Facile Preparation of Metal-Organic Framework (MIL-125)/Chitosan Beads for Adsorption of Pb(II) from Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
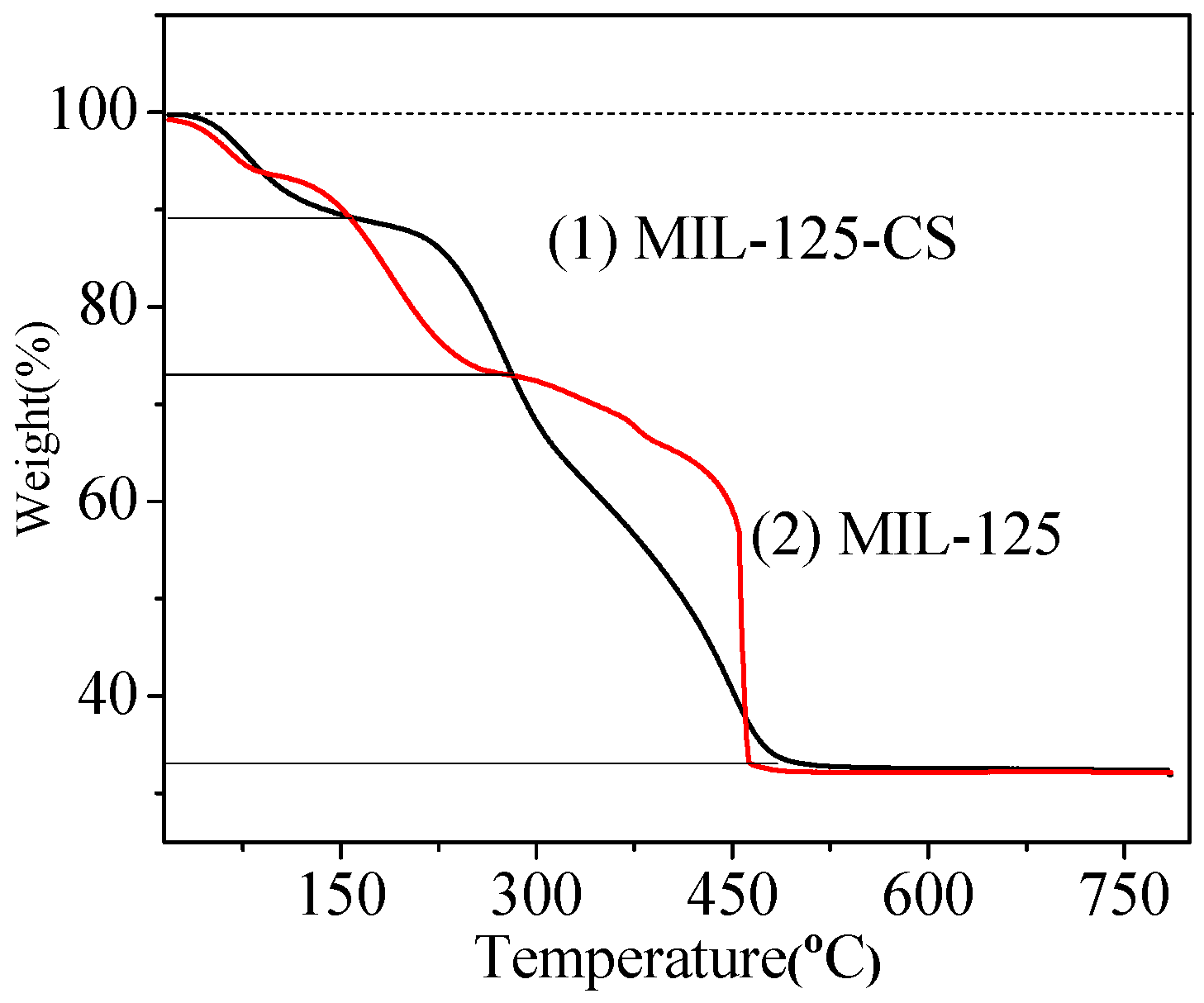
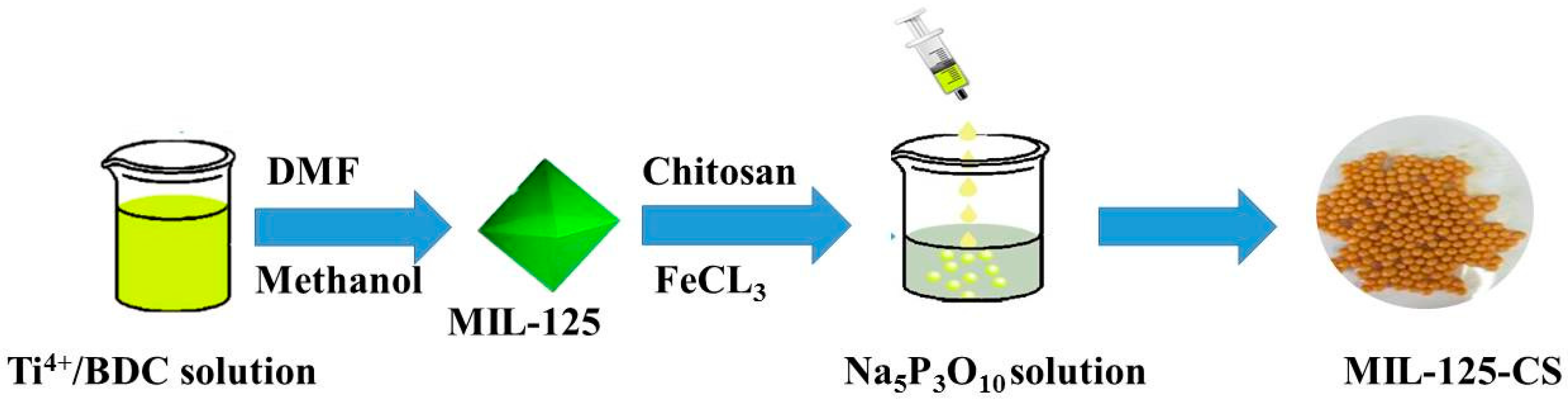
2.1. Synthesis and Characterization of MIL-125-CS
2.2. Adsorption Studies
2.2.1. Effect of the Contact Time
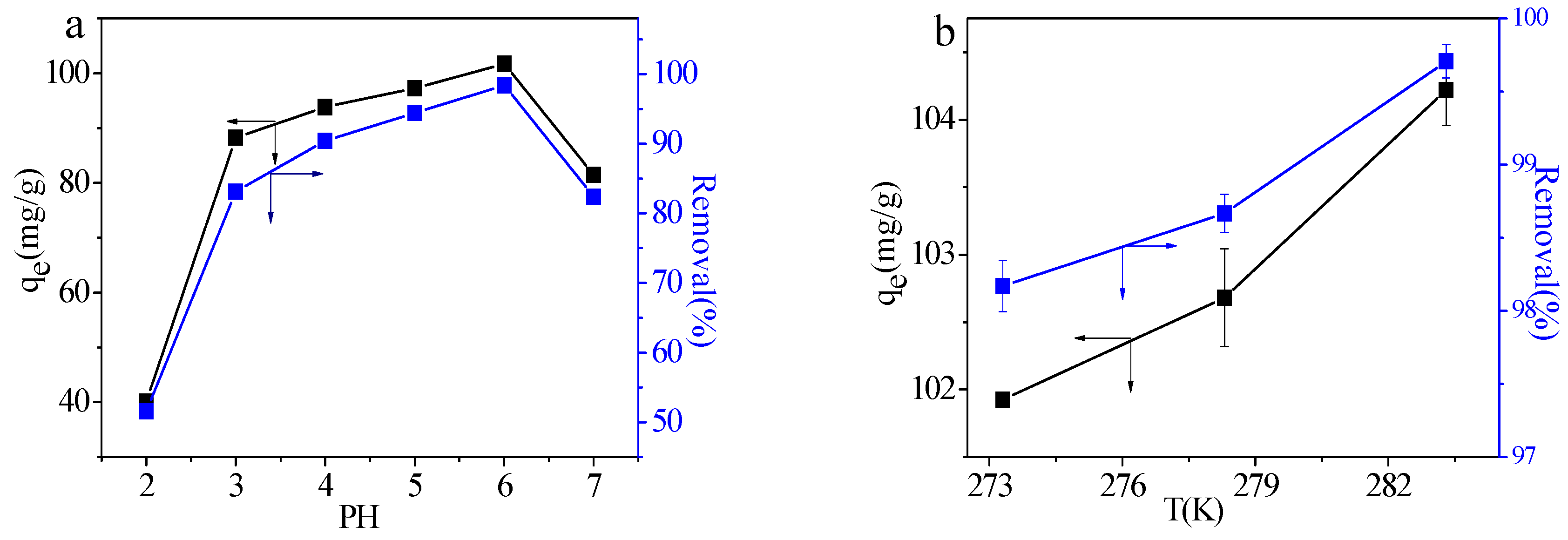
2.2.2. Effect of pH
2.2.3. Effect of the Temperature
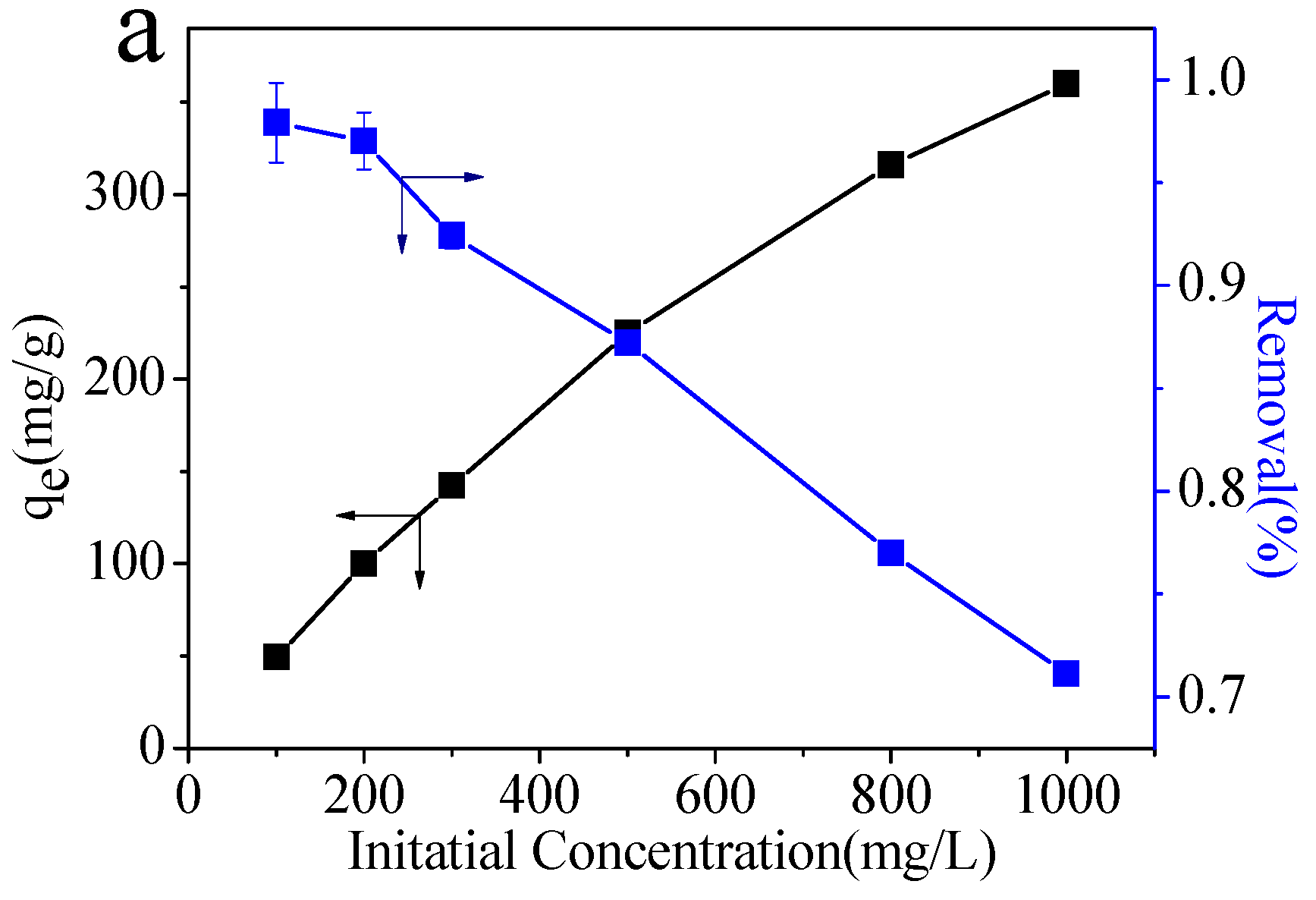
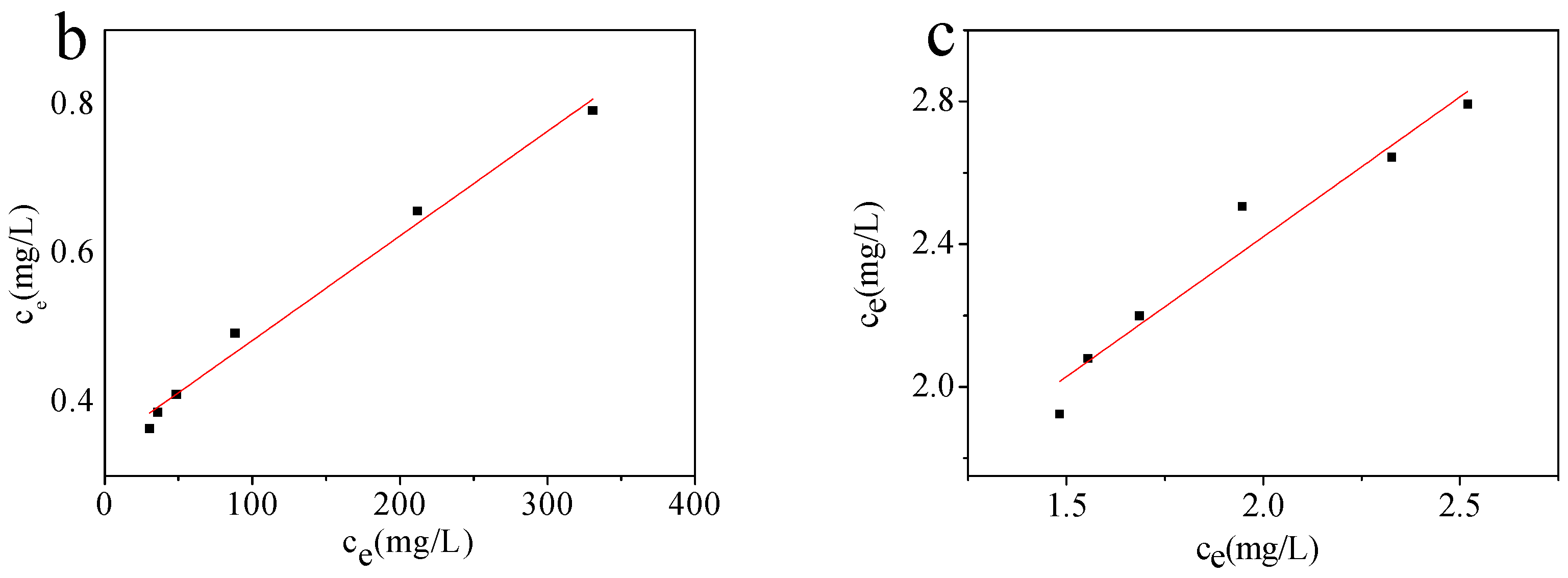
2.2.4. Effect of Pb(II) Concentration
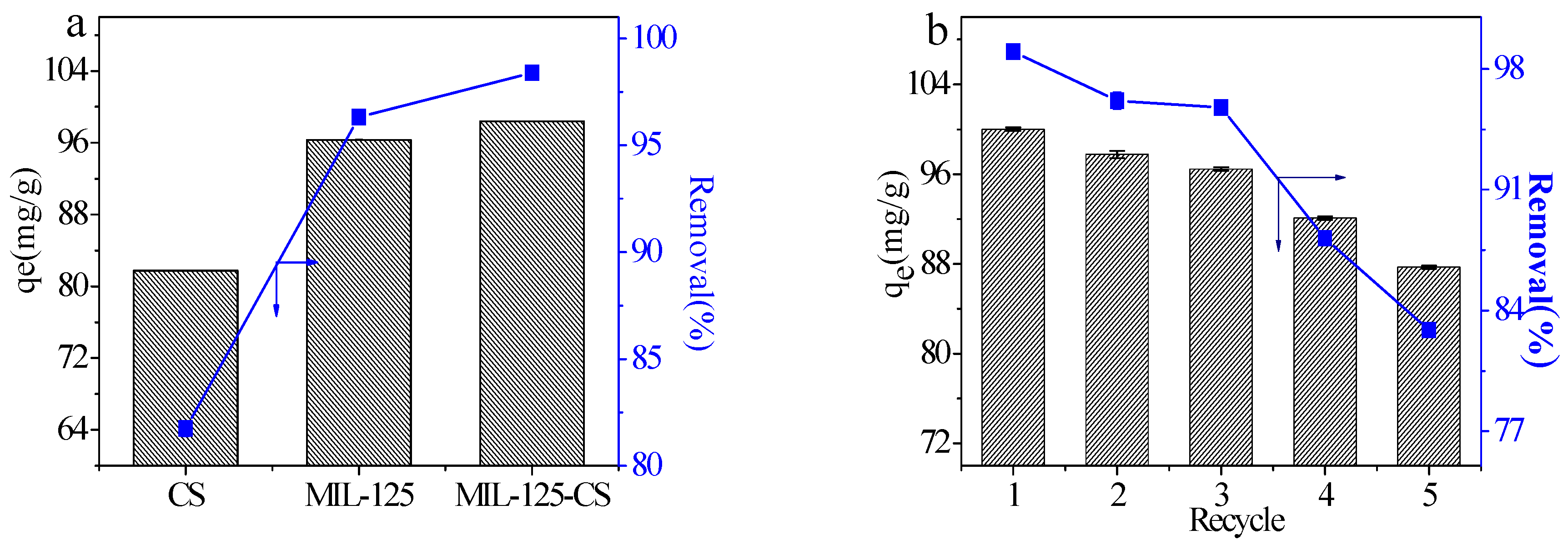
2.3. Comparison of the Adsorption Capacities of the Adsorbents
2.4. Reusability of MIL-125-CS Beads
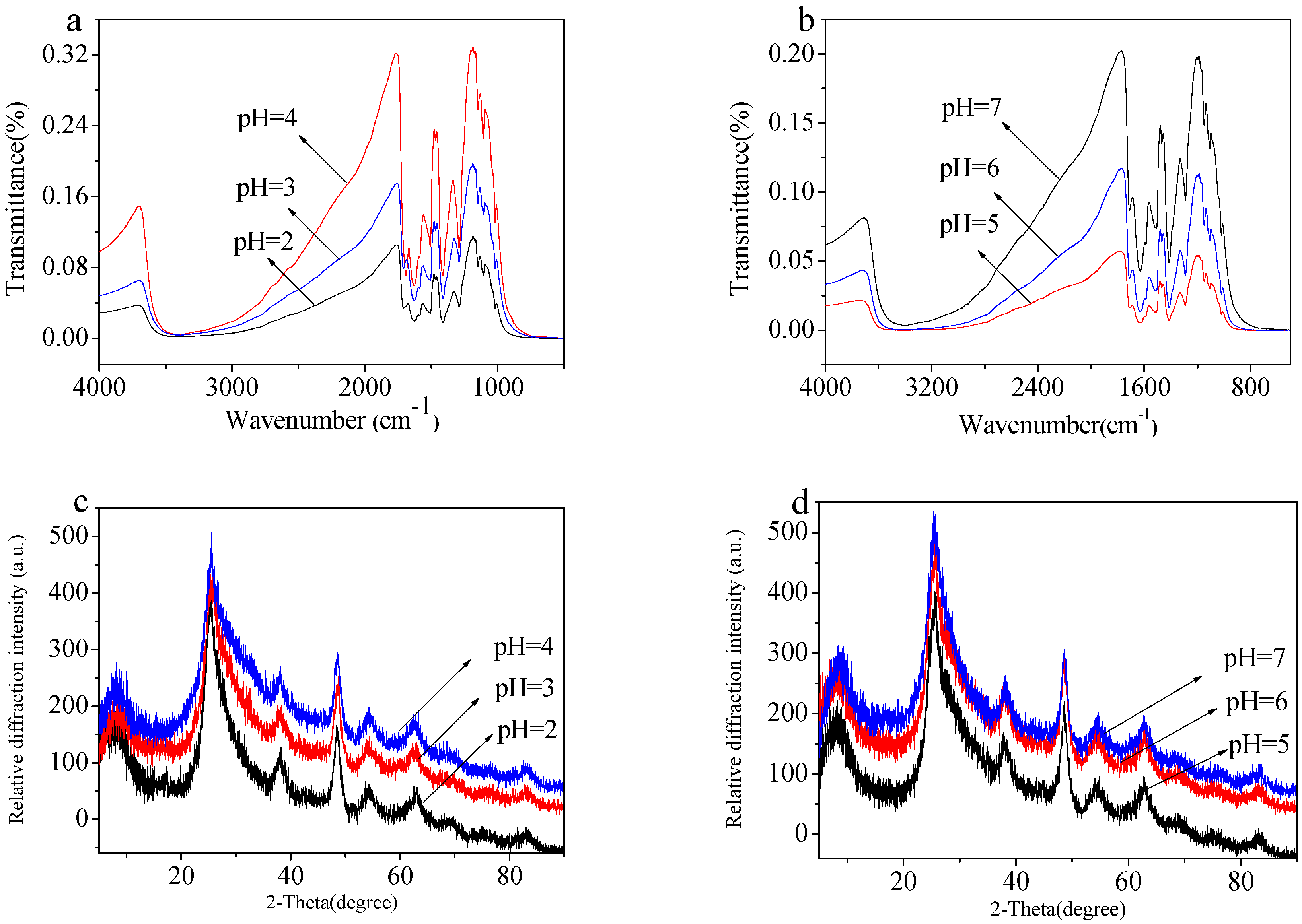
2.5. Stability of MIL-125
3. Materials and Methods
3.1. Materials
3.2. Preparation of MIL-125 and the MIL-125-CS Beads
3.3. Characterization
3.4. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, Z.H.; Omer, A.M.; Ouyang, X.K.; Yu, D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2018, 108, 149–157. [Google Scholar] [CrossRef] [PubMed]
- OuYang, X.K.; Jin, R.N.; Yang, L.P.; Wang, Y.G.; Yang, L.Y. Bamboo-derived porous bioadsorbents and their adsorption of Cd(II) from mixed aqueous solutions. RSC Adv. 2014, 4, 28699–28706. [Google Scholar] [CrossRef]
- Bindler, R.; Renberg, I.; Klaminder, J.; Emteryd, O. Tree rings as Pb pollution archives? A comparison of 206Pb/207Pb isotope ratios in pine and other environmental media. Sci. Total Environ. 2004, 319, 173–183. [Google Scholar] [CrossRef]
- Yang, H.; Linge, K.; Rose, N. The Pb pollution fingerprint at Lochnagar: The historical record and current status of Pb isotopes. Environ. Pollut. 2007, 145, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cheng, Z.; Ma, W. Adsorption of Pb(II) from glucose solution on thiol-functionalized cellulosic biomass. Bioresour. Technol. 2012, 104, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Shahzad, F.; Afzal, S.; Khan, M.I.; Ali, K. Assessment of Cd, Ni, Cu, and Pb pollution in Lahore, Pakistan. Environ. Int. 1998, 24, 761–766. [Google Scholar] [CrossRef]
- Odukoya, O.O.; Arowolo, T.A.; Bamgbose, O. Pb, Zn, and Cu levels in tree barks as indicator of atmospheric pollution. Environ. Int. 2000, 26, 11–16. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal-Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. ChemInform Abstract: Synthesis of Metal—Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 43, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Hermes, S.; Schröter, M.K.; Schmid, R.; Khodeir, L.; Muhler, M.; Tissler, A.; Fischer, R.W.; Fischer, R.A. Metal@MOF: Loading of Highly Porous Coordination Polymers Host Lattices by Metal Organic Chemical Vapor Deposition. Angew. Chem. Int. Ed. 2005, 44, 6237–6241. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jin, R.N.; Omer, A.M.; Ouyang, X.K. Adsorption of Pb(II) from fish sauce using carboxylated cellulose nanocrystal: Isotherm, kinetics, and thermodynamic studies. Int. J. Biol. Macromol. 2017, 102, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’Arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the optical response of the titanium-MIL-125 metal-organic framework through ligand functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.N.; Kim, J.; Kim, H.Y.; Cho, H.Y.; Ahn, W.S. Adsorption/catalytic properties of MIL-125 and NH 2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- Guo, H.; Lin, F.; Chen, J.; Li, F.; Weng, W. Metal–organic framework MIL-125(Ti) for efficient adsorptive removal of Rhodamine B from aqueous solution. Appl. Organomet. Chem. 2015, 29, 12–19. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Takeshita, S.; Konishi, A.; Takebayashi, Y.; Yoda, S.; Otake, K. Aldehyde Approach to Hydrophobic Modification of Chitosan Aerogels. Biomacromolecules 2017, 18, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, H.; Riazi, M.; Tabaei, M.; Kazemzadeh, Y.; Sharifi, M. Experimental investigation of interfacial properties in the EOR mechanisms by the novel synthesized Fe3O4@Chitosan nanocomposites. Colloids Surf. A 2018, 544, 15–27. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Yang, T.B.; Sun, L.X.; Xu, F.; Wang, Z.Q.; Zou, Y.J.; Chu, H.L. Synthesis of MIL-125/Graphene Oxide Composites and Hydrogen Storage Properties. Key Eng. Mater. 2017, 727, 683–687. [Google Scholar] [CrossRef]
- Martis, M.; Mori, K.; Fujiwara, K.; Ahn, W.S.; Yamashita, H. Amine-Functionalized MIL-125 with Imbedded Palladium Nanoparticles as an Efficient Catalyst for Dehydrogenation of Formic Acid at Ambient Temperature. J. Phys. Chem. C 2013, 117, 22805–22810. [Google Scholar] [CrossRef]
- McNamara, N.D.; Neumann, G.T.; Masko, E.T.; Urban, J.A.; Hicks, J.C. Catalytic performance and stability of (V) MIL-47 and (Ti) MIL-125 in the oxidative desulfurization of heterocyclic aromatic sulfur compounds. J. Catal. 2013, 305, 217–226. [Google Scholar] [CrossRef]
- Naceur, B.; Abdelkader, E.; Nadjia, L.; Sellami, M.; Noureddine, B. Synthesis and characterization of Bi1.56Sb1.48Co0.96O7 pyrochlore sun-light-responsive photocatalyst. Mater. Res. Bull. 2016, 74, 491–501. [Google Scholar] [CrossRef]
- Kordić, B.; Kovačević, M.; Sloboda, T.; Vidović, A.; Jović, B. FT-IR and NIR Spectroscopic Investigation of Hydrogen Bonding in Indole-Ether Systems. J. Mol. Struct. 2017, 1144, 159–165. [Google Scholar] [CrossRef]
- Guo, X.; Du, B.; Qin, W.; Jian, Y.; Hu, L.; Yan, L.; Xu, W. Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J. Hazard. Mater. 2014, 278, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J.Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, Y.; Chen, J.; Li, Y.; Zhao, J.; Gao, K.; Na, P. Effective elimination of As(III) via simultaneous photocatalytic oxidation and adsorption by a bifunctional cake-like TiO2 derived from MIL-125(Ti). Catal. Sci. Technol. 2018, 8, 1936–1944. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Xiao, F.P.; Wu, L.Y.; Li, C.Y.; Chen, Z.H. Effect of Heat Treatment on the Microstructure of TiO2 Nanofibers Prepared by Refluxing in Basic Solution. Key Eng. Mater. 2008, 368–372, 800–802. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Liang, X.; Lei, C.; Cui, Y.; Wu, W.; Yang, Y.; Zhang, Z.; Lei, Z. Construction of heterostructured MIL-125/Ag/g-C3N4 nanocomposite as an efficient bifunctional visible light photocatalyst for the organic oxidation and reduction reactions. Appl. Catal. B Environ. 2017, 205, 42–54. [Google Scholar] [CrossRef]
- Badiea, A.M.; Mohana, K.N. Corrosion Mechanism of Low-Carbon Steel in Industrial Water and Adsorption Thermodynamics in the Presence of Some Plant Extracts. J. Mater. Eng. Perform. 2009, 18, 1264–1271. [Google Scholar] [CrossRef]
- Ivanchikova, I.D.; Lee, J.S.; Maksimchuk, N.V.; Shmakov, A.N.; Chesalov, Y.A.; Ayupov, A.B.; Hwang, Y.K.; Chang, J.S.; Kholdeeva, O.A. Highly Selective H2O2-Based Oxidation of Alkylphenols to p-Benzoquinones Over MIL-125 Metal–Organic Frameworks. Eur. J. Inorg. Chem. 2014, 2014, 132–139. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem. Eng. J. 2012, 198–199, 219–227. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Zaidi, Z.; Siddiqui, S.I. Isotherm, kinetic and thermodynamics of arsenic adsorption onto Iron-Zirconium Binary Oxide-Coated Sand (IZBOCS): Modelling and process optimization. J. Mol. Liq. 2017, 229, 230–240. [Google Scholar] [CrossRef]
- Mojzsis, S.J. Confirmation of mass-independent isototope effects in archent(2.5 3.8 GA) sedimentary sulfides as determined by ion microprobe analysis. J. Chromatogr. A 2008, 1189, 19–31. [Google Scholar]
- Zhang, Y.; Hidajat, K.; Ray, A.K. Determination of competitive adsorption isotherm parameters of pindolol enantiomers on α1-acid glycoprotein chiral stationary phase. J.Chromatogr. A 2006, 1131, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Qian, J.; Xin, X.; Hu, S.; Zhang, S.; Wei, J. An exploratory study on low-concentration hexavalent chromium adsorption by Fe(III)-cross-linked chitosan beads. R. Soc. Open Sci. 2017, 4, 170905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danhardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Férey, G. A new photoactive crystalline highly porous titanium(IV) dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




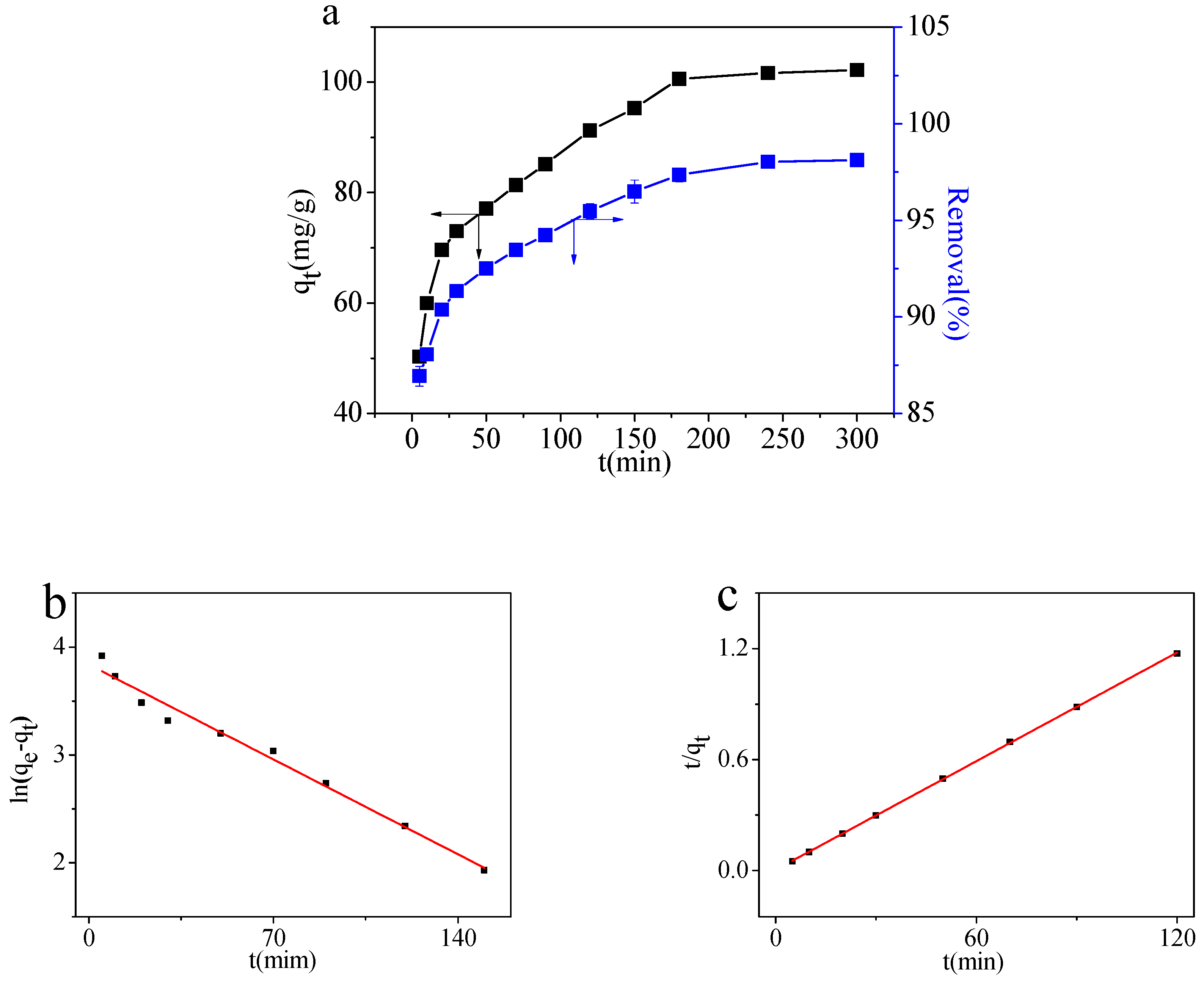
| qe,exp | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| qe,cal | k1 | R2 | qe,cal | k2 | R2 | |
| 99.94 | 46.45 | 0.01256 | 0.97978 | 102.04 | 0.0098 | 0.9999 |
| ΔG (kJ/mol) T (K) | ΔH (kJ/mol) | ΔS (J/mol∙K) | ||
|---|---|---|---|---|
| 293.2 | 298.2 | 303.2 | 8.343 | 28.00 |
| −5.78 | −5.92 | −6.13 | ||
| T (K) | Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | b (L/mg) | R2 | KF | n | R2 | |
| 298.2 | 406.50 | 0.02 | 0.99077 | 27.53 | 1.12 | 0.94637 |
| C0 (mg/L) | 100 | 200 | 300 | 500 | 800 | 1000 |
|---|---|---|---|---|---|---|
| RL | 0.33 | 0.20 | 0.14 | 0.090 | 0.058 | 0.047 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.-X.; Wang, N.; Qu, Y.-L.; Yang, L.-Y.; Wang, Y.-G.; Ouyang, X.-K. Facile Preparation of Metal-Organic Framework (MIL-125)/Chitosan Beads for Adsorption of Pb(II) from Aqueous Solutions. Molecules 2018, 23, 1524. https://doi.org/10.3390/molecules23071524
Liang X-X, Wang N, Qu Y-L, Yang L-Y, Wang Y-G, Ouyang X-K. Facile Preparation of Metal-Organic Framework (MIL-125)/Chitosan Beads for Adsorption of Pb(II) from Aqueous Solutions. Molecules. 2018; 23(7):1524. https://doi.org/10.3390/molecules23071524
Chicago/Turabian StyleLiang, Xue-Xue, Nan Wang, You-Le Qu, Li-Ye Yang, Yang-Guang Wang, and Xiao-Kun Ouyang. 2018. "Facile Preparation of Metal-Organic Framework (MIL-125)/Chitosan Beads for Adsorption of Pb(II) from Aqueous Solutions" Molecules 23, no. 7: 1524. https://doi.org/10.3390/molecules23071524






