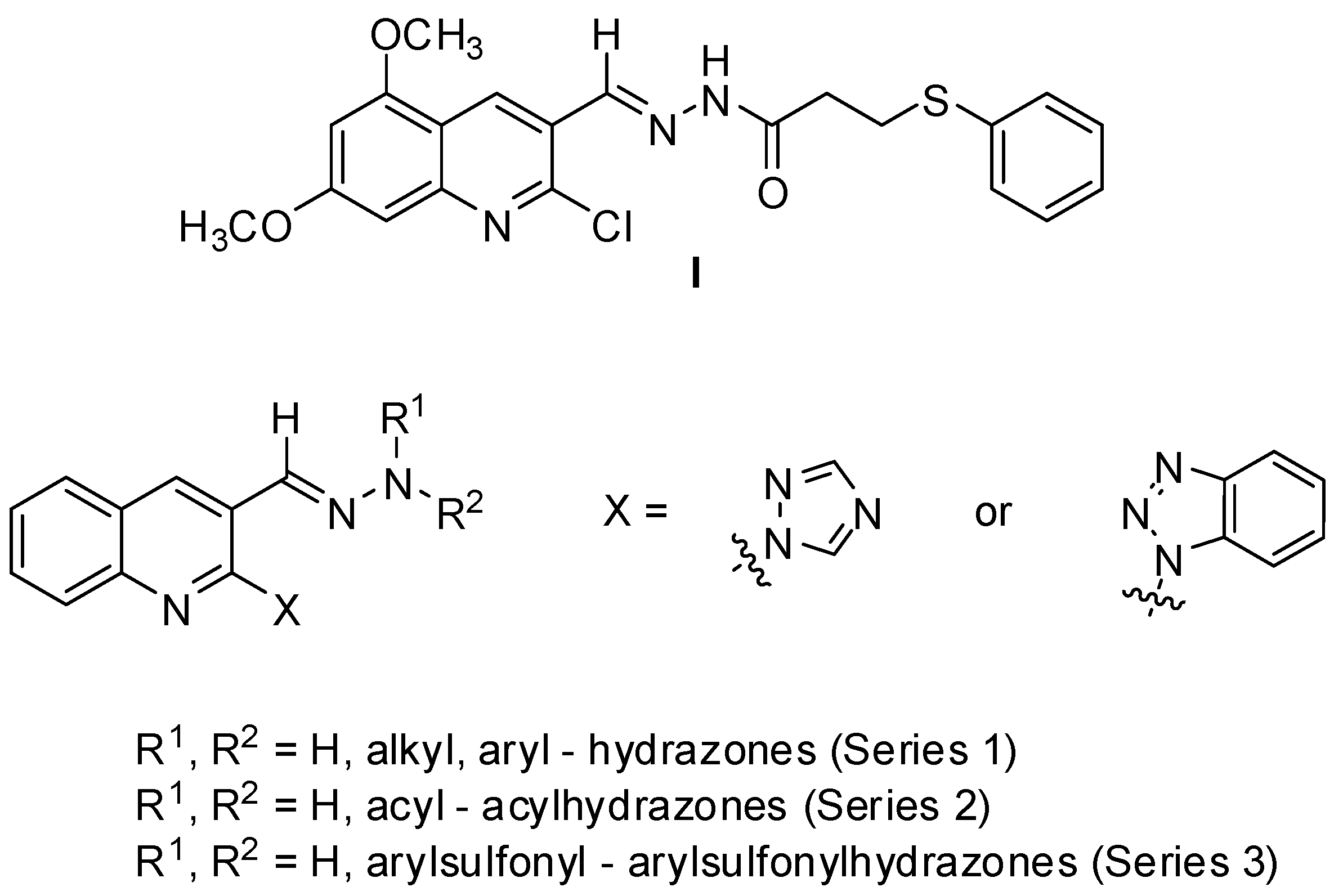
3.2. Chemistry
3.2.1. Procedure for the Preparation of 2-(1H-1,2,4-Triazol-1-yl)quinoline-3-carbaldehyde (2)
To a stirred solution of 2-chloroquinoline-3-carbaldehyde (1) (6.0 g, 31 mmol) in DMF (20 mL), potassium carbonate (8.28 g, 60 mmol) and 1,2,4-triazole (6.32 g, 93 mmol) were added and the mixture was heated at 40 °C for 6 h. Next, the mixture was poured into crushed ice and the precipitate was collected by vacuum filtration and purified by column chromatography (silica gel) eluting with CH2Cl2/AcOEt 20:1 v/v to afford the title compound 2. Yield 4.6 g (66%); m.p. 175–178 °C; IR (KBr) νmax: 3131, 3113, 3062, 2896, 1698, 1617, 1505, 1444, 1345, 1277, 1166, 1048, 984, 954, 779, 752 cm−1; 1H NMR (200 MHz, CDCl3) δ: 7.65–7.72 (m, 1H, Ar-H), 7.88–7.96 (m, 1H, Ar-H), 8.02–8.13 (m, 2H, Ar-H), 8.22 (s, 1H, triazole), 8.91 (s, 1H, 4-H, quinoline), 9.36 (s, 1H, triazole), 10.78 (s, 1H, CHO) ppm. Anal. calcd. for C12H8N4O (224.22): C, 64.28; H, 3.60; N, 24.99. Found: C, 64.39; H, 3.51; N, 24.63.
3.2.2. Procedure for the Preparation of 2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3)
To a stirred solution of 2-chloroquinoline-3-carbaldehyde (1) (6.0 g, 31 mmol) in ethanol (20 mL), benzotriazole (11.07 g, 93 mmol) in ethanol (10 mL) was added at 60 °C. After stirring at reflux for 7 h, the resulting mixture was cooled and the precipitate was collected by vacuum filtration to give the title compound 3. Yield 7.9 g (92%); m.p. 219–221 °C; IR (KBr) νmax: 3056, 2922, 1690, 1618, 1583, 1498, 1446, 1286, 1161, 1070, 1024, 785, 745 cm−1; 1H NMR (200 MHz, CDCl3) δ: 7.56 (t, J = 7.9 Hz, 1H, Ar-H), 7.69–7.74 (m, 2H, Ar-H), 7.94 (t, J = 8.2 Hz, 1H, Ar-H), 8.08 (d, J = 8.1 Hz, 1H, Ar-H), 8.18–8.23 (m, 2H, Ar-H), 8.58 (d, J = 8.3 Hz, 1H, Ar-H), 9.00 (s, 1H, 4-H, quinoline), 10.59 (s, 1H, CHO) ppm; 13C NMR (200 MHz, CDCl3) δ: 114.6, 120.6, 124.1, 126.1, 127.0, 128.6, 129.3 (two overlapping signals), 129.8, 130.1, 133.8, 135.9, 141.8, 146.9, 148.5, 189.2 ppm; MS (ESI) m/z: 275 [M + H]+. Anal. calcd. for C16H10N4O (274.28): C, 70.06; H, 3.67; N, 20.43. Found: C, 70.18; H, 3.54; N, 20.52.
3.2.3. General Procedure for the Preparation of Hydrazones 4a–e and 5a–e
To a suspension of 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) or 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) in ethanol (10 mL), the appropriate hydrazine (1 mmol) was added. After stirring for 24 h at ambient temperature (TLC control) the precipitated solid was collected by vacuum filtration, dried, and recrystallized or subjected to preparative thin layer chromatography. In this manner, the following compounds were obtained.
3-(Hydrazonomethyl)-2-(1H-1,2,4-triazol-1-yl)quinoline (4a). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 67% hydrazine hydrate (1 mmol), the title compound 4a was obtained after crystallization from ethanol. Yield 53%; m.p. 197–199 °C; IR (KBr) νmax: 3358, 3207, 3102, 1617, 1597, 1570, 1493, 1442, 1416, 1324, 1278, 1145, 1054, 984, 952, 785, 763, 725 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.39 (s, 2H, NH2), 7.66 (t, J = 7.8 Hz, 1H, Ar-H), 7.79 (t, J = 7.3 Hz, 1H, Ar-H), 7.84 (s, 1H, N=CH), 7.98 (d, J = 8.3 Hz, 1H, Ar-H), 8.13 (d, J = 7.8 Hz, 1H, Ar-H), 8.36 (s, 1H, triazole), 8.88 (s, 1H, 4-H, quinoline), 9.23 (s, 1H, triazole) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 125.4, 128.6, 128.7, 128.9 (two overlapping signals), 131.2, 131.6, 134.6, 145.4, 145.9, 146.0, 153.2; MS (ESI) m/z: 239 [M + H]+. Anal. calcd. for C12H10N6 (238.25): C, 60.50; H, 4.23; N, 35.27. Found: C, 60.32; H, 4.36; N, 35.32.
3-[(2,2-Dimethylhydrazono)methyl]-2-(1H-1,2,4-triazol-1-yl)quinoline (4b). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and dimethylhydrazine (1 mmol), the title compound 4b was obtained after preparative thin layer chromatography (eluent: CH2Cl2/AcOEt 10:1 v/v). Yield 59%; m.p. 138–141 °C; IR (KBr) νmax: 3129, 3048, 2925, 1618, 1551, 1501, 1493, 1438, 1402, 1279, 1142, 1070, 1045, 987, 915, 757 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 2.98 (s, 6H, 2xCH3), 7.44 (s, 1H, N=CH), 7.67 (t, J = 7.9 Hz, 1H, Ar-H), 7.81 (t, J = 8.3 Hz, 1H, Ar-H), 7.99 (d, J = 8.3 Hz, 1H, Ar-H), 8.15 (d, J = 7.9 Hz, 1H, Ar-H), 8.37 (s, 1H, triazole), 8.86 (s, 1H, 4-H, quinoline), 9.27 (s, 1H, triazole) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 42.7 (two overlapping signals), 125.1 (two overlapping signals), 128.3, 128.5 (three overlapping signals), 130.7, 134.0, 144.9, 145.7 (two overlapping signals), 152.8 ppm; MS (ESI) m/z: 267 [M + H]+. Anal. calcd. for C14H14N6 (266.30): C, 63.14; H, 5.30; N, 31.56. Found: C, 63.32; H, 5.42; N, 31.26.
3-[(2-Phenylhydrazono)methyl]-2-(1H-1,2,4-triazol-1-yl)quinoline (4c). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and phenylhydrazine (1 mmol), the title compound 4c was obtained after crystallization from toluene. Yield 32%; m.p. 182–185 °C; IR (KBr) νmax: 3236, 3132, 3052, 1603, 1593, 1557, 1490, 1436, 1271, 1207, 1123, 985, 959, 924, 748 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 6.80 (t, J = 7.3 Hz, 1H, Ar-H), 7.15 (d, J = 8.3 Hz, 2H, Ar-H), 7.25 (t, J = 7.8 Hz, 2H, Ar-H), 7.70 (t, J = 7.7 Hz, 1H, Ar-H), 7.82 (t, J = 7.3 Hz, 1H, Ar-H), 8.00 (d, J = 8.3 Hz, 1H, Ar-H), 8.11 (s, 1H, N=CH), 8.22 (d, J = 8.3 Hz, 1H, Ar-H), 8.42 (s, 1H, triazole), 9.12 (s, 1H, 4-H, quinoline), 9.29 (s, 1H, triazole), 10.82 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 113.0 (two overlapping signals), 120.2, 124.6, 128.6, 128.7, 128.9, 129.1, 129.9 (two overlapping signals), 131.2, 131.5, 135.3, 145.4, 145.6, 146.0, 146.1, 153.3 ppm; MS (ESI) m/z: 313 [M − H]−. Anal. calcd. for C18H14N6 (314.34): C, 68.78; H, 4.49; N, 26.74. Found: C, 68.59; H, 4.38; N, 27.03.
2-{2-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]hydrazinyl}ethanol (4d). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 2-hydrazinylethanol (1 mmol), the title compound 4d was obtained after crystallization from ethanol. Yield 51%; m.p. 173–175 °C; IR (KBr) νmax: 3345, 2946, 1618, 1599, 1580, 1492, 1449, 1428, 1392, 1287, 1178, 1056, 1023, 910, 786 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 3.21–3.24 (m, 2H, CH2), 3.56–3.57 (m, 2H, CH2), 4.67 (s, 1H, OH), 7.64 (t, J = 7.3 Hz, 1H, Ar-H), 7.72 (s, 1H, N=CH), 7.76 (t, J = 7.8 Hz, 1H, Ar-H), 7.90 (t, 1H, NH), 7.96 (d, J = 8.3 Hz, 1H, Ar-H), 8.12 (d, J = 7.8 Hz, 1H, Ar-H), 8.35 (s, 1H, triazole), 8.84 (s, 1H, 4-H, quinoline), 9.23 (s, 1H, triazole) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 51.6, 60.0, 125.6, 127.4, 128.6, 128.7, 128.8, 128.9, 130.9, 134.1, 145.2, 145.9 (two overlapping signals), 153.1 ppm; MS (ESI) m/z: 281 [M − H]−. Anal. calcd. for C14H14N6O (282.30): C, 59.56; H, 5.00; N, 29.77. Found: C, 59.38; H, 5.17; N, 29.65.
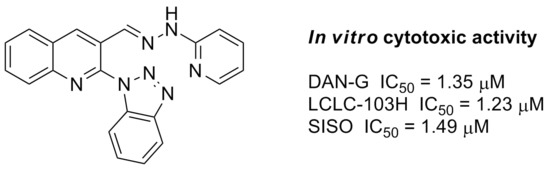
3-[(2-(Pyridin-2-yl)hydrazono)methyl]-2-(1H-1,2,4-triazol-1-yl)quinoline (4e). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 2-hydrazinylpyridine (1 mmol), the title compound 4e was obtained after crystallization from a DMF–methanol mixture. Yield 20%; m.p. 248–252 °C; IR (KBr) νmax: 3186, 3118, 3069, 3024, 1595, 1560, 1540, 1490, 1458, 1442, 1306, 1278, 1129, 1123, 991, 753 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 6.79 (t, J = 5.9 Hz, 1H, Ar-H), 7.32 (d, J = 8.3 Hz, 1H, Ar-H), 7.64–7.71 (m, 2H, Ar-H), 7.83 (t, J = 7.3 Hz, 1H, Ar-H), 8.01 (d, J = 8.3 Hz, 1H, Ar-H), 8.13 (d, J = 4.4 Hz, 1H, Ar-H), 8.18 (d, J = 8.3 Hz, 1H, Ar-H), 8.33 (s, 2H, N=CH and CH triazole), 9.07 (s, 1H, 4-H, quinoline), 9.22 (s, 1H, triazole), 10.98 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 107.6, 116.2, 124.4, 128.5, 128.6, 129.0, 129.1, 131.6, 134.5, 136.4, 138.5, 145.7, 146.0, 146.4, 148.5, 153.2, 157.6 ppm. Anal. calcd. for C17H13N7 (315.33): C, 64.75; H, 4.16; N, 31.09. Found: C, 64.59; H, 3.98; N, 31.43.
2-(1H-Benzo[d][1,2,3]triazol-1-yl)-3-(hydrazonomethyl)quinoline (5a). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 65% hydrazine hydrate (1 mmol), the title compound 5a was obtained after crystallization from a DMF–methanol mixture. Yield 75%; m.p. 239–241 °C; IR (KBr) νmax: 3416, 3282, 3180, 3060, 1617, 1591, 1493, 1461, 1400, 1286, 1217, 1067, 1022, 1013, 928, 760, 737 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 7.39 (s, 2H, NH2), 7.52–7.64 (m, 2H, Ar-H), 7.66–7.91 (m, 3H, Ar-H and N=CH), 8.05 (t, 2H, Ar-H), 8.25 (t, 2H, Ar-H), 9.03 (s, 1H, 4-H, quinoline) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 113.1, 120.1, 125.7, 126.2, 128.6, 128.8, 128.9, 129.0, 129.7, 131.3, 132.5, 133.8, 135.5, 145.8, 145.9, 146.2 ppm; MS (ESI) m/z: 289 [M + H]+. Anal. calcd. for C16H12N6 (288.31): C, 66.66; H, 4.20; N, 29.15. Found: C, 66.21; H, 4.29; N, 29.50.
2-(1H-Benzo[d][1,2,3]triazol-1-yl)-3-[(2,2-dimethylhydrazono)methyl]quinoline (5b). Starting from 2-(1H[d]-benzo[1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and dimethylhydrazine (1 mmol), the title compound 5b was obtained after preparative thin layer chromatography (eluent: CH2Cl2/AcOEt 10:1 v/v). Yield 80%; m.p. 165–167 °C; IR (KBr) νmax: 3045, 2914, 2859, 1542, 1490, 1421, 1283, 1062, 1022, 739 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 2.91 (s, 6H, 2xCH3), 7.31 (s, 1H, N=CH), 7.53 (t, J =7.9 Hz, 1H, Ar-H), 7.70 (t, J = 7.9 Hz, 2H, Ar-H), 7.82 (t, J = 8.3 Hz, 1H, Ar-H), 8.04 (d, J = 8.3 Hz, 2H, Ar-H), 8.17–8.27 (m, 2H, Ar-H), 8.94 (s, 1H, 4-H, quinoline) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 42.7 (two overlapping signals), 113.2, 119.9, 125.1, 125.4, 126.0, 128.3, 128.5, 128.6, 128.8, 129.3, 130.8, 133.3, 134.6, 145.2, 145.5, 145.9 ppm. Anal. calcd. for C18H16N6 (316.36): C, 68.56; H, 5.21; N, 26.23. Found: C, 68.47; H, 5.21; N, 26.32.
2-(1H-Benzo[d][1,2,3]triazol-1-yl)-3-[(2-phenylhydrazono)methyl]quinoline (5c). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and phenylhydrazine (1 mmol), the title compound 5c was obtained after crystallization from ethanol. Yield 51%; m.p. 106–110 °C; IR (KBr) νmax: 3253, 3055, 1600, 1551, 1491, 1425, 1286, 1262, 1131, 1090, 1021, 1010, 929, 783, 749 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 6.78 (t, J = 7.3 Hz, 1H, Ar-H), 7.05 (d, J = 7.8 Hz, 2H, Ar-H), 7.22 (t, J = 7.8 Hz, 2H, Ar-H), 7.56 (t, J = 7.3 Hz, 1H, Ar-H), 7.69–7.76 (m, 2H, Ar-H), 7.85 (t, J = 7.8 Hz, 1H, Ar-H), 8.02 (s, 1H, N=CH), 8.05–8.08 (m, 2H, Ar-H), 8.27 (d, J = 8.8 Hz, 2H, Ar-H), 9.21 (s, 1H, 4-H, quinoline), 10.76 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 113.0 (two overlapping signals), 113.5, 120.1, 120.2, 125.5, 125.9, 128.6, 128.9, 129.1 (two overlapping signals), 129.8 (three overlapping signals), 131.3, 131.6, 133.7, 135.4, 145.3, 145.8, 145.9, 146.2 ppm; MS (ESI) m/z: 363 [M − H]−. Anal. calcd. for C22H16N6 (364.40): C, 72.51; H, 4.43; N, 23.06. Found: C, 72.27; H, 4.58; N, 23.15.
2-{2-[(2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]hydrazinyl}ethanol (5d). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and hydrazinylethanol (1 mmol), the title compound 5d was obtained after crystallization from ethanol. Yield 61%; m.p. 177–179 °C; IR (KBr) νmax: 3344, 3139, 2934, 2877, 1596, 1571, 1509, 1493, 1441, 1328, 1284, 1211, 1141, 1071, 989, 753 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 3.15–3.19 (m, 2H, CH2), 3.50–3.53 (m, 2H, CH2), 4.64 (t, J = 5.3 Hz, 1H, OH), 7.55 (t, J = 7.8 Hz, 1H, Ar-H), 7.63 (s, 1H, N=CH), 7.66–7.70 (m, 2H, Ar-H), 7.79 (t, J = 7.3 Hz, 1H, Ar-H), 7.87 (t, J = 4.8 Hz, 1H, NH), 8.00 (d, J = 9.3 Hz, 2H, Ar-H), 8.18 (d, J = 7.8 Hz, 1H, Ar-H), 8.23 (d, J = 8.3 Hz, 1H, Ar-H), 8.95 (s, 1H, 4-H, quinoline) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 51.6, 60.0, 113.3, 120.1, 125.8. 126.4, 127.4, 128.6, 128.7, 128.9, 129.0, 129.7, 131.0, 133.6, 134.6, 145.5, 145.7, 146.0 ppm; MS (ESI) m/z: 331 [M − H]−. Anal. calcd. for C18H16N6O (332.36): C, 65.05; H, 4.85; N, 25.29. Found: C, 64.89; H, 5.06; N, 25.11.
2-(1H-Benzo[d][1,2,3]triazol-1-yl)-3-[2-(pyridin-2-yl)hydrazonomethyl]quinoline (5e). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 2-hydrazinylpyridine (1 mmol), the title compound 5e was obtained after crystallization from a DMF/methanol mixture. Yield 54%; m.p. 266–268 °C; IR (KBr) νmax: 3199, 3159, 3024, 2926, 2862, 1601, 1558, 1492, 1444, 1308, 1290, 1133, 1058, 1021, 756, 738 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 6.82 (t, J = 6.2 Hz, 1H, Ar-H), 7.23 (d, J = 8.7 Hz, 1H, Ar-H), 7.57–7.82 (m, 4H, Ar-H), 7.91 (t, J = 6.6 Hz, 1H, Ar-H), 8.09–8.13 (m, 3H, Ar-H), 8.24 (s, 1H, N=CH), 8.30 (d, J = 8.3 Hz, 2H, Ar-H), 9.26 (s, 1H, 4-H, quinoline), 11.18 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 107.1, 113.3, 116.0, 119.9, 124.8, 125.6, 128.2, 128.7, 128.9, 129.0, 129.5, 131.6, 133.3, 133.9, 136.5, 138.3, 145.6, 145.9, 146.2, 148.2, 157.0 ppm; MS (ESI) m/z: 364 [M − H]−. Anal. calcd. for C21H15N7 (365.39): C, 69.03; H, 4.14; N, 26.83. Found: C, 68.94; H, 3.96; N, 27.10.
3.2.4. General Procedure for the Preparation of N′-Acylhydrazones 6a–h and 7a–h
A mixture of 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) or 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and appropriate hydrazide (1 mmol) in the presence of a catalytic amount of acetic acid in dichloromethane (5 mL) was heated under reflux for 5–8 h. The progress of the reaction was controlled by TLC. The mixture was then evaporated under reduced pressure to dryness and the crude product thus obtained was purified as described below. In this manner, the following compounds were obtained.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]benzohydrazide (6a). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and benzohydrazide (1 mmol), the title compound 6a was obtained after washing with hot ethanol. Yield 70%; m.p. 254–255 °C; IR (KBr) νmax: 3219, 3131, 3038, 1651, 1602, 1546, 1491, 1442, 1280, 1132, 983, 759 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.53 (t, J = 7.8 Hz, 2H, Ar-H), 7.60 (t, J = 7.3 Hz, 1H, Ar-H), 7.75 (t, J = 7.8 Hz, 1H, Ar-H), 7.90–7.93 (m, 3H, Ar-H), 8.06 (d, J = 8.3 Hz, 1H, Ar-H), 8.30 (d, J = 8.3 Hz, 1H, Ar-H), 8.43 (s, 1H, N=CH), 8.76 (s, 1H, triazole), 9.16 (s, 1H, triazole), 9.36 (s, 1H, 4-H, quinoline), 12.19 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 122.7, 128.1, 128.4 (three overlapping signals), 128.9, 129.0, 129.3 (two overlapping signals), 129.6, 132.8, 133.5, 138.0, 143.9, 145.9, 146.5, 146.7, 153.5, 164.3 ppm; MS (ESI) m/z: 341 [M − H]−. Anal. calcd. for C19H14N6O (342.35): C, 66.66; H, 4.12; N, 24.55. Found: C, 66.78; H, 4.01; N, 24.38.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-4-methylbenzohydrazide (6b). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-methylbenzohydrazide (1 mmol), the title compound 6b was obtained after washing with hot ethanol. Yield 68%; m.p. 250–254 °C; IR (KBr) νmax: 3243, 3081, 1663, 1559, 1507, 1492, 1444, 1275, 1209, 1118, 982, 958, 762 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 2.37 (s, 3H, CH3), 7.33 (d, J = 8.3 Hz, 2H, Ar-H), 7.74 (t, J = 7.3 Hz, 1H, Ar-H), 7.84 (d, J = 7.8 Hz, 2H, Ar-H), 7.91 (t, J = 7.3 Hz, 1H, Ar-H), 8.06 (d, J = 8.8 Hz, 1H, Ar-H), 8.30 (d, J = 8.3 Hz, 1H, Ar-H), 8.43 (s, 1H, N=CH), 8.76 (s, 1H, triazole), 9.14 (s, 1H, triazole), 9.36 (s, 1H, 4-H, quinoline), 12.12 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.7, 123.1, 128.2, 128.6, 128.8, 128.9 (two overlapping signals), 129.5, 129.6 (two overlapping signals), 129.9, 131.2, 132.5, 137.9, 142.5, 145.8, 146.7, 146.8, 153.5, 164.5 ppm; MS (ESI) m/z: 355 [M − H]−. Anal. calcd. for C20H16N6O (356.38): C, 67.40; H, 4.53; N, 23.58. Found: C, 67.38; H, 4.72; N, 23.15.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-4-methoxybenzohydrazide (6c). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-methoxybenzohydrazide (1 mmol), the title compound 6c was obtained after crystallization from a DMF–methanol mixture. Yield 29%; m.p. 262–263 °C; IR (KBr) νmax: 3216, 3132, 3029, 2958, 2835, 1646, 1602, 1508, 1440, 1254, 1174, 987, 863, 762 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 3.84 (s, 3H, OCH3), 7.07 (d, J = 8.7 Hz, 2H, Ar-H), 7.75 (t, J = 7.8 Hz, 1H, Ar-H), 7.89–7.96 (m, 3H, Ar-H), 8.08 (d, J = 8.3 Hz, 1H, Ar-H), 8.32 (d, J = 7.9 Hz, 1H, Ar-H), 8.44 (s, 1H, N=CH), 8.74 (s, 1H, triazole), 9.15 (s, 1H, triazole), 9.37 (s, 1H, 4-H, quinoline), 12.06 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.1, 114.5 (two overlapping signals), 122.9, 125.5, 128.1, 128.9, 129.0, 129.5, 130.4, 132.7, 137.9, 143.2, 145.9 (two overlapping signals), 146.5, 146.7, 153.5, 162.9, 163.7 ppm; MS (ESI) m/z: 371 [M − H]−. Anal. calcd. for C20H16N6O2 (372.38): C, 64.51; H, 4.33; N, 22.57. Found: C, 64.41; H, 4.21; N, 22.83.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-4-chlorobenzohydrazide (6d). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-chlorobenzohydrazide (1 mmol), the title compound 6d was obtained after washing with hot ethanol. Yield 21%; m.p. 272–274 °C; IR (KBr) νmax: 3221, 3087, 1672, 1597, 1560, 1490, 1445, 1273, 1210, 1115, 981, 958, 752 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 7.60–7.66 (m, 2H, Ar-H), 7.76 (t, J = 7.9 Hz, 1H, Ar-H), 7.89–7.98 (m, 3H, Ar-H), 8.06–8.10 (m, 1H, Ar-H), 8.32 (d, J = 7.9 Hz, 1H, Ar-H), 8.44 (s, 1H, N=CH), 8.78 (s, 1H, triazole), 9.16 (s, 1H, triazole), 9.39 (s, 1H, 4-H, quinoline), 12.24 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 122.6, 128.1, 128.9, 129.0, 129.3 (two overlapping signals), 129.6, 130.4 (two overlapping signals), 132.3, 132.8, 137.6, 138.1, 144.3, 145.9, 146.6, 146.7, 153.5, 163.3 ppm; MS (ESI) m/z: 375 [M − H]−. Anal. calcd. for C19H13ClN6O (376.80): C, 60.56; H, 3.48; N, 22.30. Found: C, 60.42; H, 3.41; N, 22.17.
N′-[(2-(1H-1,2,4-triazol-1-yl)quinolin-3-yl)methylene]-4-fluorobenzohydrazide (6e). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-fluorobenzohydrazide (1 mmol), the title compound 6e was obtained after preparative thin layer chromatography (eluent: CH2Cl2/AcOEt 10:1 v/v). Yield 42%; m.p. 261–263 °C; IR (KBr) νmax: 3217, 3132, 3038, 1652, 1601, 1503, 1493, 1346, 1282, 1223, 1158, 1119, 983, 852, 759 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 7.38 (t, J = 8.7 Hz, 2H, Ar-H), 7.75 (t, J = 7.9 Hz, 1H, Ar-H), 7.88–8.09 (m, 4H, Ar-H), 8.31 (d, J = 7.9 Hz, 1H, Ar-H), 8.44 (s, 1H, N=CH), 8.78 (s, 1H, triazole), 9.15 (s, 1H, triazole), 9.37 (s, 1H, 4-H, quinoline), 12.21 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 115.7, 116.1, 122.5, 127.9, 128.6 (two overlapping signals), 129.4, 130.0, 130.8, 131.0, 132.4, 137.6, 143.5, 145.7, 146.3, 146.5, 153.3, 162.6, 167.1 ppm; MS (ESI) m/z: 359 [M − H]−. Anal. calcd. for C19H13FN6O (360.34): C, 63.33; H, 3.64; N, 23.32. Found: C, 63.52; H, 3.51; N, 23.21.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]furan-2-carbohydrazide (6f). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and furan-2-carbohydrazide (1 mmol), the title compound 6f was obtained after washing with hot ethanol. Yield 31%; m.p. 248–250 °C; IR (KBr) νmax: 3176, 3105, 3036, 1659, 1643, 1599, 1507, 1491, 1444, 1357, 1290, 1280, 1190, 1122, 1086, 1023, 984, 859, 783, 755 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 6.71–6.74 (m, 1H, Ar-H), 7.21–7.45 (m, 1H, Ar-H), 7.75 (t, J = 7.9 Hz, 1H, Ar-H), 7.89–7.98 (m, 2H, Ar-H and N=CH), 8.07 (d, J = 8.3 Hz, 1H, Ar-H), 8.31 (d, J = 8.3 Hz, 1H, Ar-H), 8.45 (s, 1H, triazole), 8.76 (br. s, 1H, Ar-H), 9.13 (s, 1H, 4-H, quinoline), 9.38 (s, 1H, triazole), 12.22 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 112.6, 115.8, 122.5, 127.9, 128.6 (two overlapping signals), 129.4, 132.4, 137.6, 138.8, 143.5, 145.6, 146.3, 146.5, 146.6, 153.3, 154.3 ppm; MS (ESI) m/z: 355 [M + Na]+. Anal. calcd. for C17H12N6O2 (332.32): C, 61.44; H, 3.64; N, 25.29. Found: C, 61.58; H, 3.51; N, 25.34.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]thiophene-2-carbohydrazide (6g). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and thiophene-2-carbohydrazide (1 mmol), the title compound 6g was obtained without further purification. Yield 36%; m.p. 265–267 °C; IR (KBr) νmax: 3164, 3096, 2985, 1633, 1601, 1511, 1499, 1374, 1313, 1181, 1118, 1036, 982, 757, 740 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 7.25 (t, J = 4.2 Hz, 1H, Ar-H), 7.76 (t, J = 7.1 Hz, 1H, Ar-H), 7.89–8.10 (m, 4H, Ar-H and N=CH), 8.30 (d, J = 7.9 Hz, 1H, Ar-H), 8.46 (s, 1H, triazole), 8.76 (br. s, 1H, Ar-H), 9.14 (s, 1H, 4-H, quinoline), 9.39 (s, 1H, triazole), 12.21 (br. s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 122.4, 126.5, 127.8, 128.6 (two overlapping signals), 129.4, 129.9, 132.4, 135.7, 137.7, 140.5, 143.0, 145.7, 146.2, 146.5, 153.3, 158.0 ppm, MS (ESI) m/z: 347 [M − H]−. Anal. calcd. for C17H12N6OS (348.38): C, 58.61; H, 3.47; N, 24.12. Found: C, 58.49; H, 3.35; N, 24.23.
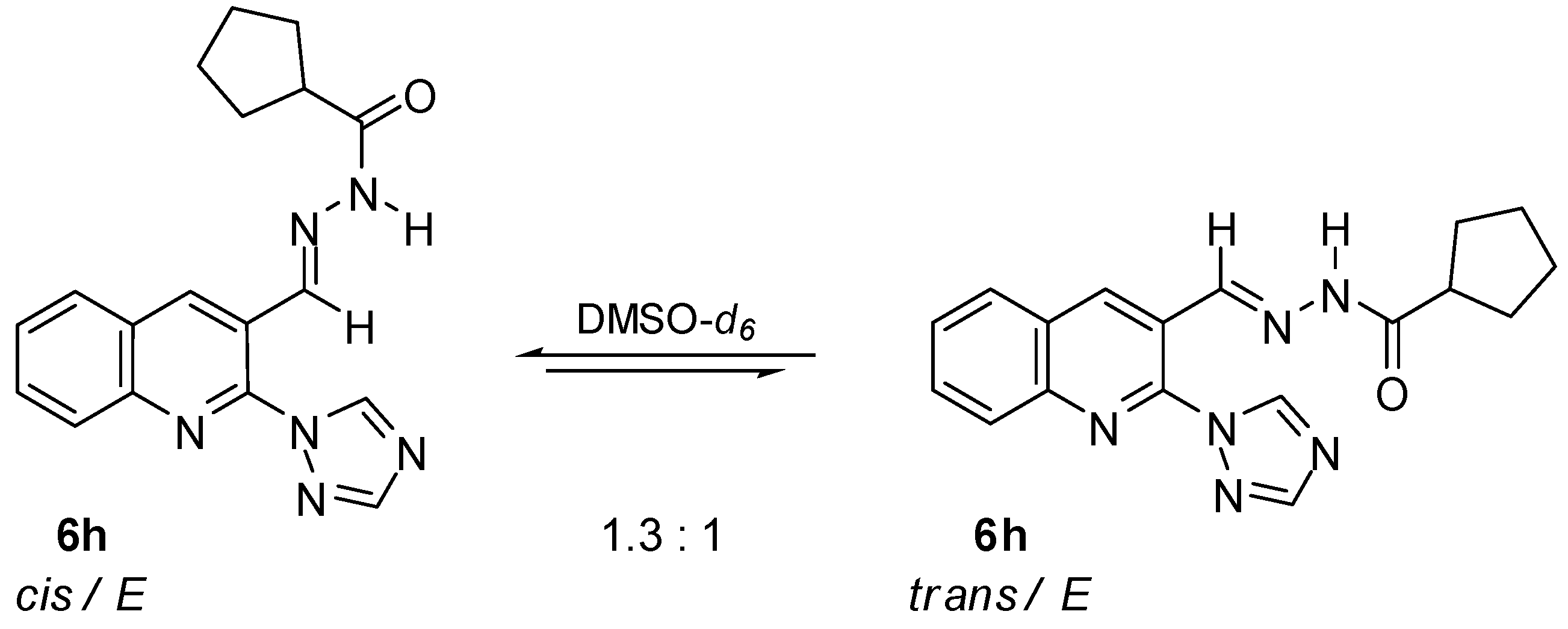
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]cyclopentanecarbohydrazide (6h). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and cyclopentanecarbohydrazide (1 mmol), the title compound 6h was obtained as a mixture of cis/trans conformers after preparative thin layer chromatography (eluent: CH2Cl2:AcOEt 10:1 v/v). Yield 42%; m.p. 239–241 °C; IR (KBr) νmax: 3196, 3103, 2959, 2866, 1661, 1599, 1491, 1442, 1395, 1257, 1140, 1124, 982, 950, 760 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 1.66–1.98 (m, 16H, 8xCH2), 2.62–2.69 and 3.53–4.21 (m, 1H, CH), 7.73 (t, J = 7.5 Hz, 2H, 2xCH), 7.90 (t, J = 8.3 Hz, 2H, 2xCH), 8.03–8.07 (m, 2H, 2xCH), 8.24–8.31 (m, 3H, 3xCH), 8.40 and 8.44 (s, 1H, triazole), 8.51 (s, 1H, N=CH), 9.02 and 9.06 (s, 1H, 4-H, quinoline), 9.32 and 9.36 (s, 1H, triazole), 11.42 and 11.67 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 26.2, 26.3, 29.8, 30.4, 43.5, 127.8, 128.5, 129.2, 132.3, 137.3, 137.4, 138.4, 141.4, 145.5, 145.6, 153.1, 153.3, 172.5 and 177.8 ppm; MS (ESI) m/z: 333 [M − H]−. Anal. calcd. for C18H18N6O (334.38): C, 64.66; H, 5.43; N, 25.13. Found: C, 64.72; H, 5.51; N, 25.17.
N′-[(2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]benzohydrazide (7a). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and benzohydrazide (1 mmol), the title compound 7a was obtained after crystallization from methanol. Yield 77%; m.p. 236–239 °C; IR (KBr) νmax: 3234, 3047, 1654, 1545, 1491, 1378, 1283, 1068, 1017, 784, 748, 739 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.51 (t, J = 7.8 Hz, 2H, Ar-H), 7.57–7.61 (m, 2H, Ar-H), 7.73–7.80 (m, 2H, Ar-H), 7.90–7.95 (m, 3H, Ar-H), 8.12 (d, J = 8.3 Hz, 1H, Ar-H), 8.23 (d, J = 8.3 Hz, 1H, Ar-H), 8.27 (d, J = 8.3 Hz, 1H, Ar-H), 8.36 (d, J = 7.8 Hz, 1H, Ar-H), 8.75 (s, 1H, N=CH), 9.26 (s, 1H, 4-H, quinoline), 12.17 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 114.1, 120.2, 123.6, 126.2, 128.0, 128.4 (two overlapping signals), 129.0, 129.1, 129.2 (two overlapping signals), 129.7, 130.0 (two overlapping signals), 132.7, 133.4, 133.8, 138.2, 143.8, 146.0, 146.8, 147.2, 164.0 ppm; MS (ESI) m/z: 391 [M − H]−. Anal. calcd. for C23H16N6O (392.41): C, 70.40; H, 4.11; N, 21.42. Found: C, 70.52; H, 4.21; N, 21.67.
N′-[2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-methylbenzohydrazide (7b). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-methylbenzohydrazide (1 mmol), the title compound 7b was obtained after crystallization from n-butyl alcohol. Yield 40%; m.p. 226–230 °C; IR (KBr) νmax: 3233, 3047, 2922, 1655, 1546, 1491, 1462, 1444, 1378, 1284, 1068, 1018, 749 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 2.37 (s, 3H, CH3), 7.32 (d, J = 8.3 Hz, 2H, Ar-H), 7.61 (t, J = 7.8 Hz, 1H, Ar-H), 7.74–7.79 (m, 2H, Ar-H), 7.82 (d, J = 8.3 Hz, 2H, Ar-H), 7.95 (t, J = 8.3 Hz, 1H, Ar-H), 8.13 (d, J = 8.3 Hz, 1H, Ar-H), 8.23 (d, J = 8.3 Hz, 1H, Ar-H), 8.28 (d, J = 8.3 Hz, 1H, Ar-H), 8.37 (d, J = 8.3 Hz, 1H, Ar-H), 8.74 (s, 1H, N=CH), 9.27 (s, 1H, 4-H, quinoline), 12.09 (s, 1H, NH) ppm; MS (ESI) m/z: 405 [M − H]−. Anal. calcd. for C24H18N6O (406.44): C, 70.92; H, 4.46; N, 20.68. Found: C, 70.86; H, 4.62; N, 20.55.
N′-[2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-methoxybenzohydrazide (7c). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-methoxybenzohydrazide (1 mmol), the title compound 7c was obtained after crystallization from n-butyl alcohol. Yield 35%; m.p. 242–245 °C; IR (KBr) νmax: 3217, 3043, 2989, 2964, 2835, 1647, 1603, 1544, 1490, 1460, 1368, 1288, 1262, 1070, 1016, 784 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 3.82 (s, 3H, OCH3), 7.04 (d, J = 8.8 Hz, 2H, Ar-H), 7.60 (t, J = 7.8 Hz, 1H, Ar-H), 7.73–7.80 (m, 2H, Ar-H), 7.90 (d, J = 8.8 Hz, 2H, Ar-H), 7.92–7.95 (m, 1H, Ar-H), 8.12 (d, J = 8.3 Hz, 1H, Ar-H), 8.22 (d, J = 8.3 Hz, 1H, Ar-H), 8.28 (d, J = 8.3 Hz, 1H, Ar-H), 8.35 (d, J = 7.8 Hz, 1H, Ar-H), 8.73 (s, 1H, N=CH), 9.25 (s, 1H, 4-H, quinoline), 12.04 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 55.9, 113.7, 114.1 (three overlapping signals), 119.9, 123.5, 125.5, 125.9, 127.7, 128.7 (two overlapping signals), 129.4, 129.7, 130.1, 132.4, 133.2, 137.8, 142.8, 145.6, 146.5, 146.9, 162.6, 163.0 ppm; MS (ESI) m/z: 421 [M − H]−. Anal. calcd. for C24H18N6O2 (422.44): C, 68.24; H, 4.29; N, 19.89. Found: C, 68.43; H, 4.18; N, 19.60.
N′-[2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-chlorobenzohydrazide (7d). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-chlorobenzohydrazide (1 mmol), the title compound 7d was obtained after crystallization from n-butyl alcohol. Yield 80%; m.p. 238–240 °C; IR (KBr) νmax: 3234, 3067, 1656, 1593, 1547, 1490, 1462, 1375, 1297, 1282, 1066, 1017, 785, 748 cm−1; 1H NMR (200 MHz, CDCl3) δ: 7.26–7.43 (m, 3H, Ar-H), 7.50–7.67 (m, 2H, Ar-H), 7.72–7.88 (m, 3H, Ar-H), 7.96–8.09 (m, 3H, Ar-H), 8.24 (d, J = 8.0 Hz, 1H, Ar-H), 9.00 (s, 1H, N=CH), 9.22 (s, 1H, 4-H, quinoline), 10.62 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 114.1, 120.2, 123.5, 126.2, 128.0, 129.0, 129.1, 129.3 (two overlapping signals), 129.7, 130.0, 130.4 (two overlapping signals), 132.5, 132.7, 133.4, 137.5, 138.2, 144.2, 145.9, 146.8, 147.2, 162.9 ppm; MS (ESI) m/z: 425 [M − H]−. Anal. calcd. for C23H15ClN6O (426.86): C, 64.72; H, 3.54; N, 19.69. Found: C, 64.46; H, 3.89; N, 20.00.
N′-[2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-fluorobenzohydrazide (7e). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-fluorobenzohydrazide (1 mmol), the title compound 7e was obtained after preparative thin layer chromatography (eluent: CH2Cl2/AcOEt 10:1 v/v). Yield 61%; m.p. 256–258 °C; IR (KBr) νmax: 3202, 3066, 2924, 1655, 1600, 1555, 1505, 1491, 1462, 1378, 1288, 1228, 1067, 1018, 748 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 7.37 (t, J = 8.3 Hz, 2H, Ar-H), 7.61 (t, J = 8.3 Hz, 1H, Ar-H), 7.72–7.83 (m, 2H, Ar-H), 7.97–8.04 (m, 3H, Ar-H), 8.14 (d, J = 8.3 Hz, 1H, Ar-H), 8.24–8.38 (m, 3H, Ar-H), 8.76 (s, 1H, N=CH), 9.27 (s, 1H, 4-H, quinoline), 12.19 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 113.8, 115.7, 116.1, 119.9, 123.3, 125.9, 127.7, 128.8 (two overlapping signals), 129.4, 129.7, 129.9, 130.8, 131.0, 132.4, 133.1, 137.9, 143.6, 145.7, 146.5, 146.9, 162.6, 167.1 ppm; MS (ESI) m/z: 409 [M − H]−. Anal. calcd. for C23H15FN6O (410.40): C, 67.31; H, 3.68; N, 20.48. Found: C, 67.23; H, 3.47; N, 20.27.
N′-[2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]furan-2-carbohydrazide (7f). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and furan-2-carbohydrazide (1 mmol), the title compound 7f was obtained after washing with hot ethanol. Yield 53%; m.p. 283–285 °C; IR (KBr) νmax: 3254, 3147, 3072, 1663, 1597, 1565, 1544, 1492, 1467, 1301, 1200, 1069, 1017, 784, 751 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 6.69–6.70 (m, 1H, Ar-H), 7.28–7.34 (m, 1H, Ar-H), 7.60 (t, J = 7.3 Hz, 1H, Ar-H), 7.72–7.78 (m, 2H, Ar-CH), 7.91–7.94 (m, 2H, Ar-H and N=CH), 8.11 (d, J = 8.3 Hz, 1H, Ar-H), 8.22 (d, J = 8.3 Hz, 1H, Ar-H), 8.27 (d, J = 8.3 Hz, 1H, Ar-H), 8.34 (d, J = 8.3 Hz, 1H, Ar-H), 8.74 (s, 1H, Ar-H), 9.22 (s, 1H, 4-H, quinoline), 12.18 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 112.9, 114.0, 116.1, 120.2, 123.5, 126.1, 128.0, 129.0, 129.1, 129.7, 130.0 (two overlapping signals), 132.7, 133.4, 138.2, 143.9, 146.0, 146.8, 147.1, 147.2, 155.0 ppm; MS (ESI) m/z: 381 [M − H]−. Anal. calcd. for C21H14N6O2 (382.37): C, 65.96; H, 3.69; N, 21.98. Found: C, 65.87; H, 3.75; N, 22.11.
N′-[2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]thiophene-2-carbohydrazide (7g). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and thiophene-2-carbohydrazide (1 mmol), the title compound 7g was obtained after washing with hot ethanol. Yield 53%; m.p. 237–239 °C; IR (KBr) νmax: 3243, 3106, 1647, 1596, 1548, 1492, 1426, 1381, 1284, 1064, 1015, 784, 750 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.18–7.24 (m, 1H, Ar-H), 7.59 (t, J = 7.8 Hz, 1H, Ar-H), 7.73–7.84 (m, 2H, Ar-H), 7.89–7.97 (m, 3H, Ar-H), 8.12 (d, J = 8.8 Hz, 1H, Ar-H), 8.24–8.28 (m, 2H, Ar-H and N=CH), 8.33 (d, J = 7.8 Hz, 1H, Ar-H), 8.73 (br. s, 1H, Ar-H), 9.22 (s, 1H, 4-H, quinoline), 12.17 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 113.9, 119.9 (two overlapping signals), 123.2, 125.9 (two overlapping signals), 127.6, 128.7 (two overlapping signals), 129.4, 129.7 (two overlapping signals), 132.4, 133.1, 135.5, 138.0, 138.5, 143.2, 145.7, 146.5, 146.9 ppm; MS (ESI) m/z: 397 [M − H]−. Anal. calcd. for C21H14N6OS (398.44): C, 63.30; H, 3.54; N, 21.09. Found: C, 63.15; H, 3.27; N, 21.44.
N′-[2-(1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]cyclopentanecarbohydrazide (7h). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and cyclopentanecarbohydrazide (1 mmol), the title compound 7h was obtained as a mixture of cis/trans conformers after preparative thin layer chromatography (eluent: CH2Cl2: AcOEt 10:1 v/v). Yield 71%; m.p. 202–204 °C; IR (KBr) νmax: 3199, 3057, 2954, 2867, 1665, 1619, 1560, 1493, 1463, 1447, 1384, 1288, 1214, 1062, 1020, 784, 747 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 1.60–1.68 (m, 16H, 8xCH2), 2.60–2.63 and 3.19–3.42 (m, 1H, CH), 7.48–7.62 (m, 2H, 2xCH), 7.65–7.82 (m, 4H, 4xCH), 7.94 (t, J = 7.9 Hz, 2H, 2xCH), 8.11–8.15 (m, 3H, 3xCH), 8.27–8.32 (m, 6H, 6xCH), 8.48 (s, 1H, N=CH), 9.11 and 9.18 (s, 1H, 4-H, quinoline), 11.36 and 11.64 (s, 1H, NH) ppm; 13C NMR (50 MHz, DMSO-d6) δ: 26.2 (two overlapping signals), 29.7, 30.4, 43.6, 113.8, 119.9, 125.9, 127.7, 128.7, 129.2, 129.5, 129.7, 132.3, 133.1, 137.8, 138.2, 138.6, 141.6, 145.6, 146.4, 172.5 and 177.7 ppm; MS (ESI) m/z: 383 [M − H]−. Anal. calcd. for C22H20N6O (384.43): C, 68.73; H, 5.24; N, 21.86. Found: C, 68.82; H, 5.32; N, 21.47.
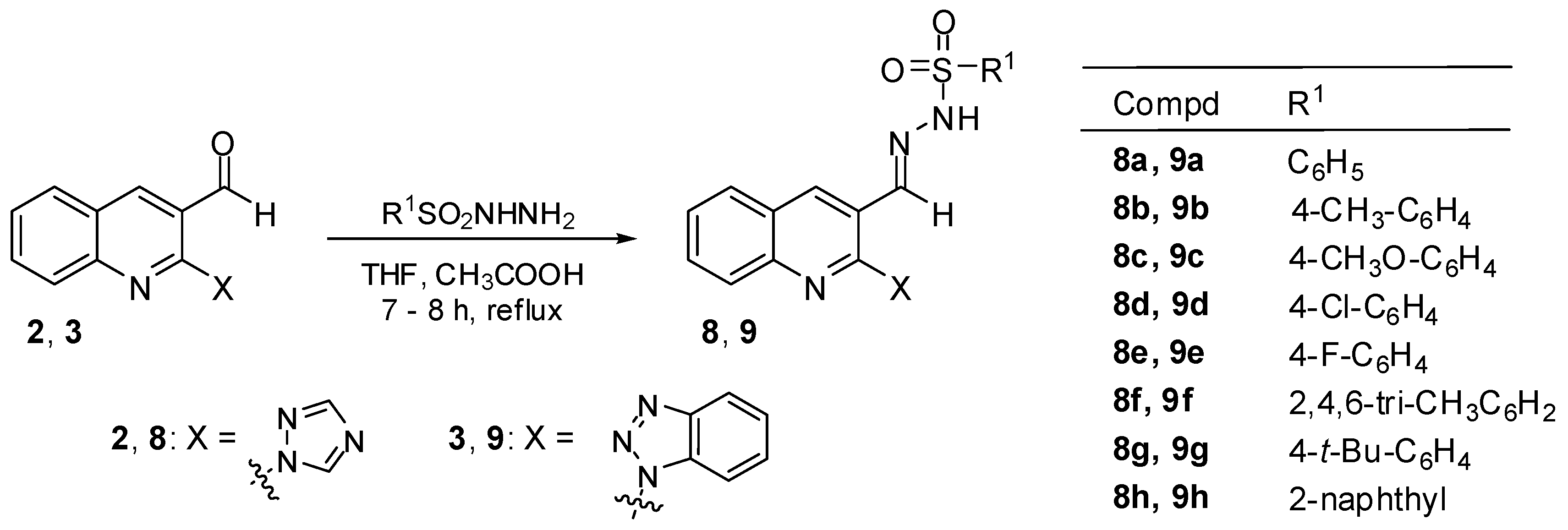
3.2.5. General Procedure for the Preparation of N′-Sulfonylhydrazones 8a–h and 9a–h
A mixture of 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) or 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and appropriate sulfonohydrazide (1 mmol) in the presence of a catalytic amount of acetic acid in THF (5 mL) was heated under reflux for 7–8 h. The progress of the reaction was controlled by TLC. The mixture was then evaporated under reduced pressure and the crude product thus obtained was purified as described below. In this manner, the following compounds were obtained.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]benzenesulfonohydrazide (8a). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and benzenesulfonohydrazide (1 mmol), the title compound 8a was obtained after washing with hot methanol. Yield 35%; m.p. 193–196 °C; IR (KBr) νmax: 3115, 3072, 2978, 2910, 1620, 1605, 1566, 1511, 1495, 1442, 1338, 1284, 1164, 1063, 1048, 938, 895, 760 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 7.62 (d, J = 8.3 Hz, 2H, Ar-H), 7.76 (t, J = 7.9 Hz, 1H, Ar-H), 7.90–8.00 (m, 4H, Ar-H), 8.06–8.10 (m, 1H, Ar-H), 8.33 (d, J = 7.9 Hz, 1H, Ar-H), 8.45 (s, 1H, N=CH), 8.78 (s, 1H, triazole), 9.17 (s, 1H, 4-H, quinoline), 9.38 (s, 1H, triazole), 12.25 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 121.8, 127.8 (three overlapping signals), 128.8, 128.9, 129.5, 130.1 (two overlapping signals), 132.8, 134.0, 137.7, 139.6, 143.4, 145.8, 146.3, 146.4, 153.4 ppm. Anal. calcd. for C18H14N6O2S (378.41): C, 57.13; H, 3.73; N, 22.21. Found: C, 56.83; H, 3.65; N, 22.65.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-4-methylbenzenesulfonohydrazide (8b). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-methylbenzenesulfonohydrazide (1 mmol), the title compound 8b was obtained after washing with hot methanol. Yield 44%; m.p. 180–186 °C; IR (KBr) νmax: 3110, 2916, 1620, 1599, 1512, 1493, 1441, 1345, 1284, 1165, 1067, 951, 902, 759 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 2.36 (s, 3H, CH3), 7.44 (d, J = 7.9 Hz, 2H, Ar-H), 7.75 (t, J = 7.1 Hz, 1H, Ar-H), 7.77–7.93 (m, 3H, Ar-H), 8.03 (d, J = 7.1 Hz, 1H, Ar-H), 8.25–8.30 (m, 2H, Ar-H and N=CH), 8.38 (s, 1H, triazole), 8.87 (s, 1H, 4-H, quinoline), 9.30 (s, 1H, triazole), 11.38 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.7, 121.9, 127.8, 127.9 (two overlapping signals), 128.8, 128.9, 129.5, 130.5 (two overlapping signals), 132.8, 136.7, 137.6, 143.2, 144.6, 145.8, 146.3, 146.4, 153.4 ppm; MS (ESI) m/z: 415 [M + Na]+. Anal. calcd. for C19H16N6O2S (392.43): C, 58.15; H, 4.11; N, 21.42. Found: C, 57.93; H, 3.95; N, 21.71.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-4-methoxybenzenesulfonohydrazide (8c). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-methoxybenzenesulfonohydrazide (1 mmol), the title compound 8c was obtained after washing with hot methanol. Yield 52%; m.p. 185–188 °C; IR (KBr) νmax: 3110, 3058, 2882, 2799, 1618, 1567, 1512, 1490, 1441, 1338, 1282, 1176, 1167, 1065, 992, 944, 787, 762 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 3.82 (s, 3H, OCH3), 7.15 (d, J = 9.1 Hz, 2H, Ar-H), 7.74 (t, J = 7.1 Hz, 1H, Ar-H), 7.85–7.94 (m, 3H, Ar-H), 8.01–8.06 (m, 1H, Ar-H), 8.25–8.30 (m, 2H, Ar-H and N=CH), 8.38 (s, 1H, triazole), 8.87 (s, 1H, 4-H, quinoline), 9.30 (s, 1H, triazole), 11.74 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.4, 115.2 (two overlapping signals), 121.9, 127.9, 128.8, 128.9, 129.5, 130.2 (two overlapping signals), 131.1, 132.8, 137.6, 143.0, 145.8, 146.3, 146.4, 153.4, 163.4 ppm. Anal. calcd. for C19H16N6O3S (408.43): C, 55.87; H, 3.95; N, 20.58. Found: C, 55.97; H, 4.15; N, 20.58.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-4-chlorobenzenesulfonohydrazide (8d). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-chlorobenzenesulfonohydrazide (1 mmol), the title compound 8d was obtained after washing with hot methanol. Yield 71%; m.p. 196–200 °C; IR (KBr) νmax: 3110, 3058, 2882, 2799, 1618, 1567, 1512, 1490, 1441, 1338, 1282, 1176, 1167, 1065, 944, 787, 762 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 7.69–7.73 (m, 3H, Ar-H), 7.87–7.90 (m, 1H, Ar-H), 7.94 (d, J = 8.8 Hz, 2H, Ar-H), 8.01–8.02 (m, 1H, Ar-H), 8.22 (d, J = 8.3 Hz, 1H, Ar-H), 8.34 (s, 1H, triazole), 8.36 (s, 1H, N=CH), 8.84 (s, 1H, 4-H, quinoline), 9.27 (s, 1H, triazole), 11.89 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 121.9, 127.9, 128.8, 128.9, 129.0, 129.6 (two overlapping signals), 130.2 (two overlapping signals), 132.7, 137.8, 138.7, 138.8, 144.0, 145.8, 146.4, 146.6, 153.5 ppm. Anal. calcd. for C18H13ClN6O2S (412.85): C, 52.37; H, 3.17; N, 20.36. Found: C, 52.45; H, 3.37; N, 20.14.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-4-fluorobenzenesulfonohydrazide (8e). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-fluorobenzenesulfonohydrazide (1 mmol), the title compound 8e was obtained after crystallization from n-butyl alcohol. Yield 38%; m.p. 184–186 °C; IR (KBr) νmax: 3110, 3066, 2909, 2799, 1619, 1592, 1511, 1492, 1441, 1330, 1284, 1231, 1171, 1066, 944, 832, 757 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.48 (t, J = 8.8 Hz, 2H, Ar-H), 7.71 (t, J = 7.8 Hz, 1H, Ar-H), 7.87 (t, J = 7.8 Hz, 1H, Ar-H), 8.00–8.02 (m, 3H, Ar-H), 8.24 (d, J = 8.3 Hz, 1H, Ar-H), 8.32 (s, 1H, N=CH), 8.37 (s, 1H, triazole), 8.85 (s, 1H, 4-H, quinoline), 9.30 (s, 1H, triazole), 11.94 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 117.2, 117.4, 121.8, 127.9, 128.8, 129.6, 131.0, 131.1, 132.7, 136.0, 137.7, 143.8, 145.8, 146.4, 146.5, 153.3, 164.3, 166.3 ppm; MS (ESI) m/z: 397 [M + H]+. Anal. calcd. for C18H13FN6O2S (396.40): C, 54.54; H, 3.31; N, 21.20. Found: C, 54.37; H, 3.18; N, 21.58.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-2,4,6-trimethylbenzenesulfonohydrazide (8f). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 2,4,6-trimethylbenzenesulfonohydrazide (1 mmol), the title compound 8f was obtained after crystallization from n-butyl alcohol. Yield 65%; m.p. 184–186 °C; IR (KBr) νmax: 3115, 3071, 2909, 1604, 1511, 1495, 1442, 1338, 1284, 1164, 1063, 1048, 938, 895, 760 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 2.21 (s, 3H, CH3), 2.64 (s, 6H, 2xCH3), 7.04 (s, 2H, Ar-H), 7.69 (t, J = 7.3 Hz, 1H, Ar-H), 7.85 (t, J = 7.3 Hz, 1H, Ar-H), 7.99 (d, J = 8.3 Hz, 1H, Ar-H), 8.10 (d, J = 8.3 Hz, 1H, Ar-H), 8.28 (s, 1H, N=CH), 8.35 (s, 1H, triazole), 8.65 (s, 1H, 4-H, quinoline), 9.26 (s, 1H, triazole), 11.97 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.0, 23.2 (two overlapping signals), 122.3, 127.9, 128.9 (two overlapping signals), 129.2 (two overlapping signals), 132.3 (three overlapping signals), 134.4, 137.2, 139.9, 141.4, 143.1, 145.6, 146.3, 146.5, 153.3 ppm. Anal. calcd. for C21H20N6O2S (420.49): C, 59.98; H, 4.79; N, 19.99. Found: C, 60.13; H, 4.87; N, 19.67.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]-4-tert-butylbenzenesulfonohydrazide (8g). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and 4-tert-butylbenzenesulfonohydrazide (1 mmol), the title compound 8g was obtained after washing with hot methanol. Yield 35%; m.p. 142–146 °C; IR (KBr) νmax: 3113, 3067, 2962, 2798, 1619, 1597, 1566, 1491, 1440, 1338, 1283, 1168, 1066, 944, 787, 761 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 1.27 (s, 9H, 3xCH3), 7.63–7.71 (m, 3H, Ar-H), 7.85–7.90 (m, 3H, Ar-H), 8.01–8.05 (m, 1H, Ar-H), 8.25–8.38 (m, 3H, Ar-H, N=CH and CH-triazole), 8.88 (s, 1H, 4-H, quinoline), 9.30 (s, 1H, triazole), 11.88 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 31.4 (three overlapping signals), 35.6, 121.8, 127.0 (two overlapping signals), 127.8 (two overlapping signals), 127.9, 128.8, 129.9, 129.5, 132.8, 136.8, 137.6, 143.1, 145.8, 146.3, 146.4, 153.4, 157.0 ppm; MS (ESI) m/z: 457 [M + Na]+. Anal. calcd. for C22H22N6O2S (434.51): C, 60.81; H, 5.10; N, 19.34. Found: C, 60.63; H, 4.98; N, 19.66.
N′-[(2-(1H-1,2,4-Triazol-1-yl)quinolin-3-yl)methylene]naphthalene-2-sulfonohydrazide (8h). Starting from 2-(1H-1,2,4-triazol-1-yl)quinoline-3-carbaldehyde (2) (0.224 g, 1 mmol) and naphthalene-2-sulfonohydrazide (1 mmol), the title compound 8h was obtained after washing with hot methanol. Yield 59%; m.p. 183–187 °C; IR (KBr) νmax: 3111, 3056, 2907, 2795, 1619, 1602, 1511, 1492, 1441, 1338, 1283, 1165, 1066, 954, 812, 747 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.65–7.71 (m, 3H, Ar-H), 7.86 (t, J = 8.3 Hz, 1H, Ar-H), 7.95 (dd, J = 8.8 Hz, J = 1.5 Hz, 1H, Ar-H), 7.97–8.02 (m, 2H, Ar-H), 8.15 (d, J = 8.8 Hz, 1H, Ar-H), 8.22 (d, J = 7.8 Hz, 1H, Ar-H), 8.27 (d, J = 8.8 Hz, 1H, Ar-H), 8.31 (s, 1H, N=CH), 8.35 (s, 1H, triazole), 8.67 (s, 1H, Ar-H), 8.87 (s, 1H, 4-H, quinoline), 9.27 (s, 1H, triazole), 11.97 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 106.3, 121.8, 123.2, 127.9, 128.4, 128.6, 128.8, 129.2, 129.5, 129.8, 130.0, 130.1, 132.5, 132.7, 135.1, 136.7, 137.6, 143.4, 145.8, 146.4, 146.5, 153.5 ppm. Anal. calcd. for C22H16N6O2S (428.47): C, 61.67; H, 3.76; N, 19.61. Found: C, 61.43; H, 3.67; N, 19.30.
N′-[(2-1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]benzenesulfonohydrazide (9a). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and benzenesulfonohydrazide (1 mmol), the title compound 9a was obtained after washing with methanol. Yield 53%; m.p. 118–121 °C; IR (KBr) νmax: 3101, 3059, 2923, 2853, 1621, 1603, 1496, 1449, 1363, 1324, 1286, 1164, 1091, 1065, 947, 750 cm−1; 1H NMR (400 MHz, DMSO-d6) δ: 7.58–7.68 (m, 4H, Ar-H), 7.70–7.80 (m, 2H, Ar-H), 7.94–7.96 (m, 3H, Ar-H), 8.12 (d, J = 8.3 Hz, 1H, Ar-H), 8.22–8.26 (m, 2H, Ar-H), 8.28 (s, 1H, N=CH), 8.33 (d, J = 8.1 Hz, 1H, Ar-H), 8.98 (s, 1H, 4-H, quinoline), 11.90 (s, 1H, NH) ppm. MS (ESI): m/z: 451 [M + Na]+. Anal. calcd. for C22H16N6O2S (428.47): C, 61.67; H, 3.76; N, 19.61. Found: C, 61.87; H, 3.98; N, 19.29.
N′-[(2-1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-methylbenzenesulfonohydrazide (9b). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-methylbenzenesulfonohydrazide (1 mmol), the title compound 9b was obtained after preparative thin layer chromatography (eluent: CH2Cl2: AcOEt 10:1 v/v). Yield 41%; m.p. 193–197 °C; IR (KBr) νmax: 3214, 3064, 2955, 2855, 2772, 1597, 1149, 1447, 1370, 1324, 1286, 1163, 1049, 1020, 943, 763 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 2.35 (s, 3H, CH3), 7.42 (d, J = 7.8 Hz, 2H, Ar-H), 7.58 (t, J = 8.3 Hz, 1H, Ar-H), 7.71 (t, J = 7.8 Hz, 1H, Ar-H), 7.76 (t, J = 7.8 Hz, 1H, Ar-H), 7.79 (d, J = 8.3 Hz, 2H, Ar-H), 7.92 (t, J = 8.3 Hz, 1H, Ar-H), 8.09 (d, J = 8.3 Hz, 1H, Ar-H), 8.20 (d, J = 8.3 Hz, 1H, Ar-H), 8.24–8.25 (m, 2H, Ar-H and N=CH), 8.31 (d, J = 8.3 Hz, 1H, Ar-H), 8.96 (s, 1H, 4-H, quinoline), 11.80 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.6, 113.9, 120.1, 122.9, 126.1, 127.7, 127.8 (two overlapping signals), 129.0 (two overlapping signals), 129.4, 129.9, 130.4 (two overlapping signals), 132.7, 133.4, 137.0, 138.0 (two overlapping signals), 143.3, 144.3, 145.9, 146.8 ppm. MS (ESI): m/z: 441 [M − H]−. Anal. calcd. for C23H18N6O2S (442.49): C, 62.43; H, 4.10; N, 18.99. Found: C, 62.27; H, 3.98; N, 19.35.
N′-[(2-1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-methoxybenzenesulfonohydrazide (9c). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-methoxybenzenesulfonohydrazide (1 mmol), the title compound 9c was obtained after washing with hot methanol. Yield 63%; m.p. 198–202 °C; IR (KBr) νmax: 3149, 3069, 2860, 2760, 1595, 1578, 1493, 1426, 1352, 1290, 1264, 1163, 1022, 953, 785, 745 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 3.81 (s, 3H, OCH3), 7.13 (d, J = 8.8 Hz, 2H, Ar-H), 7.58 (t, J = 7.8 Hz, 1H, Ar-H), 7.72 (t, J = 7.8 Hz, 1H, Ar-H), 7.76 (t, J = 7.8 Hz, 1H, Ar-H), 7.85 (d, J = 8.8 Hz, 2H, Ar-H), 7.92 (t, J = 7.8 Hz, 1H, Ar-H), 8.10 (d, J = 8.3 Hz, 1H, Ar-H), 8.20 (d, J = 8.3 Hz, 1H, Ar-H), 8.23–8.26 (m, 2H, Ar-H and N=CH), 8.31 (d, J = 8.3 Hz, 1H, Ar-H), 8.96 (s, 1H, 4-H, quinoline), 11.71 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 56.4, 114.2, 115.2 (two overlapping signals), 120.2, 122.9, 126.1, 127.7, 129.0, 129.1, 129.6, 129.9, 130.1 (two overlapping signals), 131.3, 132.7, 133.3, 137.9, 143.1, 145.9, 146.7, 146.9, 163.4 ppm; MS (ESI): m/z: 457 [M − H]−. Anal. calcd. for C23H18N6O3S (458.49): C, 60.25; H, 3.96; N, 18.33. Found: C, 60.12; H, 3.76; N, 18.65.
N′-[(2-1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-chlorobenzenesulfonohydrazide (9d). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-chlorobenzenesulfonohydrazide (1 mmol), the title compound 9d was obtained after washing with hot methanol. Yield 45%; m.p. 202–206 °C; IR (KBr) νmax: 3190, 3064, 2875, 1598, 1587, 1494, 1431, 1355, 1320, 1173, 1091, 1067, 1022, 952, 785, 757 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.57 (t, J = 7.3 Hz, 1H, Ar-H), 7.66–7.74 (m, 3H, Ar-H), 7.75 (t, J = 7.3 Hz, 1H, Ar-H), 7.77–7.93 (m, 3H, Ar-H), 8.09 (d, J = 8.3 Hz, 1H, Ar-H), 8.19 (d, J = 8.3 Hz, 1H, Ar-H), 8.22 (d, J = 8.3 Hz, 1H, Ar-H), 8.26 (d, J = 7.8 Hz, 1H, Ar-H), 8.32 (s, 1H, N=CH), 8.93 (s, 1H, 4-H, quinoline), 11.78 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 114.1, 120.2, 122.8, 126.0, 127.8, 128.9, 129.0, 129.5, 129.7 (two overlapping signals), 129.8 (two overlapping signals), 130.1 (two overlapping signals), 132.7, 133.4, 138.2, 138.8, 144.1, 146.0, 146.9, 147.0 ppm. Anal. calcd. for C22H15ClN6O2S (462.91): C, 57.08; H, 3.27; N, 18.15. Found: C, 56.87; H, 3.15; N, 18.54.
N′-[(2-1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-fluorobenzenesulfonohydrazide (9e). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-fluorobenzenesulfonohydrazide (1 mmol), the title compound 9e was obtained after washing with hot methanol. Yield 56%; m.p. 205–209 °C; IR (KBr) νmax: 3071, 2869, 2771, 1618, 1590, 1493, 1424, 1326, 1289, 1238, 1170, 1056, 1024, 941, 838, 757, 748 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.45 (t, J = 8.8 Hz, 2H, Ar-H), 7.57 (t, J = 7.8 Hz, 1H, Ar-H), 7.71 (t, J = 7.3 Hz, 1H, Ar-H), 7.75 (t, J = 7.3 Hz, 1H, Ar-H), 7.92 (t, J = 7.8 Hz, 1H, Ar-H), 7.97–8.00 (m, 2H, Ar-H), 8.09 (d, J = 8.3 Hz, 1H, Ar-H), 8.20 (d, J = 8.3 Hz, 1H, Ar-H), 8.23 (d, J = 8.3 Hz, 1H, Ar-H), 8.27–8.29 (m, 2H, Ar-H and N=CH), 8.94 (s, 1H, 4-H, quinoline), 11.82 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 114.2, 117.1, 117.3, 120.2, 122.8, 126.1, 127.7, 128.9, 129.0, 129.6, 129.9, 131.0, 132.7, 133.3, 136.2, 138.2, 144.0, 146.0, 146.8, 147.0, 164.2, 166.2 ppm. Anal. calcd. for C22H15FN6O2S (446.46): C, 59.18; H, 3.39; N, 18.82. Found: C, 59.38; H, 3.16; N, 18.56.
N′-[(2-1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-2,4,6-trimethylbenzenesulfonohydrazide (9f). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 2,4,6-trimethylbenzenesulfonohydrazide (1 mmol), the title compound 9f was obtained after washing with hot methanol. Yield 57%; m.p. 187–191 °C; IR (KBr) νmax: 3213, 3060, 2938, 1601, 1493, 1448, 1424, 1316, 1164, 1052, 1022, 941, 889, 750 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 2.23 (s, 3H, CH3), 2.64 (s, 6H, 2xCH3), 7.05 (s, 2H, Ar-H), 7.58 (t, J = 7.8 Hz, 1H, Ar-H), 7.70–7.76 (m, 2H, Ar-H), 7.91 (t, J = 8.3 Hz, 1H, Ar-H), 8.08 (d, J = 8.3 Hz, 1H, Ar-H), 8.17–8.25 (m, 3H, Ar-H), 8.26 (s, 1H, N=CH), 8.80 (s, 1H, 4-H, quinoline), 11.92 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.1, 23.4 (two overlapping signals), 114.2, 120.2, 123.0, 126.1, 127.6, 128.9, 129.0, 129.5, 129.9, 132.4 (three overlapping signals), 132.6, 133.2, 134.1, 137.4, 139.8, 141.6, 143.1, 145.9, 146.6, 146.9 ppm. Anal. calcd. for C25H22N6O2S (470.55): C, 63.81; H, 4.71; N, 17.86. Found: C, 63.61; H, 4.58; N, 17.58.
N′-[(2-1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]-4-tert-butylbenzenesulfonohydrazide (9g). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and 4-tert-butylbenzenesulfonohydrazide (1 mmol), the title compound 9g was obtained after washing with hot methanol. Yield 57%; m.p. 132–136 °C; IR (KBr) νmax: 3187, 3061, 2965, 2870, 2771, 1593, 1495, 1463, 1429, 1357, 1321, 1290, 1164, 1064, 1027, 944, 783 cm−1; 1H NMR (200 MHz, DMSO-d6) δ: 1.26 (s, 9H, 3xCH3), 7.58 (t, J = 7.8 Hz, 1H, Ar-H), 7.64 (d, J = 8.8 Hz, 2H, Ar-H), 7.72 (t, J = 7.8 Hz, 1H, Ar-H), 7.77 (t, J = 7.8 Hz, 1H, Ar-H), 7.84 (d, J = 8.8 Hz, 2H, Ar-H), 7.92 (t, J = 8.3 Hz, 1H, Ar-H), 8.10 (d, J = 8.3 Hz, 1H, Ar-H), 8.21 (d, J = 8.3 Hz, 1H, Ar-H), 8.25–8.26 (m, 2H, Ar-H and N=CH), 8.31 (d, J = 7.8 Hz, 1H, Ar-H), 8.98 (s, 1H, 4-H, quinoline), 11.85 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 31.4 (three overlapping signals), 35.5, 113.9, 120.1, 123.0, 126.0, 126.7 (two overlapping signals), 127.7 (two overlapping signals), 127.8, 129.0 (two overlapping signals), 129.4, 129.9 (two overlapping signals), 132.7, 133.4, 137.1, 138.1, 143.3, 146.0, 146.9, 157.1 ppm; MS (ESI): m/z: 483 [M − H]−. Anal. calcd. for C26H24N6O2S (484.57): C, 64.44; H, 4.99; N, 17.34. Found: C, 64.34; H, 4.78; N, 17.71.
N′-[(2-1H-Benzo[d][1,2,3]triazol-1-yl)quinolin-3-yl)methylene]naphthalene-2-sulfonohydrazide (9h). Starting from 2-(1H-benzo[d][1,2,3]triazol-1-yl)quinoline-3-carbaldehyde (3) (0.274 g, 1 mmol) and naphthalene-2-sulfonohydrazide (1 mmol), the title compound 9h was obtained after washing with hot methanol. Yield 52%; m.p. 196–200 °C; IR (KBr) νmax: 3179, 3055, 2915, 1618, 1587, 1493, 1446, 1427, 1328, 1289, 1164, 1051, 1021, 958, 783, 752 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.56 (t, J = 7.3 Hz, 1H, Ar-H), 7.66–7.71 (m, 3H, Ar-H), 7.75 (t, J = 7.8 Hz, 1H, Ar-H), 7.89–7.94 (m, 2H, Ar-H), 8.02 (d, J = 7.8 Hz, 1H, Ar-H), 8.07 (d, J = 8.3 Hz, 1H, Ar-H), 8.14–8.19 (m, 2H, Ar-H), 8.23 (d, J = 8.3 Hz, 1H, Ar-H), 8.26–8.29 (m, 3H, Ar-H and N=CH), 8.65 (s, 1H, Ar-H), 8.98 (s, 1H, 4-H, quinoline), 11.95 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 113.9, 120.1, 122.9, 123.1, 126.0, 127.7, 128.4, 128.5, 129.9 (two overlapping signals), 129.0, 129.4, 129.7, 129.8 (two overlapping signals), 129.9, 130.0, 132.5, 132.7, 133.4, 135.2, 136.9, 138.2, 143.6, 146.0, 146.8 ppm. Anal. calcd. for C26H18N6O2S (478.53): C, 65.26; H, 3.79; N, 17.56. Found: C, 65.17; H, 3.58; N, 17.96.