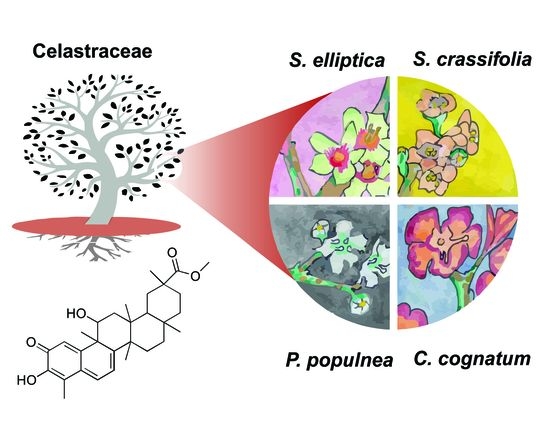
Cytotoxic Triterpenes from Salacia crassifolia and Metabolite Profiling of Celastraceae Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dose-Response Results
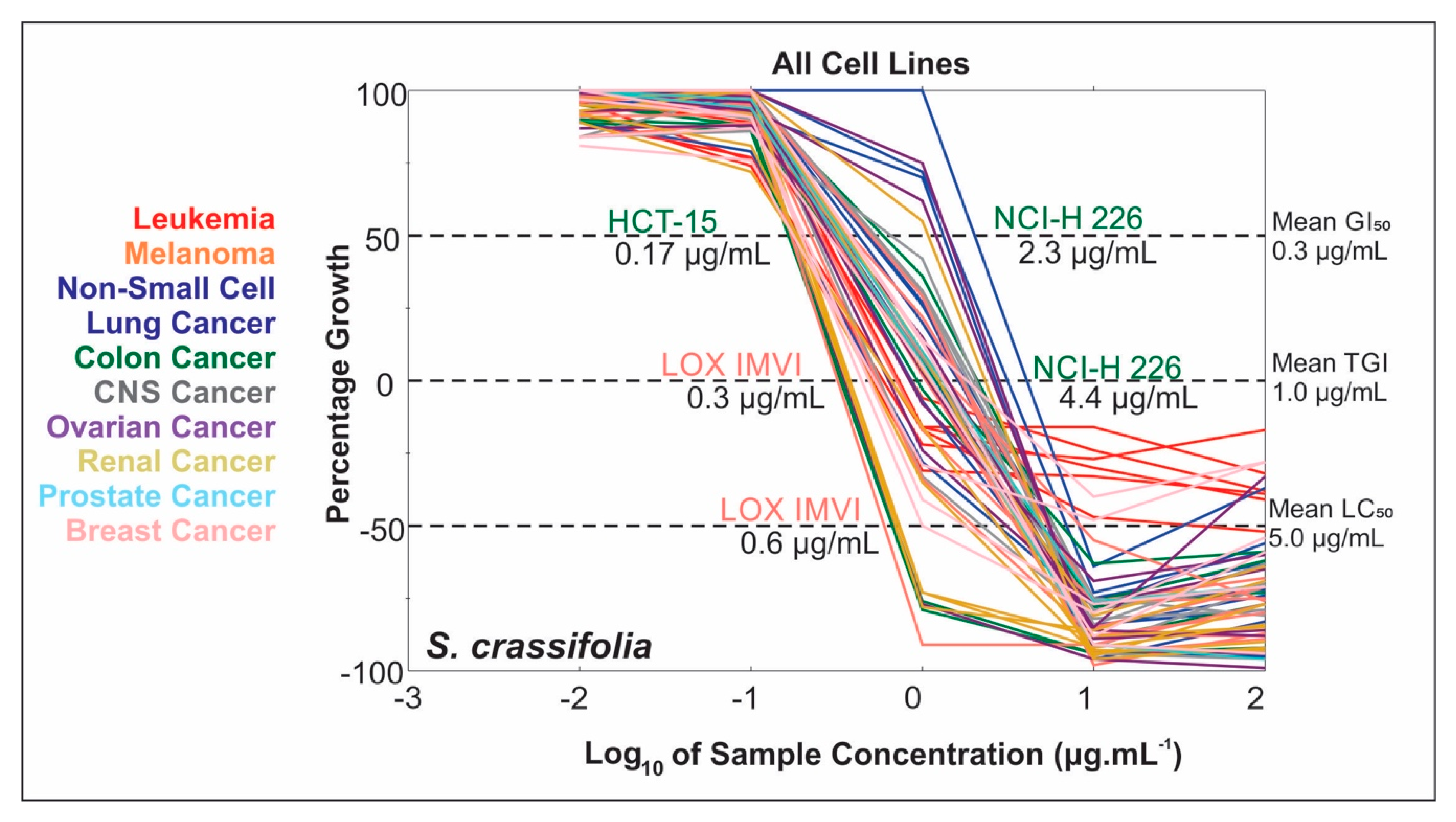
2.2. Extract NCI-60 Results
2.3. Bioassay-Guided Fractionation of S. crassifolia
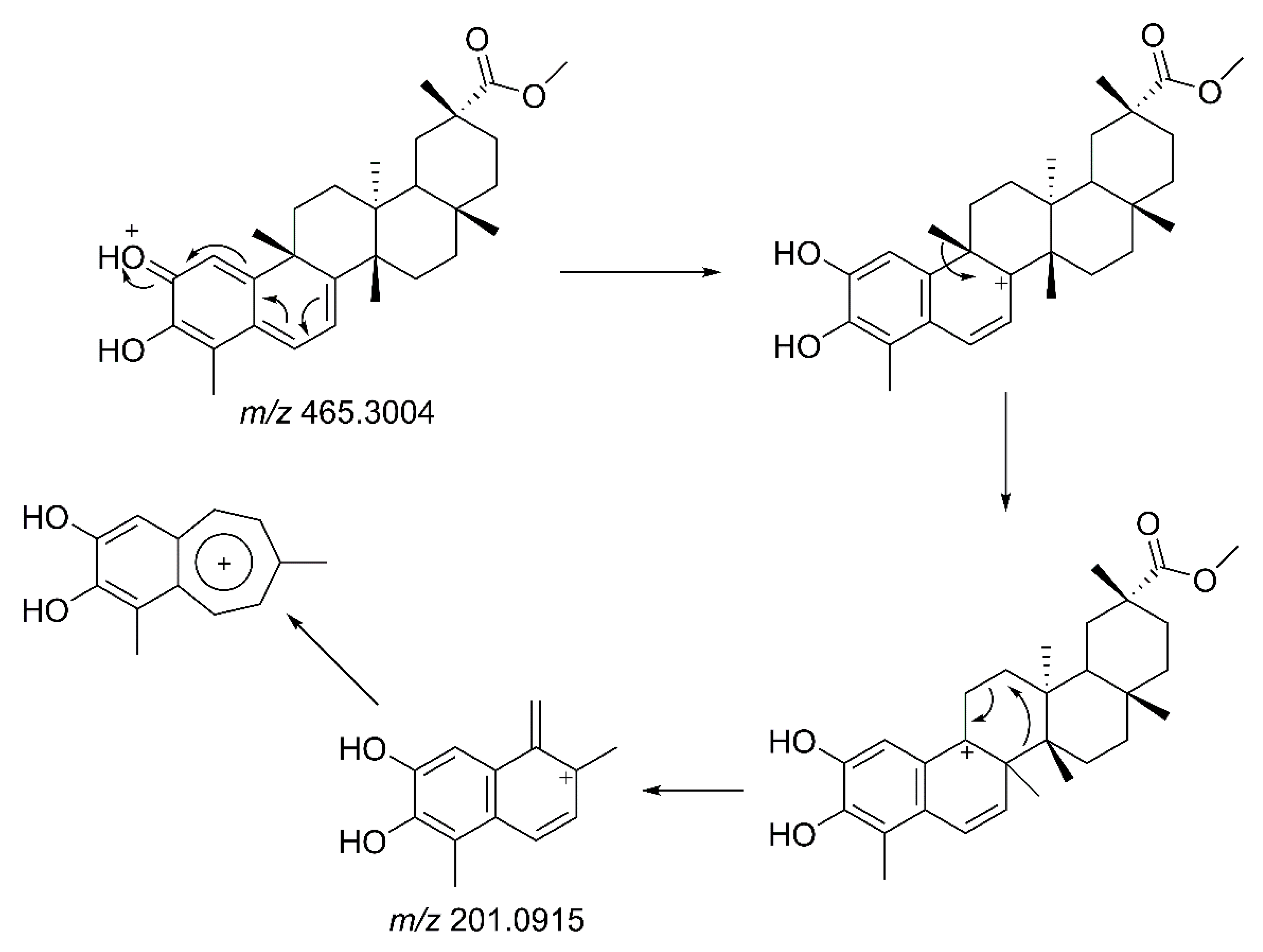
2.4. Identification of Isolated Compounds from Salacia crassifolia
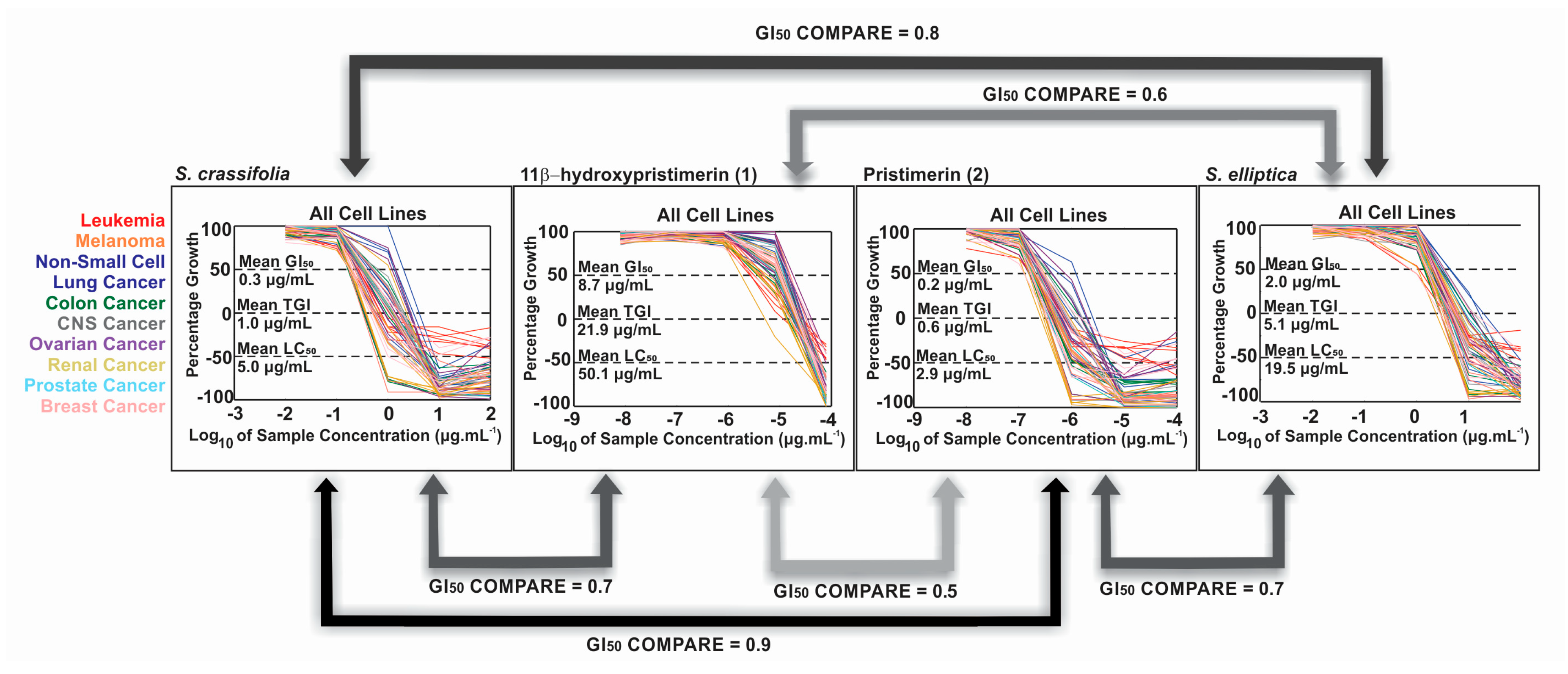
2.5. Biological Activity of Isolated Compounds
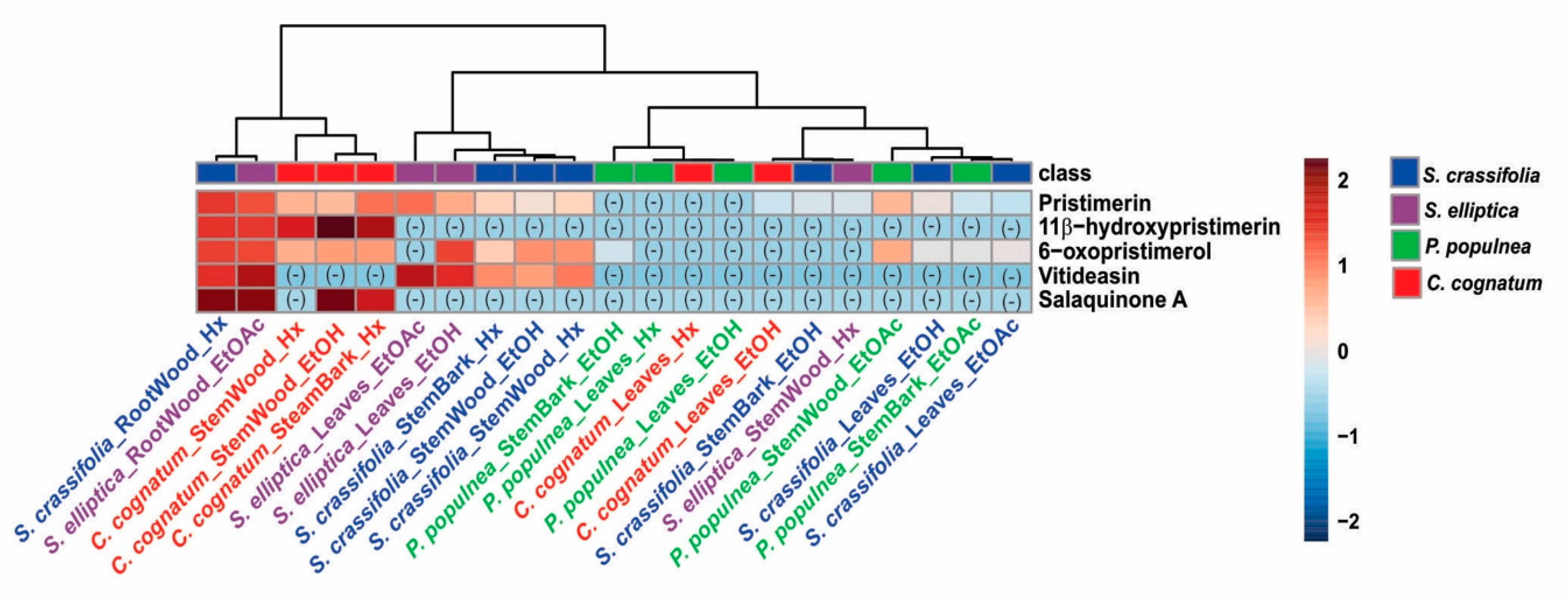
2.6. LC-MS/MS Analysis of Extracts of Species from the Celastraceae Family
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Extracts
3.3. Antiproliferative Bioassay
3.4. Bioassay-Guided Isolation of Compounds
3.5. LC-MS/MS Analysis of Isolated Compounds
3.6. LC-MS/MS Analysis of Extracts of Species from the Celastraceae Family: Targeted Search for Pristimerin and Derivatives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New horizons for old drugs and drug leads. J. Nat. Prod. 2014, 77, 703–723. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, M.L.; de Paula, J.E.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V.; Grougnet, R.; Michel, S.; Tillequin, F.; Espindola, L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethnopharmacol 2009, 123, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Attuch, I.M. Conhecimentos tradicionais do Cerrado: Sobre a memória de Dona Flor, raizeira e parteira. Master Thesis, Universidade de Brasília, Brasília, Brazil, 2006. [Google Scholar]
- Rodrigues, V.G.; Duarte, L.P.; Silva, R.R.; Silva, G.D.F.; Mercadante-Simoes, M.O.; Takahashi, J.A.; Matildes, B.L.G.; Fonseca, T.H.S.; Gomes, M.A.; Vieira, S.A. Salacia crassifolia (Celastraceae): Chemical constituents and antimicrobial activity. Quim. Nova 2015, 38, 237–242. [Google Scholar] [CrossRef]
- Shan, W.G.; Zhang, L.W.; Xiang, J.G.; Zhan, Z.J. Natural friedelanes. Chem. Biodivers. 2013, 10, 1392–1434. [Google Scholar] [CrossRef] [PubMed]
- Corsino, J.; de Carvalho, P.R.; Kato, M.J.; Latorre, L.R.; Oliveira, O.M.; Araujo, A.R.; Bolzani, V.D.; Franca, S.C.; Pereira, A.M.; Furlan, M. Biosynthesis of friedelane and quinonemethide triterpenoids is compartmentalized in Maytenus aquifolium and Salacia campestris. Phytochemistry 2000, 55, 741–748. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nature Reviews. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves de Lima, O.; Coelho, J.S.B.; Maciel, G.M.; Heringer, E.P.; Gonçalves de Lima, C. Substancias antimicrobianas de plantas superiores-Comunicação XXXIX-Identificação de pristimerina como um componente ativo do “Bacupari” do Araguaia, Salacia crassifolia (Mart.) G. Don. (Hippocrateaceae). Rev. Inst. Antibiót. (Recife) 1972, 12, 19–24. [Google Scholar]
- Carvalho, P.R.; Silva, D.H.; Bolzani, V.S.; Furlan, M. Antioxidant quinonemethide triterpenes from Salacia campestris. Chem. Biodivers. 2005, 2, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves de Lima, O.; Coelho, J.S.B.; Weigert, E.; D’Albuquerque, I.L.; Lima, D.A.; Souza, M.A.M. Substancias antimicrobianas de plantas superiores-Comunicação XXXVI-Sobre a presença de maitenina e pristimerina na parte cortical das raízes de Maytenus ilicifolia, procedente do Brasil Meridional. Rev. Inst. Antibiót. (Recife) 1971, 11, 35–38. [Google Scholar]
- Gunatilaka, A.A.L.; Chandrasiri, H.; Kikuchi, T.; Tezuka, Y. 1H and 13C NMR analysis of three Quinone-Methide Triterpenoids. Magn. Reson. Chem. 1989, 27, 803–811. [Google Scholar] [CrossRef]
- Shirota, O.; Morita, H.; Takeya, K.; Itokawa, H. Cytotoxic aromatic triterpenes from Maytenus ilicifolia and Maytenus chuchuhuasca. J. Nat. Prod. 1994, 57, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.T.; Rios-Luci, C.; Padron, J.M.; Palermo, J.A. Antiproliferative terpenoids and alkaloids from the roots of Maytenus vitis-idaea and Maytenus spinosa. Phytochemistry 2010, 71, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Goncalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 14–10. [Google Scholar] [CrossRef] [PubMed]
- Dirsch, V.M.; Kiemer, A.K.; Wagner, H.; Vollmar, A.M. The triterpenoid quinonemethide pristimerin inhibits induction of inducible nitric oxide synthase in murine macrophages. Eur. J. Pharmacol. 1997, 336, 211–217. [Google Scholar] [CrossRef]
- Yousef, B.A.; Hassan, H.M.; Zhang, L.Y.; Jiang, Z.Z. Anticancer potential and molecular targets of pristimerin: A mini-review. Curr. Cancer Drug Targets 2017, 17, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Nanjundaiah, S.M.; Venkatesha, S.H.; Astry, B.; Yu, H.; Moudgil, K.D. Pristimerin, a naturally occurring triterpenoid, protects against autoimmune arthritis by modulating the cellular and soluble immune mediators of inflammation and tissue damage. Clin. Immunol. 2014, 155, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Nossack, A.C.; Celeghini, R.M.D.; Lancas, F.M.; Yariwake, J.H. HPLC-UV and LC-MS analysis of quinonemethides triterpenes in hydroalcoholic extracts of “espinheira santa” (Maytenus aquifolium Martius, Celastraceae) leaves. J. Braz. Chem. Soc. 2004, 15, 582–586. [Google Scholar] [CrossRef]
- Carneiro, C.C.; Silva, C.R.; Menezes, A.C.; Pérez, C.N.; Chen-Chen, L. Assessment of genotoxic, cytotoxic, and protective effects of Salacia crassifolia (Mart. Ex. Schult.) G. Don. stem bark fractions in mice. Genet. Mol. Res. 2013, 12, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Ransom, T.T.; Sigmund, J.; Tran, T.; Cichewicz, R.H.; Goetz, M.; Beutler, J.A. Growth inhibition of colon cancer and melanoma cells by versiol derivatives from a Paraconiothyrium species. J. Nat. Prod. 2017, 80, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espindola, L.S.; Dusi, R.G.; Demarque, D.P.; Braz-Filho, R.; Yan, P.; Bokesch, H.R.; Gustafson, K.R.; Beutler, J.A. Cytotoxic Triterpenes from Salacia crassifolia and Metabolite Profiling of Celastraceae Species. Molecules 2018, 23, 1494. https://doi.org/10.3390/molecules23061494
Espindola LS, Dusi RG, Demarque DP, Braz-Filho R, Yan P, Bokesch HR, Gustafson KR, Beutler JA. Cytotoxic Triterpenes from Salacia crassifolia and Metabolite Profiling of Celastraceae Species. Molecules. 2018; 23(6):1494. https://doi.org/10.3390/molecules23061494
Chicago/Turabian StyleEspindola, Laila S., Renata G. Dusi, Daniel P. Demarque, Raimundo Braz-Filho, Pengcheng Yan, Heidi R. Bokesch, Kirk R. Gustafson, and John A. Beutler. 2018. "Cytotoxic Triterpenes from Salacia crassifolia and Metabolite Profiling of Celastraceae Species" Molecules 23, no. 6: 1494. https://doi.org/10.3390/molecules23061494
APA StyleEspindola, L. S., Dusi, R. G., Demarque, D. P., Braz-Filho, R., Yan, P., Bokesch, H. R., Gustafson, K. R., & Beutler, J. A. (2018). Cytotoxic Triterpenes from Salacia crassifolia and Metabolite Profiling of Celastraceae Species. Molecules, 23(6), 1494. https://doi.org/10.3390/molecules23061494