Applications of Metal-Organic Frameworks in Food Sample Preparation
Abstract
:1. Introduction
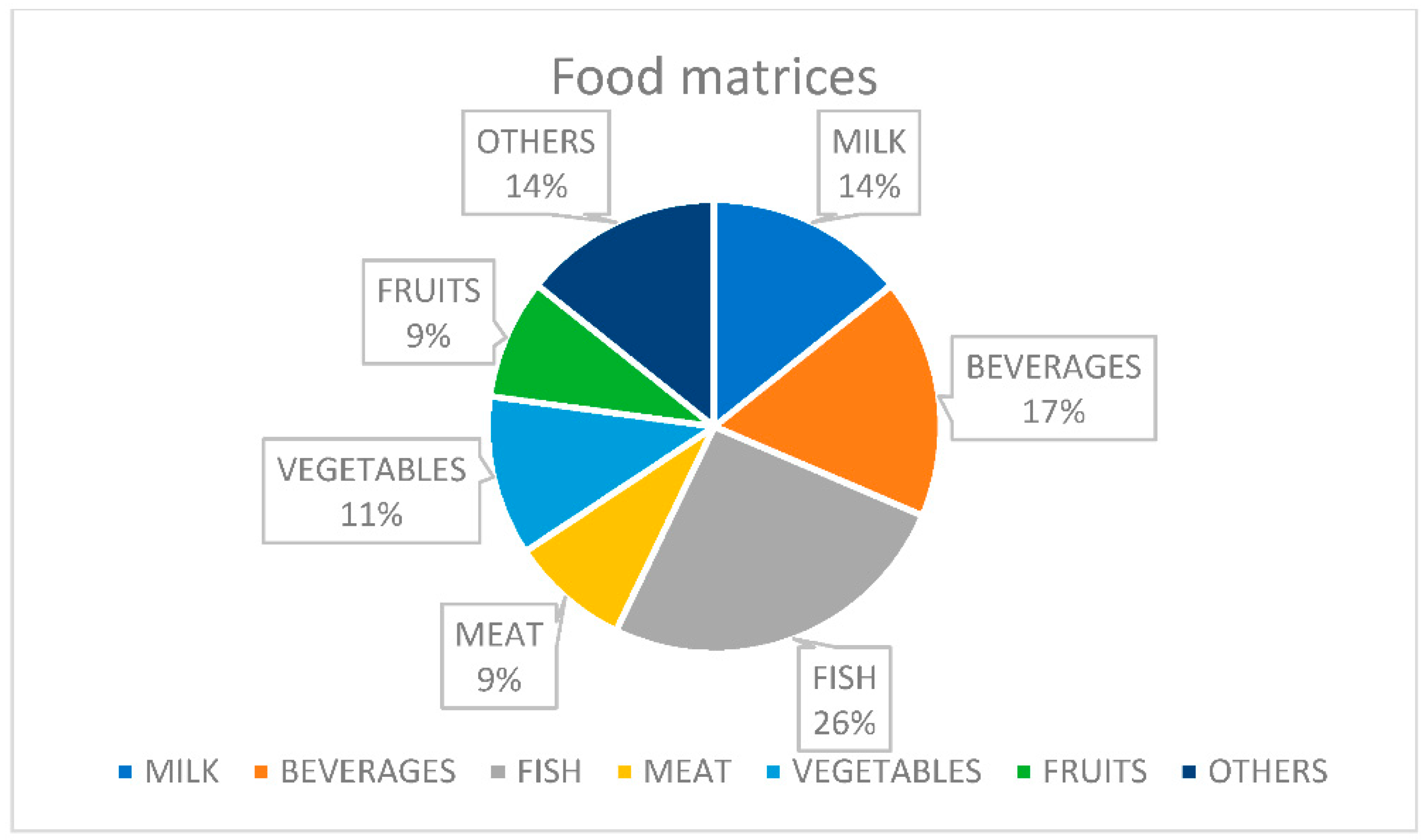
2. Food Matrices
2.1. Milk Samples
2.2. Beverages
2.3. Fish
2.4. Meat, Chicken and Shrimps
2.5. Fruits and Vegetables
2.6. Other Food Samples
3. MOF-Derived Carbon Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manousi, N.; Raber, G. Recent advances in microextraction techniques of antipsychotics in biological fluids prior to liquid chromatography analysis. Separations 2017, 4, 18. [Google Scholar] [CrossRef]
- Green Chemistry. Available online: http://www.epa.gov/greenchemistry (accessed on 30 September 2018).
- Arthur, L.C.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Ioannou, K.I. On-line sequential injection dispersive liquid-liquid microextraction system for flame atomic absorption spectrometric determination of copper and lead in water samples. Talanta 2009, 30, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, F.V.; Karageorgou, E. Carbon nanotubes in sample preparation. Curr. Org. Chem. 2012, 16, 1645–16699. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Graphene oxide and its application as an adsorbent for wastewater treatment. J. Chem. Technol. Biotechnol. 2014, 89, 196–205. [Google Scholar] [CrossRef]
- Maya, F.; Cabello, C.P.; Frizzarin, R.M.; Estela, J.M.; Palomino, G.T.; Cerda, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Rostami, S.; Pour, A.N.; Salimi, A.; Abolghasempour, A. Hydrogen adsorption in metal-organic frameworks (MOFs): Effects of adsorbent architecture. Int. J. Hydrog. Energy 2018, 43, 7072–7080. [Google Scholar] [CrossRef]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Zhou, H.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, B.; Zohrabi, P.; Raza, N.; Kim, K. Metal-organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. Trends Anal. Chem. 2017, 97, 65–82. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yagh, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W.; Zhang, Y.; Deng, Z.; Zhao, W.; Du, H.; Ma, X.; Yin, D.; Xie, F.; Chen, W.; et al. In situ preparation of core-shell magnetic porous aromatic framework nanoparticles for mixed-mode solid-phase extraction of trace multitarget analytes. J. Chromatogr. A 2018, 1556, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Lou, X.W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, C. Synthesis, functionalization, and applications of metal-organic frameworks in biomedicine. Dalton Trans. 2018, 47, 2114–2133. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, D.; Dougherty, C.A.; Lu, W.; Wu, H.; He, X.; Cai, T.; Van Dort, M.E.; Ross, B.D.; Hong, H. In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal-organic frameworks nanomaterials. ACS Nano 2017, 25, 4315–4327. [Google Scholar] [CrossRef] [PubMed]
- Peller, M.; Böll, K.; Zimpel, A.; Wuttke, S. Metal-organic framework nanoparticles for magnetic resonance imaging. Inorg. Chem. Front. 2018, 5, 1760–1779. [Google Scholar] [CrossRef]
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.S.; Clancy, Y.L.; Lobkovsky, E.B.; Yaghi, O.M.; Dai, S. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew. Chem. 2006, 45, 1390–1393. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-Y.; Yan, X.-P. Metal-organic framework MIL-101 for high-resolution gas chromatographic separation of xylene isomers and ethylbenzene. Angew. Chem. 2010, 49, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-Y.; Jiang, J.-Q.; Yan, X.-P. Fabrication of isoreticular metal-organic framework coated capillary columns for high-resolution gas chromatographic separation of persistent organic pollutants. Anal. Chem. 2011, 83, 5093–5100. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Gu, Z.-Y.; Wang, H.-F.; Yan, X.-P. Metal-organic frameworks-based tandem molecular sieves as a dual platform for selective microextraction and high resolution gas chromatographic separation of n-alkanes in complex matrixes. Anal. Chem. 2011, 83, 7094–7101. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-X.; Yan, X.-P. Metal-organic framework MIL-101(Cr) for high-performance liquid chromatographic separation of substituted aromatics. Anal. Chem. 2011, 83, 7144–7150. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-S.; Yang, C.-X.; Wang, S.-W.; Yan, X.-P. Metal-organic frameworks for reverse-phase high-performance liquid chromatography. Analyst 2012, 137, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-X.; Chen, Y.-J.; Wang, H.-F.; Yan, X.-P. High-performance separation of fullerenes on metal-organic framework MIL-101(Cr). Chem. Eur. J. 2011, 17, 11734–11737. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-Y.; Yang, C.X. Metal-organic frameworks for analytical chemistry: From sample collection to chromatographic separation. Acc. Chem. Res. 2012, 45, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Chaikittisilp, W.; Ariga, K.; Yamauchi, Y. A new family of carbon materials: Synthesis of MOF-derived nanoporous carbons and their promising applications. J. Mater. Chem. A 2013, 1, 14–19. [Google Scholar] [CrossRef]
- Tolika, E.P.; Samanidou, V.F.; Papadoyannis, I.N. Development and validation of an HPLC method for the determination of ten sulfonamide residues in milk according to 2002/657/EC. J. Sep. Sci. 2011, 34, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Bitas, D.; Samanidou, V. Molecularly imprinted polymers as extracting media for the chromatographic determination of antibiotics in milk. Molecules 2018, 23, 316. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, P.; Ye, X.; Zhang, L.; Wang, T.; Chen, Q.; Hou, X. A novel metal-organic framework composite MIL-101(Cr)@GO as an efficient sorbent in dispersive micro-solid phase extraction coupling with UHPLC-MS/MS for the determination of sulfonamides in milk samples. Talanta 2017, 169, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Lirio, S.; Liu, W.-L.; Lin, C.-L.; Lin, C.-H.; Huang, H.Y. Aluminum based metal-organic framework-polymer monolith in solid-phase microextraction of penicillins in river water and milk samples. J. Chromatogr. A 2016, 1428, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Q.; Yang, C.-X.; Yan, X.-P. Zeolite imidazolate framework-8 as sorbent for on-line solid-phase extraction coupled with high-performance liquid chromatography for the determination of tetracyclines in water and milk samples. J. Chromatogr. A 2013, 1304, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Pan, D.; Sun, Y.; Guo, Y.; Wu, Z. Thin metal-organic frameworks coatings by cathodic electrodeposition for solid-phase microextraction and analysis of trace exogenous estrogens in milk. Anal. Chim. Acta 2016, 937, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rocío-Bautista, P.; Pino, V.; Ayala, J.H.; Pasán, J.; Ruiz-Pérez, C.; Afonso, A.M. A magnetic-based dispersive micro-solid-phase extraction method using the metal-organic framework HKUST-1 and ultra-high-performance liquid chromatography with fluorescence detection for determining polycyclic aromatic hydrocarbons in waters and fruit tea infusions. J. Chromatogr. A 2016, 1436, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; He, X.; Wang, T.; Liu, S.; Hou, X. Magnetic solid-phase extraction using MIL-101(Cr)-based composite combined with dispersive liquid-liquid microextraction based on solidification of a floating organic droplet for the determination of pyrethroids in environmental water and tea samples. Microchem. J. 2018, 137, 449–455. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Ge, H.; Chen, H.; Ye, G.; Hu, X. Fabrication of metal-organic frameworks and graphite oxide hybrid composites for solid-phase extraction and preconcentration of luteolin. Talanta 2014, 122, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, G.; Wei, F.; Song, Q.; Tang, T.; Wang, X.; Hu, Q. Determination of Hg (II) in tea and mushroom samples based on metal-organic frameworks as solid phase extraction sorbents. Microporous Mesoporous Mater. 2016, 235, 204–210. [Google Scholar] [CrossRef]
- Lin, S.; Gan, N.; Qiao, L.; Zhang, J.; Cao, Y.; Chen, Y. Magnetic metal-organic frameworks coated stir bar sorptive extraction coupled with GC-MS for determination of polychlorinated biphenyls in fish samples. Talanta 2015, 144, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gan, N.; Zhang, J.; Qiao, L.; Chen, Y.; Cao, Y. Aptamer-functionalized stir bar sorptive extraction coupled with gas chromatography–mass spectrometry for selective enrichment and determination of polychlorinated biphenyls in fish samples. Talanta 2016, 149, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Huang, Z.; Liao, J.; Li, G. Chemical bonding approach for fabrication of hybrid magnetic metal-organic framework-5: High efficient adsorbents for magnetic enrichment of trace analytes. Anal. Chem. 2013, 85, 6885–6893. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Fu, Y.; Qin, Q.; Lu, X.; Shia, X.; Zhao, C.; Xu, G. Synthesis of magnetic mesoporous metal-organic framework-5 for the effective enrichment of malachite green and crystal violet in fish samples. J. Chromatogr. A 2018, 1560, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.R.; Matbouie, Z.; Asgharinezhad, A.A.; Dehghani, A. Solid phase extraction of Cd(II) and Pb(II) using a magnetic metal-organic framework, and their determination by FAAS. Microchim. Acta 2013, 180, 589–597. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Asgharinezhad, A.A.; Pooladi, M.; Barzin, M.; Abbaszadeh, A.; Tadjarodi, A. A novel magnetic metal-organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim. Acta 2013, 180, 1073–1084. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Abbaszadeh, A. A magnetic nanocomposite prepared from chelator-modified magnetite (Fe3O4) and HKUST-1 (MOF-199) for separation and preconcentration of mercury(II). Microchim. Acta 2016, 183, 1391–1399. [Google Scholar] [CrossRef]
- Ghorbani-Kalhor, E. A metal-organic framework nanocomposite made from functionalized magnetite nanoparticles and HKUST-1 (MOF-199) for preconcentration of Cd(II), Pb(II), and Ni(II). Microchim. Acta 2016, 183, 2639–2647. [Google Scholar] [CrossRef]
- Xia, L.; Liu, L.; Lv, X.; Qu, F.; Li, G.; You, J. Towards the determination of sulfonamides in meat samples: A magnetic and mesoporous metal-organic framework as an efficient sorbent for magnetic solid phase extraction combined with high-performance liquid chromatography. J. Chromatogr. A 2017, 1500, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, X.; He, X.; Chen, L.; Hou, X. MIL-101(Cr)@GO for dispersive micro-solid-phase extraction of pharmaceutical residue in chicken breast used in microwave-assisted coupling with HPLC-MS/MS detection. J. Pharm. Biomed. Anal. 2017, 145, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Barreto, A.S.; da Silva, R.L.; Dos Santos Silva, S.C.; Rodrigues, M.O.; de Simone, C.A.; de Sá, G.F.; Júnior, S.A.; Navickiene, S.; de Mesquita, M.E. Potential of a metal-organic framework as a new material for solid-phase extraction of pesticides from lettuce (Lactuca sativa), with analysis by gas chromatography-mass spectrometry. J. Sep. Sci. 2010, 33, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wu, M.; Hu, B.; Yao, M.; Zhang, L.; Lu, Q.; Pang, J. Electrospun UiO-66/polyacrylonitrile nanofibers as efficient sorbent for pipette tip solid phase extraction of phytohormones in vegetable samples. J. Chromatogr. A 2018, 1542, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, L.; Wu, C.; Qu, F.; Li, G.; Sun, Z.; You, J. Zirconium(IV)-based metal-organic framework(UIO-67) as efficient sorbent in dispersive solid phase extraction of plant growth regulator from fruits coupled with HPLC fluorescence detection. Talanta 2016, 154, 23–30. [Google Scholar] [CrossRef] [PubMed]
- You, L.; He, M.; Chen, B.; Hu, B. One-pot synthesis of zeolitic imidazolate framework-8/poly (methylmethacrylate-ethyleneglycol dimethacrylate) monolith coating for stir bar sorptive extraction of phytohormones from fruit samples followed by high performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2017, 1524, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, X.; Huang, P.; Wang, J.; Du, T.; Dua, X.; Lu, X. Magnetic Cu-MOFs embedded within graphene oxide nanocomposites for enhanced preconcentration of benzenoid-containing insecticides. Talanta 2018, 181, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, Z.; Gao, J.; Tong, P.; Liu, W.; Zhang, L. Metal-organic framework UiO-66 modified magnetite@silica core-shell magnetic microspheres for magnetic solid-phase extraction of domoic acid from shellfish sample. J. Chromatogr. A 2015, 1400, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wang, X.; Sun, Y.; Ma, P.; Li, X.; Piao, H.; Jiang, Y.; Song, D. Magnetic solid-phase extraction of triazine herbicides from rice using metal-organic framework MIL-101(Cr) functionalized magnetic particles. Talanta 2019, 179, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-R.; Chen, X.-L.; Hao, Y.-L.; Li, L.; Xu, H.-J.; Wang, M.-M. Magnetic metal-organic frameworks for fast and efficient solid-phase extraction of six Sudan dyes in tomato sauce. J. Chromatogr. B 2018, 1086, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Z.; Zhang, L.; Nian, L.; Lei, L.; Yang, X.; Zhang, H.; Yu, A. Liquid-phase extraction coupled with metal-organic frameworks-based dispersive solid phase extraction of herbicides in peanuts. Talanta 2014, 128, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Tokalıoglu, S.; Yavuz, E.; Demir, S.; Patat, S. Zirconium-based highly porous metal-organic framework (MOF-545) as an efficient adsorbent for vortex assisted-solid phase extraction of lead from cereal, beverage and water samples. Food Chem. 2017, 237, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Costa, H.; Albuquuerque, T.; Ramos, F.; Castilho, M.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Petricorena, Z.C. Chemical Composition of Fish and Fishery Products. In Handbook of Food Chemistry; Cheung, P., Ed.; Springer: Heidelberg/Berlin, Germany, 2014; pp. 1–28. [Google Scholar]
- Samanidou, V.; Charitonos, S.; Papadoyannis, I.; Bitas, D. On the extraction of antibiotics from shrimps prior to chromatographic analysis. Separations 2016, 3, 8. [Google Scholar] [CrossRef]
- Sources of Meat. Available online: www.fao.org/ag/againfo/themes/en/meat/backgr_composition.html (accessed on 30 October 2018).
- 2.2 Chemical Composition. Available online: http://www.fao.org/docrep/V5030E/V5030E06.htm (accessed on 30 October 2018).
- Introduction to Inorganic Chemistry/Coordination Chemistry and Crystal Field Theory. Available online: https://en.wikibooks.org/wiki/Introduction_to_Inorganic_Chemistry/Coordination_Chemistry_and_Crystal_Field_Theory (accessed on 27 September 2018).
- An Underused Framework for Simpler Sample Prep? Available online: https://theanalyticalscientist.com/issues/0618/an-underused-framework-for-simpler-sample-prep/ (accessed on 1 October 2018).
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 30, 5390–5391. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, C.; Wu, Q.; Wang, Z. Magnetic porous carbon-based solid-phase extraction of carbamates prior to HPLC analysis. Microchim. Acta 2015, 183, 415–421. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Wu, Q.; Wang, Z. Metal-organic framework-templated synthesis of magnetic nanoporous carbon as an efficient absorbent for enrichment of phenylurea herbicides. Anal. Chim. Acta 2015, 870, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, C.; Wu, Q.; Li, Z.; Zang, X.; Wang, Z. Metal-organic framework derived magnetic nanoporous carbon: Novel adsorbent for magnetic solid-phase extraction. Anal. Chem. 2014, 86, 12199–12205. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, J.; Jiao, C.; Wang, C.; Wu, Q.; Wang, Z. Magnetic porous carbon derived from a Zn/Co bimetallic metal-organic framework as an adsorbent for the extraction of chlorophenols from water and honey tea samples. J. Sep. Sci. 2016, 39, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, T.; Wang, C.; Hao, L.; Wang, C.; Wu, Q.; Wang, C. A metal-organic framework-derived nanoporous carbon/iron composite for enrichment of endocrine disrupting compounds from fruit juices and milk samples. Anal. Methods 2016, 8, 3528. [Google Scholar] [CrossRef]
- Hao, L.; Liu, X.-L.; Wang, J.-T.; Wang, C.; Wu, Q.-H.; Wang, Z. Metal-organic framework derived magnetic nanoporous carbon as an adsorbent for the magnetic solid-phase extraction of chlorophenols from mushroom sample. Chin. Chem. Lett. 2016, 27, 783–788. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Y.; Xu, X.; Zhang, L. Metal-organic framework-derived three-dimensional porous graphitic octahedron carbon cages-encapsulated copper nanoparticles hybrids as highly efficient enrichment material for simultaneous determination of four fluoroquinolones. J. Chromatogr. A 2018, 1533, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Matrix | Analytes | Analytical Technique | MOF Material | Sample Preparation Technique | Recovery | LODs | Reference |
|---|---|---|---|---|---|---|---|
| Milk | Sulfonamides | UHPLC-MS/MS | MIL-101(Cr)@GO | d-μSPE | 79.83–103.8% | 0.012–0.145 μg/L | [31] |
| Milk | Penicillins | UHPLC-TUV | MIL-53 | In tube SPME | 80.8–90.9% | 0.06–0.26 μg/L | [32] |
| Milk | Tetracyclines | HPLC-PDA | ZIF-8 | on-line SPE | 70.3–107.4% | 1.5–8.0 μg/L | [33] |
| Milk | Estrogens | HPLC-UV | MOF-5 | SPME | 73.1–96.7% | 0.17–0.56 ng/mL | [34] |
| Fruit tea | Polycyclic aromatic hydrocarbons | UHPLC-FLD | Fe3O4@HKUST-1 | D-μSPE | On average 75% | 0.8 ng/L | [35] |
| Tea samples | Pyrethroids | GC-ECD | MIL-101(Cr) | MSPE-DLLME-SFO | >0.015 ng/mL | 78.3–103.6% | [36] |
| Chrysanthemum tea | Luteolin | Square wave anodic stripping voltammetry | Cu3(BTC)2/GO | SPE | 7.9 × 10−10 mol/L | 99.4–101.0% | [37] |
| In tea and mushroom | Hg(II) | AFS | JUC-62 | SPE | >0.58 mg/kg | On average 93.3% | [38] |
| Fish | Polychlorinated biphenyls | GC-MS | Fe3O4-MOF-5(Fe) | SBSE | 0.061–0.096 ng/g | >80% | [39] |
| Fish | Polychlorinated biphenyls | GC-MS | MOF-5 | SBSE | 0.003–0.004 ng/mL | >80% | [40] |
| Fish | Aromatic hydrocarbons and gibberellic acids | GC-MS LC-MS/MS | MOF-5 | MSPE | 0.91–1.96 ng/L for PAHs and 0.006–0.08 μg/L for GAs | 66.4–120.0% for PAHs and 90.5–127.4% for GAs | [41] |
| Fish | Triphenylmethane dyes | HPLC-MS/MS | MOF-5 | MSPE | 0.30–0.80 ng/mL | 83.15–96.53 | [42] |
| Fish | Cd(II) and Pb(II) | FAAS | MOF-199 | MSPE | 0.2–1.1 μg/L | 92.8–117% | [43] |
| Fish | Cd(II), Zn(II), Ni(II), and Pb(II) | FAAS | MOF-199 | MSPE | 0.12–1.2 ng/mL | >90% | [44] |
| Fish | Hg(II) | Cold Vapor AAS | MOF-199 | MSPE | 10 ng/L | 95–102% | [45] |
| Fish and shrimps | Cd(II), Pb(II), and Ni(II) | FAAS | Fe3O4@TAR | MSPE | 0.15–0.8 ng/mL | NA | [46] |
| Shrimp samples, chicken and pork meat | Sulfonamides | HPLC-DAD | Fe3O4@JUC-48 | MSPE | 1.73–5.23 ng/g, | 76.1–102.6% | [47] |
| Chicken breast | Drug traces | LC-MS/MS | MIL-101(Cr)@GO | d-μSPE | 0.08 and 1.02 ng/kg | 88.9–102.3% | [48] |
| Lettuce | Pesticides | GC-MS | ∞[(La0.9Eu0.1)2(DPA)3(H2O)3] | MSPD | 0.02–0.05 mg/kg | 78–107% | [49] |
| Fruits and vegetables | Phytohormones | HPLC-FLD | UiO-66 | Pipette Tip SPE | 0.01–0.02ng/mL | 88.3–105.2% | [50] |
| Fruits | Plant growth regulator | HPLC-FLD | UIO-67 | d-SPE | 89.3–102.3% | 0.21–0.57 ng/mL | [51] |
| Fruits | Phytohormones | HPLC-UV | Zeolitic imidazolate framework-8 | SBSE | 82.7–111% | 0.11–0.51μg/L | [52] |
| Fruits and vegetables | of insecticides | HPLC-UV | Fe3O4@SiO2-GO MOF | MSPE | 81.2–105.8% | 0.30–1.58 μg/L | [53] |
| Shellfish | Shellfish poisoning toxin | LC-MS/MS | Fe3O4@SiO2@UiO-66 | MSPE | 93.1% and 107.3% | 1.45 pg/mL | [54] |
| Rice | Herbicides | HPLC-UV | MIL-101(Cr) | MSPE | 83.9–103.5% | 0.010–0.080 μg/kg | [55] |
| Tomato sauce | Sudan dyes | HPLC-DAD | Fe3O4-NH2@MIL-101 | MSPE | 69.6–92.9% | 0.5–2.5 μg/kg | [56] |
| Peanuts | Herbicides | HPLC-DAD | MIL-101(Cr) | d-SPE | 89.5–102.7% | 0.98–1.9 μg/kg | [57] |
| In cereal, beverages and water samples | Lead | FAAS | MOF-545 | Vortex Assisted SPE | 91–96% | 1.78 μg/L | [58] |
| Matrix | Analytes | Analytical Technique | Precursor MOF Material | Sample Preparation Technique | Recovery | LODs | Reference |
|---|---|---|---|---|---|---|---|
| Apples | Carbamates | HPLC-UV | MOF-5 | MSPE | 89.3–109.7% | 0.1–0.2 ng/g | [67] |
| Grapes and bitter gourd | Herbicides | HPLC-UV | ZIF-67 | MSPE | 88.9–105.1% for grapes, 89.6–105.0% for bitter gourd | 0.17–0.4 ng/g for grapes, 0.23–0.46 ng/g for bitter gourd | [68] |
| Fatmelon | Neonicotinoid insecticides | HPLC-UV | ZIF-67 | MSPE | 93.0–99.3% | 0.2–0.5 ng/g | [69] |
| Honey tea | Chlorophenols | HPLC-UV | ZIF-8 | MSPE | 83.0–114.0% | 0.1–0.2 ng/mL | [70] |
| Fruit juice and milk | Endocrine disrupting compounds | UHPLC-FLD | MIL-53 | MSPE | 92.2–108.3% | 0.05–0.10 ng/mL | [71] |
| Mushrooms | Chlorophenols | HPLC-UV | MOF-5 | MSPE | 0.25–0.30 ng/g | 85.4–97.5% | [72] |
| Chicken | Fluoroquinolones | HPLC-UV | Cu based MOF | DSPE | 0.18–0.58 ng/g | 81.3–104.3% | [73] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A.; Samanidou, V.F. Applications of Metal-Organic Frameworks in Food Sample Preparation. Molecules 2018, 23, 2896. https://doi.org/10.3390/molecules23112896
Manousi N, Zachariadis GA, Deliyanni EA, Samanidou VF. Applications of Metal-Organic Frameworks in Food Sample Preparation. Molecules. 2018; 23(11):2896. https://doi.org/10.3390/molecules23112896
Chicago/Turabian StyleManousi, Natalia, George A. Zachariadis, Eleni A. Deliyanni, and Victoria F. Samanidou. 2018. "Applications of Metal-Organic Frameworks in Food Sample Preparation" Molecules 23, no. 11: 2896. https://doi.org/10.3390/molecules23112896








