic, antioxidant, and neuroprotective properties. ChemMedChem 2009, 4, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, D.; Chojnacki, J.; Du, Y.; Fu, H.; Grant, S.; Zhang, S. Design and biological characterization of hybrid compounds of curcumin and thalidomide for multiple myeloma. Org. Biomol. Chem. 2013, 11, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Decker, M. Hybrid molecules incorporating natural products: Applications in cancer therapy, neurodegenerative disorders and beyond. Curr. Med. Chem. 2011, 18, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Porwal, S.; Chauhan, S.S.; Chauhan, P.M.S.; Shakya, N.; Verma, A.; Gupta, S. Discovery of novel antileishmanial agents in an attempt to synthesize pentamidine-aplysinopsin hybrid molecule. J. Med. Chem. 2009, 52, 5793–5802. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Saekee, A.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, biological evaluation and molecular docking of novel chalcone-coumarin hybrids as anticancer and antimalarial agents. Eur. J. Med. Chem. 2014, 85, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Luxami, V.; Paul, K. Triazine as a promising scaffold for its versatile biological behavior. Eur. J. Med. Chem. 2015, 102, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. 1,3,5-Triazines: A promising scaffold for anticancer drugs. Eur. J. Med. Chem. 2017, 142, 523–549. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Faulds, D. Altretamine: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in cancer chemotherapy. Drugs 1995, 49, 932–953. [Google Scholar] [CrossRef] [PubMed]
- Coley, H.M.; Jarman, M.; Sargent, J.M.; Kubota, T.; Lee, N.C.; Goddard, P.M.; Elgie, A.W.; Williamson, C.; Taylor, C.G.; Judson, I.R. The activity of N-(hydroxymethyl) melamines in fresh human ovarian tumor cells and xenografts. Anticancer Res. 1996, 16, 1851–1855. [Google Scholar] [PubMed]
- Nozaki, S.; Maeda, M.; Tsuda, H.; Sledge, G.W., Jr. Inhibition of breast cancer regrowth and pulmonary metastasis in nude mice by anti-gastric ulcer agent, irsogladine. Breast Cancer Res. Treat. 2004, 83, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Melman, G.; LeTourneau, N.J.; Hays, A.M.; Melman, A. Synthesis and antiproliferating activity of iron chelators of hydroxyamino-1,3,5-triazine family. Bioorg. Med. Chem. Lett. 2010, 20, 458–460. [Google Scholar] [CrossRef] [PubMed]
- Arya, K.; Dandia, A. Synthesis and cytotoxic activity of trisubstituted-1,3,5-triazines. Bioorg. Med. Chem. Lett. 2007, 17, 3298–3304. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Narayanan, K.; Prasad, K.H.; Karthikeyan, D.; Chandrasekaran, L.; Atchudan, R.; Chidambaranathan, V. Synthesis, characterization, antiproliferative and apoptosis inducing effects of novel s-triazine derivatives. New J. Chem. 2018, 42, 1698–1714. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Pillai, G.G.; Bhat, H.R.; Verma, A.; Singh, U.P. Design and discovery of novel monastrol-1,3,5-triazines as potent anti-breast cancer agent via attenuating epidermal growth factor receptor tyrosine kinase. Sci. Rep. 2017, 7, 5851. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gupta, L.; Pal, P.; Khan, S.; Singh, N.; Katiyar, S.B.; Meena, S.; Sarkar, J.; Sinha, S.; Kanaujiya, J.K.; et al. Synthesis and cytotoxicity evaluation of (tetrahydro-β-carboline)-1,3,5-triazine hybrids as anticancer agents. Eur. J. Med. Chem. 2010, 45, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Moreau, D.; Jacquot, C.; Tsita, P.; Chinou, I.; Tomasoni, C.; Juge, M.; Antoniadou-Vyza, E.; Martignat, L.; Pineau, A.; Roussakis, C. Original triazine inductor of new specific molecular targets, with antitumor activity against nonsmall cell lung cancer. Int. J. Cancer 2008, 123, 2676–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riou, J.F.; Guittat, L.; Mailliet, P.; Laoui, A.; Renou, E.; Petitgenet, O.; Mégnin-Chanet, F.; Hélène, C.; Mergny, J.L. Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proc. Natl. Acad. Sci. USA 2002, 99, 2672–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popowycz, F.; Schneider, C.; DeBonis, S.; Skoufias, D.A.; Kozielski, F.; Galmarini, C.M.; Joseph, B. Synthesis and antiproliferative evaluation of pyrazolo[1,5-a]-1,3,5-triazine myoseverin derivatives. Bioorg. Med. Chem. 2009, 17, 3471–3478. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-S.; Jacobson, E.M.; Khersonsky, S.M.; Luzung, M.R.; Walsh, D.P.; Xiong, W.; Lee, J.W.; Parikh, P.B.; Lam, J.C.; Kang, T.-W.; et al. A novel microtubule destabilizing entity from orthogonal synthesis of triazine library and zebrafish embryo screening. J. Am. Chem. Soc. 2002, 124, 11608–11609. [Google Scholar] [CrossRef] [PubMed]
- Baindur, N.; Chadha, N.; Brandt, B.M.; Asgari, D.; Patch, R.J.; Schalk-Hihi, C.; Carver, T.E.; Petrounia, I.P.; Baumann, C.A.; Ott, H.; et al. 2-Hydroxy-4,6-diamino-[1,3,5]triazines: A novel class of VEGF-R2 (KDR) tyrosine kinase inhibitors. J. Med. Chem. 2005, 48, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Hodous, B.L.; Geuns-Meyer, S.D.; Hughes, P.E.; Albrecht, B.K.; Bellon, S.; Bready, J.; Caenepeel, S.; Cee, V.J.; Chaffee, S.C.; Coxon, A.; et al. Evolution of a highly selective and potent 2-(pyridin-2-yl)-1,3,5-triazine Tie-2 kinase inhibitor. J. Med. Chem. 2007, 50, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Kuo, G.H.; Deangelis, A.; Emanuel, S.; Wang, A.; Zhang, Y.; Connolly, P.J.; Chen, X.; Gruninger, R.H.; Rugg, C.; Fuentes-Pesquera, A.; et al. Synthesis and identification of [1,3,5]triazine-pyridine biheteroaryl as a novel series of potent cyclin-dependent kinase inhibitors. J. Med. Chem. 2005, 48, 4535–4546. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Perretta, C.; Erickson, P.; Margosiak, S.; Lu, J.; Averill, A.; Almassy, R.; Chu, S. Structure-based design and synthesis of novel macrocyclic pyrazolo[1,5-a] [1,3,5]triazine compounds as potent inhibitors of protein kinase CK2 and their anticancer activities. Bioorg. Med. Chem. Lett. 2008, 18, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Veerasamy, R.; Vaidya, A.; Mourya, V.; Agrawal, R.K. QSAR analysis of some novel sulfonamides incorporating 1,3,5-triazine derivatives as carbonic anhydrase inhibitors. Med. Chem. Res. 2010, 19, 1191–1202. [Google Scholar] [CrossRef]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, J.-Y.; Montero, J.-L.; Scozzafava, A.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Novel sulfonamides incorporating 1,3,5-triazine moieties as inhibitors of the cytosolic and tumour-associated carbonic anhydrase isozymes I, II and IX. Bioorg. Med. Chem. Lett. 2005, 15, 3102–3108. [Google Scholar] [CrossRef] [PubMed]
- Saluja, A.K.; Tiwari, M.; Vullo, D.; Supuran, C.T. Substituted benzene sulfonamides incorporating 1,3,5-triazinyl moieties potently inhibit human carbonic anhydrases II, IX and XII. Bioorg. Med. Chem. Lett. 2014, 24, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kontogiorgis, C.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2015–2016). Expert Opin. Ther. Pat. 2017, 27, 1201–1226. [Google Scholar] [CrossRef] [PubMed]
- Nofal, Z.M.; El-Zahar, M.I.; Abd El-Karim, S.S. Novel coumarin derivatives with expected biological activity. Molecules 2000, 5, 99–113. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.S.; Mallireddigari, M.R.; Cosenza, S.; Gumireddy, K.; Bell, S.C.; Reddy, E.P.; Reddy, M.V.R. Synthesis of new coumarin 3-(N-aryl) sulfonamides and their anticancer activity. Bioorg. Med. Chem. Lett. 2004, 14, 4093–4097. [Google Scholar] [CrossRef] [PubMed]
- Belluti, F.; Fontana, G.; Dal Bo, L.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Finn, G.J.; Creaven, B.; Egan, D.A. Study of the in vitro cytotoxic potential of natural and synthetic coumarin derivatives using human normal and neoplastic skin cell lines. Melanoma Res. 2001, 11, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef] [PubMed]
- Donnely, A.C.; Mays, J.R.; Burlison, J.A.; Nelson, J.T.; Vielhauer, G.; Holzbeierlein, J.; Blagg, B.S.J. The design, synthesis, and evaluation of coumarin ring derivatives of the novobiocin scaffold that exhibit antiproliferative activity. J. Org. Chem. 2008, 73, 8901–8920. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzales, J.S.; Prado-Garcia, H.; Aguilar-Cazares, D.; Molina-Guarneros, J.A.; Morales-Fuentes, J.; Mandoki, J.J. Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer 2004, 43, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.; Singh, R.P.; Agarwal, C.; Lee, S.; Chi, H.; Agarwal, R. A novel anticancer agent, decursin, induces G1 arrest and apoptosis in human prostate carcinoma cells. Cancer Res. 2005, 65, 1035–1044. [Google Scholar] [PubMed]
- Payne, S.L.; Rodriguez-Aristegui, S.; Bardos, J.; Cano, C.; Golding, B.T.; Hardcastle, I.R.; Peacock, M.; Parveen, N.; Griffin, R.J. Mapping the ATP-binding domain of DNA-dependent protein kinase (DNA-PK) with coumarin- and isocoumarin-derived inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3649–3653. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhou, V.; Pan, S.; Liu, Y.; Hornsby, M.; McMullan, D.; Klock, H.E.; Haugen, J.; Lesley, S.A.; Gray, N.; et al. Identification of coumarin derivatives as a novel class of allosteric MEK1 inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5467–5473. [Google Scholar] [CrossRef] [PubMed]
- Starčević, Ŝ.; Brožič, P.; Turk, S.; Cesar, J.; Rižner, T.L.; Gobec, S. Synthesis and biological evaluation of (6- and 7-phenyl) coumarin derivatives as selective nonsteroidal inhibitors of 17β-hydroxysteroid dehydrogenase type 1. J. Med. Chem. 2011, 54, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.-A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Y.; Yan, W.; Wang, C.; Bai, P.; Wang, T.; Tang, M.; Wang, X.; Yang, Z.; Ma, B.; et al. Design, synthesis, and evaluation of in vitro and in vivo anticancer activity of 4-substituted coumarins: A novel class of potent tubulin polymerization inhibitors. J. Med. Chem. 2016, 59, 5721–5739. [Google Scholar] [CrossRef] [PubMed]
- Ganina, O.G.; Daras, E.; Bourgarel-Rey, V.; Peyrot, V.; Andresyuk, A.N.; Finet, J.-P.; Fedorov, A.Y.; Beletskaya, I.P.; Combes, S. Synthesis and biological evaluation of polymethoxylated 4-heteroarylcoumarins as tubulin assembly inhibitor. Bioorg. Med. Chem. 2008, 16, 8806–8812. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-N.; Kim, N.H.; Park, Y.S.; Kim, H.; Lee, S.; Wang, Q.; Kim, Y.K. 7-Diethylamino-3(2′-benzoxazolyl)-coumarin is a novel microtubule inhibitor with antimitotic activity in multidrug resistant cancer cells. Biochem. Pharmacol. 2009, 77, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sivakumar, K.; Shin, W.-S.; Xie, F.; Wang, Q. Synthesis and anti-angiogenesis activity of coumarin derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 4596–4599. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.N.; Conalty, M.L. Anticancer Agents XIII. Synthesis and antitumour activity of 2-iminochromene derivatives. Proc. R. Ir. Acad. Sect. B 1979, 79, 87–98. [Google Scholar]
- Huang, C.-K.; Wu, F.-Y.; Ai, Y.-X. Polyhydroxylated 3-(N-phenyl) carbamoyl-2-iminochromene derivatives as potent inhibitors of tyrosine kinase p60c-src. Bioorg. Med. Chem. Lett. 1995, 5, 2423–2428. [Google Scholar] [CrossRef]
- Huang, C.-K. 2-Iminochromene Derivatives as Inhibitors of Protein Tyrosine Kinase; WO 96/40670 (PTC/US96/07295); Research Corporation Technologies, Inc.: Tucson, AZ, USA, 1996. [Google Scholar]
- Burke, T.R.; Lim, B.; Marquez, V.E.; Li, Z.H.; Bolen, J.B.; Stefanova, I.; Horak, I.D. Bicyclic compounds as ring-constrained inhibitors of protein-tyrosine kinase p56lck1. J. Med. Chem. 1993, 36, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.K.; Kumari, J.; Bariwal, J. New 2-imino-2H-chromene-3(N-aryl)carboxamides as potential cytotoxic agents. Anti-Cancer Agents Med. Chem. 2017, 17, 85–92. [Google Scholar] [CrossRef]
- Brzozowski, Z.; Sączewski, F.; Gdaniec, M. Synthesis, structural characterization and antitumor activity of novel 2,4-diamino-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2000, 35, 1053–1064. [Google Scholar] [CrossRef]
- Brzozowski, Z.; Sączewski, F. Synthesis and antitumor activity of novel 2-amino-4-(3,5,5-trimethyl-2-pyrazolino)-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2002, 37, 709–720. [Google Scholar] [CrossRef]
- Sączewski, F.; Bułakowska, A. Synthesis, structure and anticancer activity of novel alkenyl-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2006, 41, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Sączewski, F.; Bułakowska, A.; Bednarski, P.; Grunert, R. Synthesis, structure and anticancer activity of novel 2,4-diamino-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2006, 41, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, Z.; Kamiński, Z.; Kozakiewicz, I.; Angielski, S.; Rogulski, J. Synteza I właściwości hipoglikemizujące niektórych pochodnych N-(2-pirazolino-1-karbonimidoilo)-guanidyny. Acta Pol. Pharm. 1979, 36, 401–410. [Google Scholar] [PubMed]
- Pomarnacka, E.; Bednarski, P.; Grunert, R.; Reszka, P. Synthesis and anticancer activity of novel 2-amino-4-(4-phenylpiperazino)-1,3,5-triazine derivatives. Acta Pol. Pharm. Drug Res. 2004, 61, 461–467. [Google Scholar]
- Wavefunction Inc. Molecular Modelling Studies were Performed at ab Initio Level Using the Density Functional (B3LYP) Method with the 6-31G* Basis Set as Implemented into SPARTAN Program Version ’08; Wavefunction Inc.: Irvine, CA, USA; Available online: www.wavefun.com (accessed on 25 June 2018).
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Dryanska, V. An Efficient one-pot synthesis of 3-(2-benzothiazolyl)coumarins. Synth. Commun. 1987, 17, 203–209. [Google Scholar] [CrossRef]
- Bracht, K.; Boubakari; Grünert, R.; Bednarski, J.P. Correlations between the activities of 19 antitumor agents and the intracellular glutathione concentrations in a panel of 14 human cancer cell lines: Comparisons with the National Cancer Institute data. Anticancer Drugs 2006, 11, 257–261. [Google Scholar]
- Shapiro, S.L.; Parrino, V.A.; Freedman, L. Hypoglycemic agents. III. N1-alkyl- and aralkylbiguanides. J. Am. Chem. Soc. 1959, 81, 3728–3736. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Yao, Y.; Zhou, L.; Zhao, J.; Gu, Y.; Wang, K.; Li, X. Design, synthesis and anti-tumor activities of novel triphenylene-coumarin hybrids, and their interaction with Ct-DNA. Bioorg. Med. Chem. Lett. 2013, 23, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, T.; Rehman, S.U.; Husain, M.A.; Ishiqi, H.M.; Tabish, M. Interaction of coumarin with calf thymus DNA: Deciphering the mode of binding by in vitro studies. Int. J. Biol. Macromol. 2015, 73, 9–16. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 5–14 are available from the authors. |



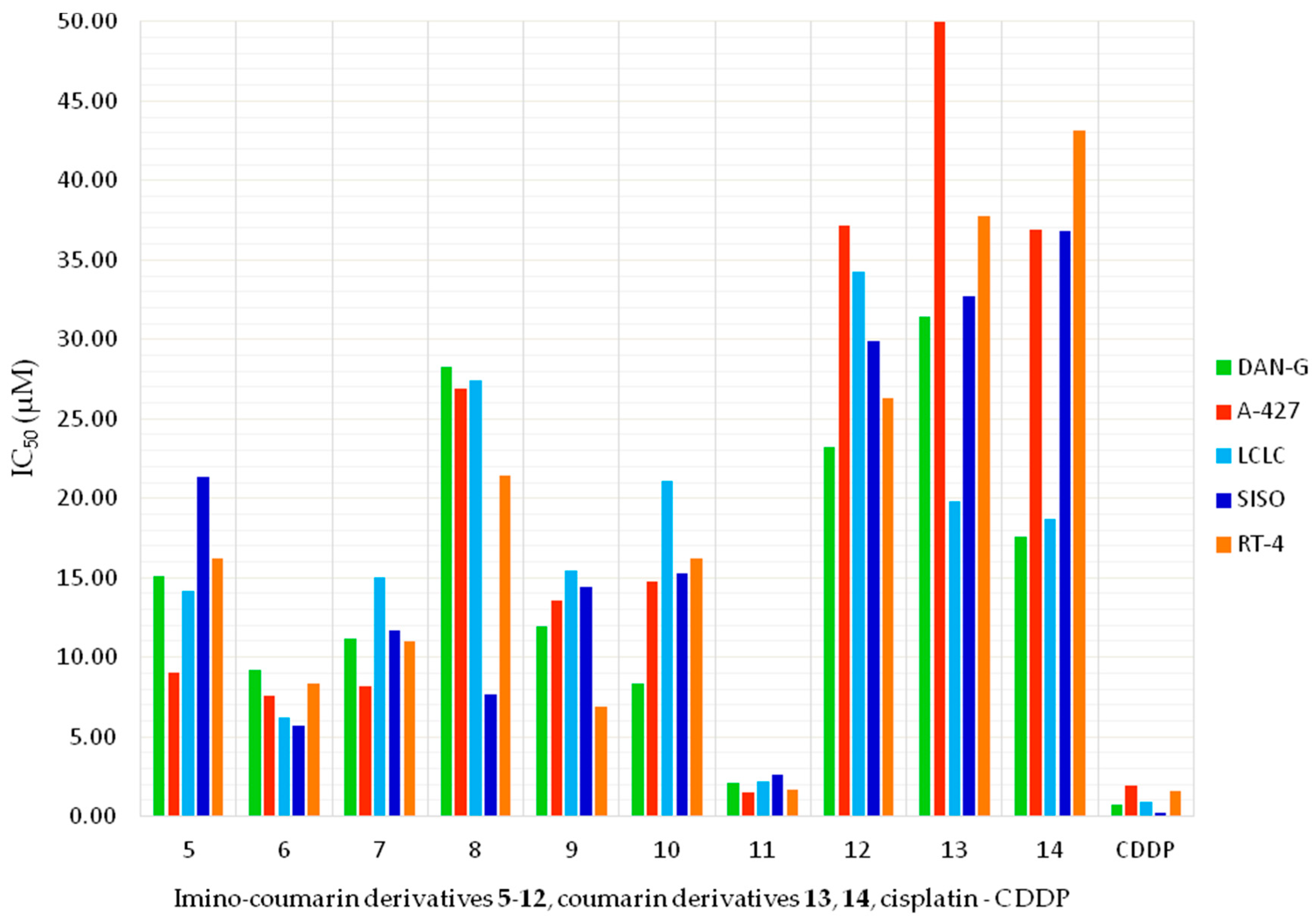

| No. | R | R1 | R2 | X | DAN-G | A-427 | LCLC | SISO | RT-4 |
|---|---|---|---|---|---|---|---|---|---|
| 5 | 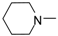 | H | H | NH | 15.12 ± 5.79 | 9.04 ± 4.55 | 14.17 ± 12.98 | 21.33 ± 2.69 | 16.24 ± 6.15 |
| 6 |  | H | (C2H5)2N | NH | 9.21 ± 0.59 | 7.62 ± 0.27 | 6.18 ± 1.26 | 5.67 ± 0.80 | 8.37 ± 1.66 |
| 7 | 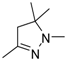 | H | (C2H5)2N | NH | 11.19 ± 0.55 | 8.16 ± 3.08 | 15.02 ± 0.82 | 11.64 ± 1.69 | 11.04 ± 6.49 |
| 8 |  | Br | H | NH | 28.25 ± 4.06 | 26.87 ± 2.45 | 27.42 ± 4.00 | 7.69 ± 1.72 | 21.40 ± 2.39 |
| 9 |  | CH3 | H | NH | 11.91 ± 0.52 | 13.56 ± 1.45 | 15.47 ± 1.84 | 14.44 ± 2.49 | 6.91 ± 0.62 |
| 10 | 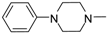 | H | H | NH | 8.35 ± 0.87 | 14.79 ± 0.45 | 21.12 ± 2.69 | 15.24 ± 0.73 | 16.24 ± 6.15 |
| 11 |  | H | (C2H5)2N | NH | 2.14 ± 0.25 | 1.51 ± 0.17 | 2.21 ± 0.39 | 2.60 ± 0.37 | 1.66 ± 0.25 |
| 12 |  | Cl | H | NH | 23.26 ± 6.62 | 37.19 ± 6.09 | 34.24 ± 3.05 | 29.86 ± 6.13 | 26.32 ± 2.99 |
| 13 |  | H | H | O | 31.44 ± 5.98 | >50 | 19.82 ± 1.12 | 32.73 ± 2.33 | 37.73 ± 3.13 |
| 14 |  | Cl | H | O | 15.87 ± 1.73 | 25.95 ± 10.95 | 17.15 ± 1.56 | 29.15 ± 7.64 | 36.14 ± 7.00 |
| cisplatin (CDDP) reference [73] | 0.73 ± 0.34 | 1.96 ± 0.54 | 0.90 ± 0.19 | 0.24 ± 0.06 | 1.61 ± 0.16 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowska, A.; Sączewski, F.; Bednarski, P.J.; Sączewski, J.; Balewski, Ł. Hybrid Molecules Composed of 2,4-Diamino-1,3,5-triazines and 2-Imino-Coumarins and Coumarins. Synthesis and Cytotoxic Properties. Molecules 2018, 23, 1616. https://doi.org/10.3390/molecules23071616
Makowska A, Sączewski F, Bednarski PJ, Sączewski J, Balewski Ł. Hybrid Molecules Composed of 2,4-Diamino-1,3,5-triazines and 2-Imino-Coumarins and Coumarins. Synthesis and Cytotoxic Properties. Molecules. 2018; 23(7):1616. https://doi.org/10.3390/molecules23071616
Chicago/Turabian StyleMakowska, Anna, Franciszek Sączewski, Patrick J. Bednarski, Jarosław Sączewski, and Łukasz Balewski. 2018. "Hybrid Molecules Composed of 2,4-Diamino-1,3,5-triazines and 2-Imino-Coumarins and Coumarins. Synthesis and Cytotoxic Properties" Molecules 23, no. 7: 1616. https://doi.org/10.3390/molecules23071616