Novel Thiazolidinone/Thiazolo[3,2-a]Benzimidazolone-Isatin Conjugates as Apoptotic Anti-proliferative Agents Towards Breast Cancer: One-Pot Synthesis and In Vitro Biological Evaluation
Abstract
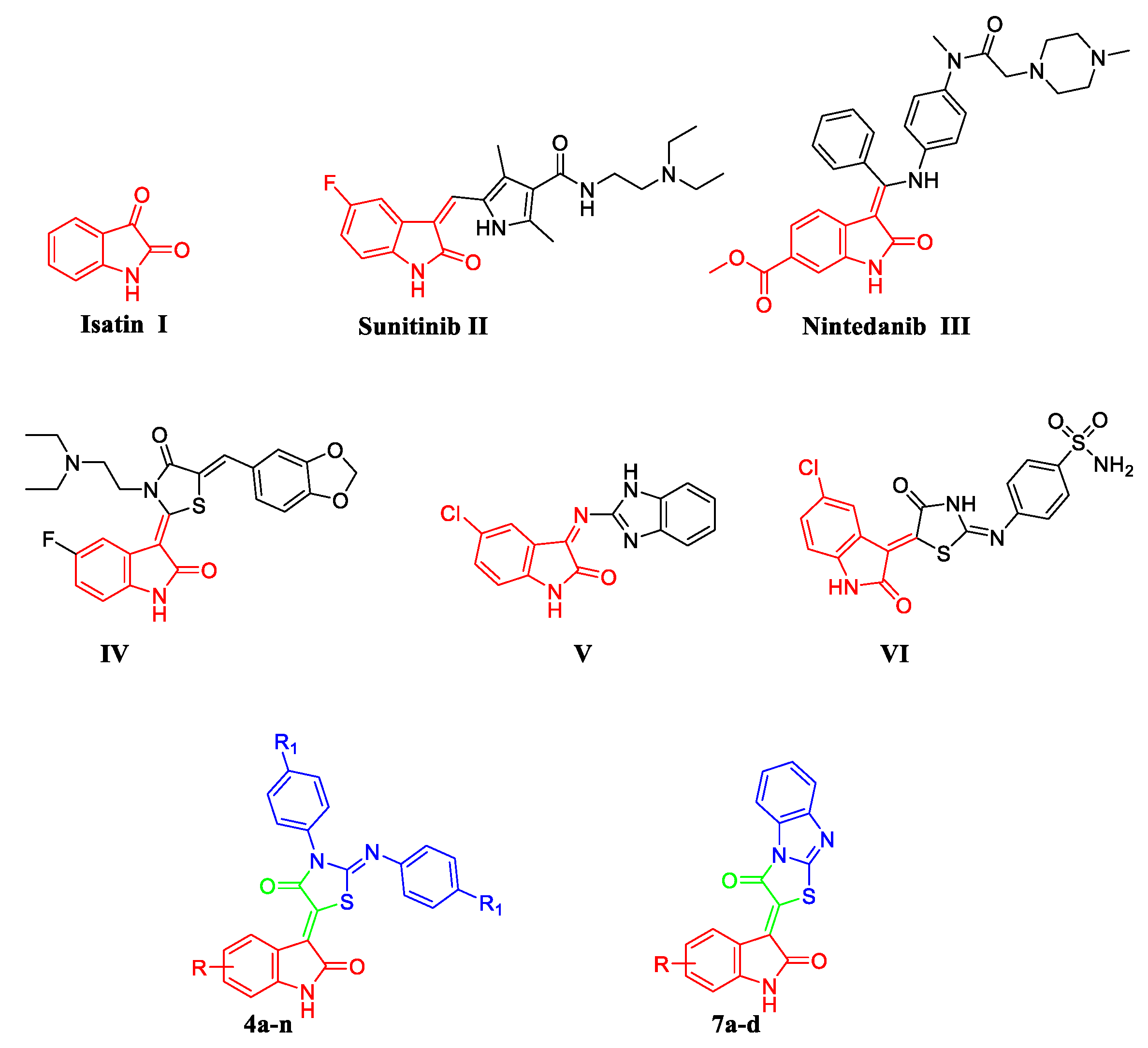
:1. Introduction
2. Results
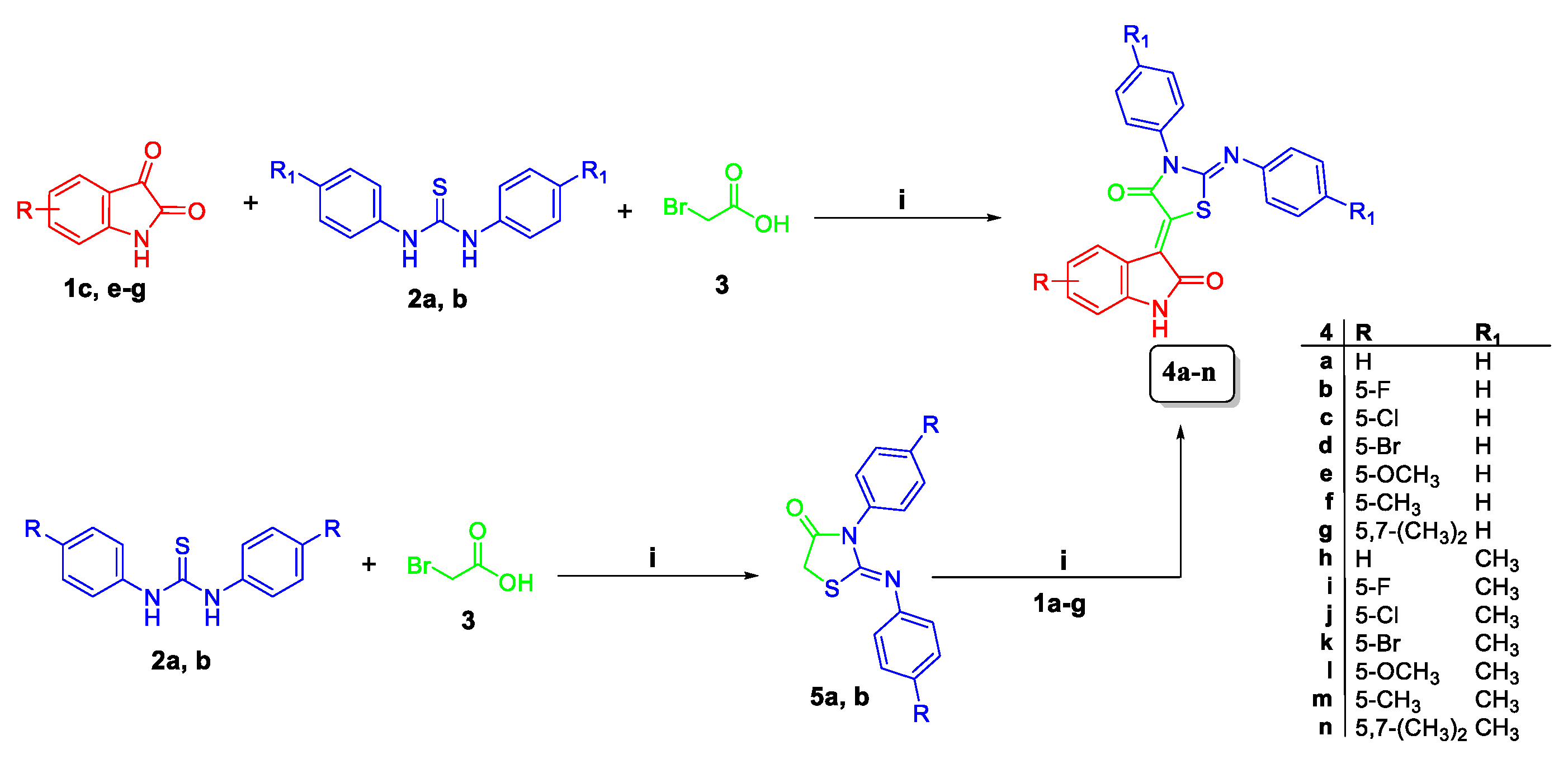
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Antiproliferative Activity
2.2.2. In Vitro Cytotoxic Activity towards Nontumorigenic Human WI-38 and MCF-10A Cells
2.2.3. Apoptosis Induction in TNBC MDA-MB-231 Cells
Effects on Mitochondrial Apoptosis Pathway Proteins Bcl-2 and Bax
Effects on the Levels of Active Caspase-3 (Key Executor of Apoptosis)
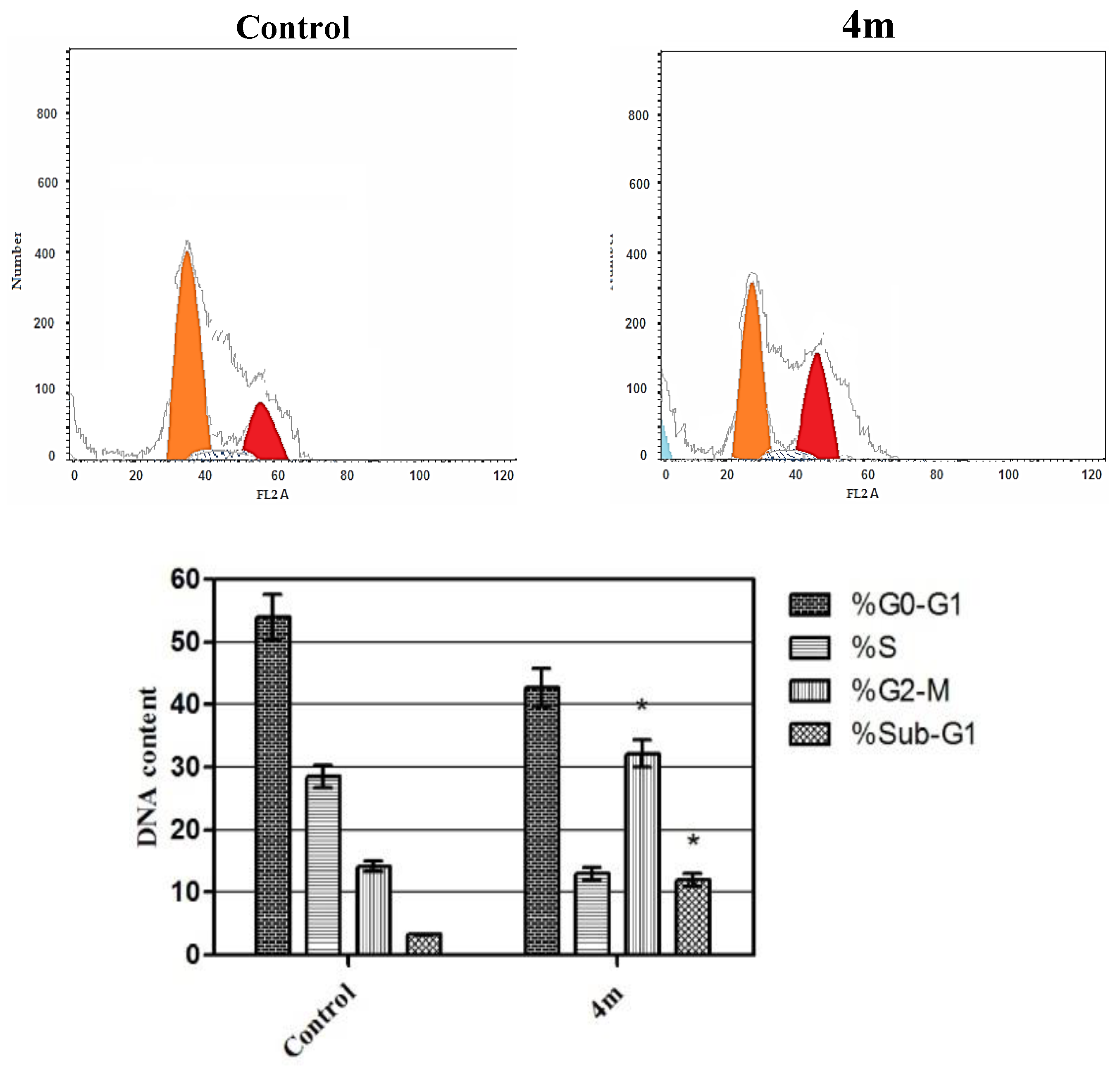
Cell Cycle Analysis
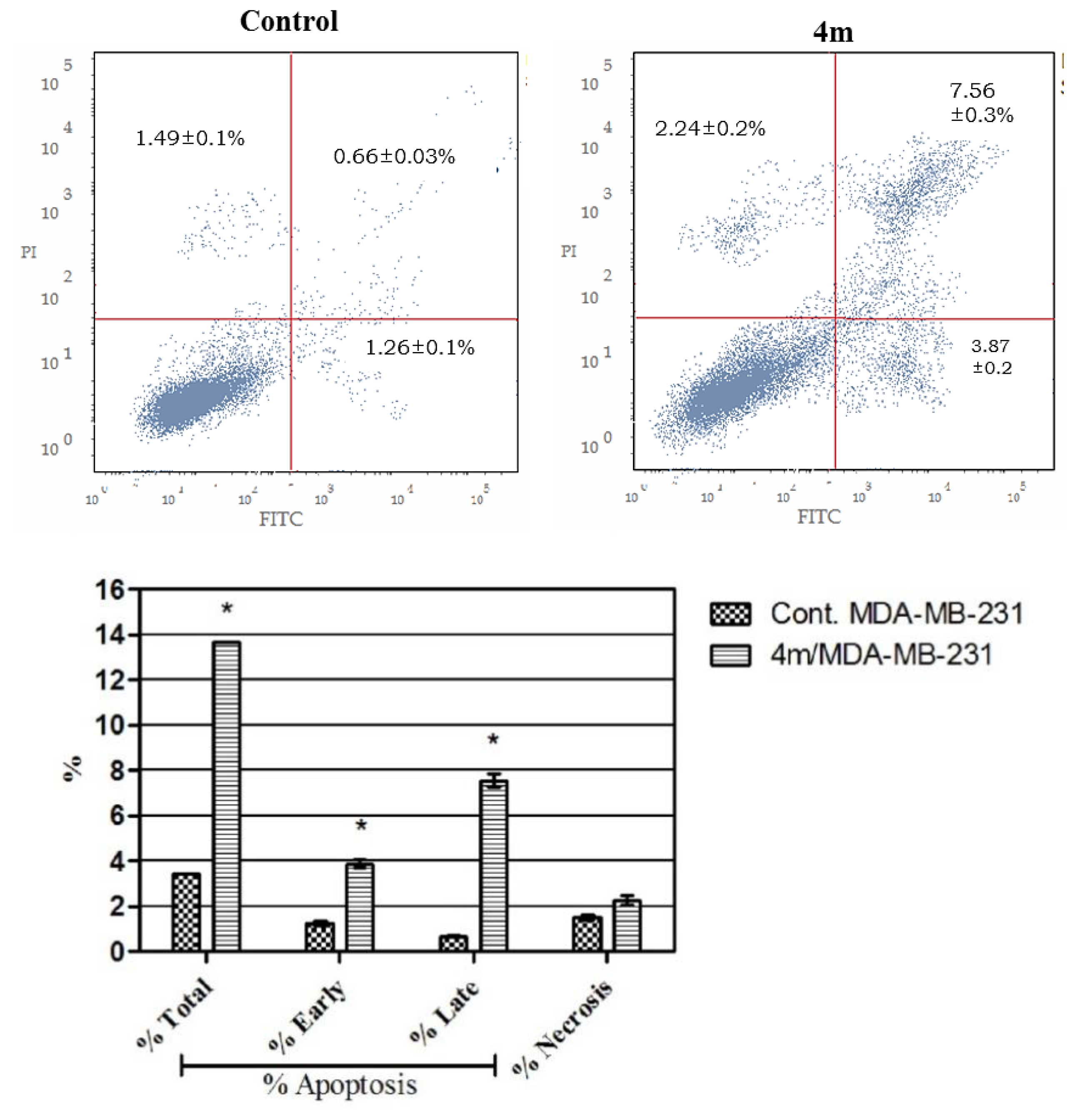
Annexin V-FITC Apoptosis Assay
2.3. In Silico ADME Profiling
3. Conclusions
4. Experimental
4.1. Chemistry
4.1.1. General
4.1.2. General Procedure for the Preparation of Target Compounds 4a–n
4.1.3. General Procedure for the Preparation of Target Compounds 7a–d
4.2. Biological Evaluation
4.2.1. Antiproliferative Activity Against Breast Cancer Cell Lines
4.2.2. In Vitro Cytotoxic Activity towards Nontumorigenic Human WI-38 and MCF-10A Cells
4.2.3. ELISA Immunoassay
4.2.4. Cell Cycle Analysis
4.2.5. Annexin V-FITC Apoptosis Assay
4.2.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. IGlobocan: Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012; IARC: Lyon, France, 2014. [Google Scholar]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Adami, H.; Hunter, D.; Trichopoulos, D. Textbook of Cancer Epidemiology; Oxford University Press: New York, NY, USA, 2002; pp. 301–373. [Google Scholar]
- Barnard, M.E.; Boeke, C.E.; Tamimi, R.M. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim. Biophys. Acta 2015, 1856, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Davis, S.R.; Pumphrey, J.G.; Bao, J.; Nau, M.M.; Meltzer, P.S.; Lipkowitz, S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res. Treat. 2009, 113, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.A.; Eldehna, W.M.; Ghabbour, H.; Al-Ansary, G.H.; Assaf, A.M.; Al-Dhfyan, A. Synthesis, crystal study, and antiproliferative activity of some 2-benzimidazolylthioacetophenones towards triple-negative breast cancer MDA-MB-468 cells as apoptosis-inducing agents. Int. J. Mol. Sci. 2016, 17, 1221. [Google Scholar] [CrossRef] [PubMed]
- Smid, M.; Wang, Y.; Zhang, Y.; Sieuwerts, A.M.; Yu, J.; Klijn, J.G.; Foekens, J.A.; Martens, J.W. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008, 68, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Ershov, P.; Ivanov, A. Isatin, an endogenous nonpeptide biofactor: A review of its molecular targets, mechanisms of actions, and their biomedical implications. BioFactors 2018, 44, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Jahan, A.; Mishra, N.K. Isatins: A diversity orientated biological profile. Med. Chem. 2014, 4, 451–468. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Al-Ansary, G.H.; Bua, S.; Nocentini, A.; Gratteri, P.; Altoukhy, A.; Ghabbour, H.; Ahmed, H.Y.; Supuran, C.T. Novel indolin-2-onebased sulfonamides as carbonic anhydrase inhibitors: Synthesis, in vitro biological evaluation against carbonic anhydrases isoforms I, II, IV and VII and molecular docking studies. Eur. J. Med. Chem. 2017, 127, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Fares, M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A. Design, synthesis and antitubercular activity of certain nicotinic Acid hydrazides. Molecules 2015, 20, 8800–8815. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.K.; Ramesh, A. Synthesis and pharmacological activities of hydrazones, Schiff and Mannich bases of isatin derivatives. Biol. Pharm. Bull. 2001, 24, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Skropeta, D. Recent highlights in the development of isatin-based anticancer agents. Adv. Anticancer Agents Med. Chem. 2013, 2, 254–312. [Google Scholar]
- Goodman, V.L.; Rock, E.P.; Dagher, R.; Ramchandani, R.P.; Abraham, S.; Gobburu, J.V.; Booth, B.P.; Verbois, S.L.; Morse, D.E.; Liang, C.Y.; et al. Approval summary: Sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin. Cancer Res. 2007, 13, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Nintedanib: First global approval. Drugs 2015, 75, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, L. Nintedanib in advanced NSCLC: Management of adverse events. Lung Cancer Manag. 2016, 5, 29–41. [Google Scholar] [CrossRef]
- Nagarsenkar, A.; Guntuku, L.; Guggilapu, S.D.; Gannoju, S.; Naidu, V.G.M.; Bathini, N.B. Synthesis and apoptosis inducing studies of triazole linked 3-benzylidene isatin derivatives. Eur. J. Med. Chem. 2016, 124, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Solomon, V.R.; Lee, H.; Trivedi, P. Design, synthesis and biological evaluation of some isatin-linked chalcones as novel anti-breast cancer agents: A molecular hybridization approach. Biomed. Prev. Nutr. 2013, 3, 325–330. [Google Scholar] [CrossRef]
- Ke, S.; Shi, L.; Yang, Z. Discovery of novel isatin–dehydroepiandrosterone conjugates as potential anticancer agents. Bioorg. Med. Chem. Lett. 2015, 25, 4628–4631. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Hu, C.; Lee, H. Hybrid pharmacophore design and synthesis of isatin–benzothiazole analogs for their anti-breast cancer activity. Bioorg. Med. Chem. 2009, 17, 7585–7592. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.A.; Elsaman, T.; Al-Dhfyan, A.; Attia, M.I.; Al-Rashood, K.A.; Al-Obaid, A.M. Synthesis and Anticancer Potential of Certain Novel 2-Oxo-N′-(2-oxoindolin-3-ylidene)-2H-chromene-3-carbohydrazides. Eur. J. Med. Chem. 2013, 70, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, R.; Anreddy, R.N.R.; Manda, S. Synthesis, anticancer and antioxidant activities of some novel N-(benzo[d]oxazol-2-yl)-2-(7- or 5-substituted-2-oxoindolin-3-ylidene) hydrazinecarboxamide derivatives. J. Enzyme Inhib. Med. Chem. 2011, 26, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Fadl, T.; Kadi, A.; Abdel-Aziz, H.A. Novel N,N′-Hydrazino-bis-isatin Derivatives with Selective Activity Against Multidrug-Resistant Cancer Cells. U.S. Patent 20120252860, 4 October 2012. [Google Scholar]
- Havrylyuk, D.; Kovach, N.; Zimenkovsky, B.; Vasylenko, O.; Lesyk, R. Synthesis and Anticancer Activity of Isatin-Based Pyrazolines and Thiazolidines Conjugates. Arch. Pharm. Chem. Life Sci. 2011, 344, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Ramshid, P.K.; Jagadeeshan, S.; Krishnan, A.; Mathew, M.; Nair, S.A.; Pillai, M.R. Synthesis and in vitro Evaluation of Some Isatin-Thiazolidinone Hybrid Analogues as Anti-Proliferative Agents. Med. Chem. 2010, 6, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, D.; Khyluk, D.; Vasylenko, O.; Zaprutko, L.; Lesky, R. A Facile Synthesis and Anticancer Activity Evaluation of Spiro[Thiazolidinone-Isatin] Conjugates. Sci. Pharm. 2011, 79, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Gzella, A.; Lesyk, R. Synthesis of New 4-Thiazolidinone-, Pyrazoline-, and Isatin-Based Conjugates with Promising Antitumor Activity. J. Med. Chem. 2012, 55, 8630–8641. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, Y.; Zhang, G.; Lv, Y.; Zhang, N.; Gong, P. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Eur. J. Med. Chem. 2011, 46, 3509–3518. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Khalil, N.A.; Ahmed, E.M. Synthesis of Novel Isatin-Thiazoline and Isatin-Benzimidazole Conjugates as Anti-Breast Cancer Agents. Arch. Pharm. Res. 2011, 34, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Almahli, H.; Al-Ansary, G.H.; Ghabbour, H.A.; Aly, M.H.; Ismael, O.E.; Al-Dhfyan, A.; Abdel-Aziz, H.A. Synthesis and in vitro antiproliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J. Enz. Inhib. Med. Chem. 2017, 32, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.A.; Eldehna, W.M.; Keeton, A.B.; Piazza, G.A.; Kadi, A.A.; Attwa, M.W.; Abdelhameed, A.S.; Attia, M.I. Isatin-benzoazine molecular hybrids as potential antiproliferative agents: Synthesis and in vitro pharmacological profiling. Drug Des. Dev. Ther. 2017, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Fares, M.; Ibrahim, H.S.; Alsherbiny, M.A.; Aly, M.H.; Ghabbour, H.A.; Abdel-Aziz, H.A. Synthesis and cytotoxic activity of biphenylurea derivatives containing indolin-2-one moieties. Molecules 2016, 21, 762. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; EL-Naggar, D.H.; Hamed, A.R.; Ibrahim, H.S.; Ghabbour, H.A.; Abdel-Aziz, H.A. One-pot three-component synthesis of novel spirooxindoles with potential cytotoxic activity against triple-negative breast cancer MDA-MB-231 cells. J. Enz. Inhib. Med. Chem. 2018, 33, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Abo-Ashour, M.F.; Ibrahim, H.S.; Al-Ansary, G.H.; Ghabbour, H.A.; Elaasser, M.M.; Ahmed, H.Y.; Safwat, N.A. Novel [(3-indolylmethylene)hydrazono]indolin-2-ones as apoptotic antiproliferativeagents: Design, synthesis and in vitro biological evaluation. J. Enz. Inhib. Med. Chem. 2018, 33, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.I.; Eldehna, W.M.; Afifi, S.A.; Keeton, A.B.; Piazza, G.A.; Abdel-Aziz, H.A. New hydrazonoindolin-2-ones: Synthesis, exploration of the possible antiproliferative mechanism of action and encapsulation into PLGA microspheres. PLoS ONE 2017, 12, e0181241. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Altoukhy, A.; Mahrous, H.; Abdel-Aziz, H.A. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential antiproliferative agents. Eur. J. Med. Chem. 2015, 90, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.A.; Ghabbour, H.A.; Eldehna, W.M.; Qabeel, M.M.; Fun, H.-K. Synthesis, crystal structure and biological activity of cis/trans amide rotomers of (Z)-N′-(2-oxoindolin-3-ylidene)formohydrazide. J. Chem. 2014, 2014, 760434. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Fares, M.; Ceruso, M.; Ghabbour, H.A.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; El Ella, D.A.A.; Supuran, C.T. Amido/ureidosubstituted benzenesulfonamides-isatin conjugates as low nanomolar/subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform XII. Eur. J. Med. Chem. 2016, 110, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldehna, W.M.; Fares, M.; Ibrahim, H.S.; Aly, M.H.; Zada, S.; Ali, M.M.; Abou-Seri, S.M.; Abdel-Aziz, H.A.; El Ella, D.A.A. Indoline ureas as potential anti-hepatocellular carcinoma agents targeting VEGFR-2: Synthesis, in vitro biological evaluation and molecular docking. Eur. J. Med. Chem. 2015, 100, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Abo-Ashour, M.F.; Nocentini, A.; Gratteri, P.; Eissa, I.H.; Fares, M.; Ismael, O.E.; Ghabbour, H.A.; Elaasser, M.M.; Abdel-Aziz, H.A.; et al. Novel 4/3-((4-oxo-5-(2-oxoindolin-3-ylidene) thiazolidin-2-ylidene) amino) benzenesulfonamides: Synthesis, carbonic anhydrase inhibitory activity, anticancer activity and molecular modelling studies. Eur. J. Med. Chem. 2017, 139, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Al-Wabli, R.I.; Almutairi, M.S.; Keeton, A.B.; Piazza, G.A.; Abdel-Aziz, H.A.; Attia, M.I. Synthesis and biological evaluation of certain hydrazonoindolin-2-one derivatives as new potent antiproliferative agents. J. Enz. Inhib. Med. Chem. 2018, 33, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.; Grabow, S.; Strasser, A.; Vaux, D.L. Thirty years of BCL-2: Translating cell death discoveries into novel cancer therapies. Nat. Rev. Cancer 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Kavanagh, J.J. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003, 4, 721–729. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S.W.G. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, P.J.; Su, D.; Feng, J.; Ma, S.L. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol. Med. Rep. 2014, 9, 2265–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Ibrahim, H.S.; Abdel-Aziz, H.A.; Farrag, N.N.; Youssef, M.M. Design, synthesis and in vitro antitumor activity of novel N-substituted-4-phenyl/benzylphthalazin-1-ones. Eur. J. Med. Chem. 2015, 89, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Accelrys Co. Ltd. Discovery Studio 4.0. Available online: http://www.accelrys.com/ (accessed on 1 June 2018).
- Fares, M.; Said, M.A.; Alsherbiny, M.A.; Eladwy, R.A.; Almahli, H.; Abdel-Aziz, M.M.; Ghabbour, H.A.; Eldehna, W.M.; Abdel-Aziz, H.A. Synthesis, biological evaluation and molecular docking of certain sulfones as potential nonazole antifungal agents. Molecules 2016, 21, 114. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zheng, M.; Wang, M.; Tong, J.; Ge, W.; Zhang, J.; Zheng, A.; Li, J.; Gao, L.; Li, J. Design and synthesis of new potent PTP1B inhibitors with the skeleton of 2-substituted imino-3-substituted-5-heteroarylidene-1, 3-thiazolidine-4-one: Part I. Eur. J. Med. Chem. 2016, 122, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, A.T.; Anichina, K.K.; Vuchev, D.I.; Tsenov, J.A.; Denkova, P.S.; Kondeva, M.S.; Micheva, M.K. Antihelminthic activity of some newly synthesized 5 (6)-(un) substituted-1H-benzimidazol-2-ylthioacetylpiperazine derivatives. Eur. J. Med. Chem. 2006, 41, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Wei, X.; Wu, X.; Chen, G.; Cao, G.; Shen, X.; Zhang, X.; Tang, Q.; Liang, G.; et al. Synthesis and biological evaluation of novel thiazolidinone derivatives as potential anti-inflammatory agents. Eur. J. Med. Chem. 2013, 64, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Soliman, D.H.; Eldehna, W.M.; Ghabbour, H.A.; Kabil, M.M.; Abdel-Aziz, M.M.; Abdel-Aziz, H.A.K. Novel 6-Phenylnicotinohydrazide Derivatives: Design, Synthesis and Biological Evaluation as a Novel Class of Antitubercular and Antimicrobial Agents. Biol. Pharm. Bull. 2017, 40, 1883–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds 4a–n and 7a–d are available from the authors. |




| Compound | R | R1 | IC50 (µM) a | |
|---|---|---|---|---|
| MDA-MB-231 | MCF-7 | |||
| 4a | H | H | NA b | NA b |
| 4b | 5-F | H | 108.3 ± 8.2 | 58.5 ± 3.2 |
| 4c | 5-Cl | H | 24.1 ± 2.1 | 29.2 ± 2.5 |
| 4d | 5-Br | H | 15.7 ± 0.9 | 17.1 ± 1.1 |
| 4e | 5-OCH3 | H | 42.6 ± 3.4 | 52.9 ± 5.0 |
| 4f | 5-CH3 | H | 60.9 ± 4.2 | 22.8 ± 2.0 |
| 4g | 5,7-(CH3)2 | H | 43.6 ± 3.1 | 47.4 ± 3.9 |
| 4h | H | CH3 | 51.7 ± 2.1 | 56.0 ± 3.7 |
| 4i | 5-F | CH3 | 152.1 ± 11.5 | 104.9 ± 7.5 |
| 4j | 5-Cl | CH3 | 50.8 ± 3.5 | NA b |
| 4k | 5-Br | CH3 | NA b | NA b |
| 4l | 5-OCH3 | CH3 | 47.1 ± 4.1 | 53.5 ± 3.6 |
| 4m | 5-CH3 | CH3 | 7.6 ± 0.5 | 8.4 ± 0.5 |
| 4n | 5,7-(CH3)2 | CH3 | 36.3 ± 2.9 | 23.7 ± 1.9 |
| 7a | 5-Cl | - | 47.2 ± 3.8 | 127.3 ± 9.9 |
| 7b | 5-OCH3 | - | 13.2 ± 1.7 | 21.7 ± 1.5 |
| 7c | 5-CH3 | - | 40.3 ± 2.5 | 48.2 ± 2.7 |
| 7d | 5,7-(CH3)2 | - | 23.5 ± 1.0 | 27.6 ± 2.4 |
| Dox. | 4.7 ± 0.4 | 3.8 ± 0.4 | ||
| Sunitinib | 5.5 ± 0.5 | 3.4 ± 0.3 | ||
| Compound | IC50 (µM) a | Selectivity Index | ||
|---|---|---|---|---|
| Lung WI-38 | Breast MCF-10A | MDA-MB-231 | ||
| 4c | 87.2 ± 5.2 | 112.6 ± 5.9 | 24.1 ± 2.1 | 4.7 |
| 4d | 65.5 ± 4.2 | 80.7 ± 3.4 | 15.7 ± 0.9 | 5.1 |
| 4e | 193.1 ± 10.6 | 89.6 ± 5.1 | 42.6 ± 3.4 | 2.1 |
| 4g | 120.0 ± 7.8 | 141.7 ± 7.9 | 43.6 ± 3.1 | 3.25 |
| 4l | 176.2 ± 11.3 | 157.4 ± 6.8 | 47.2 ± 4.1 | 3.3 |
| 4m | 49.1 ± 1.9 | 73.1 ± 2.7 | 7.6 ± 0.5 | 9.6 |
| 4n | 218.3 ± 13.5 | 154.0 ± 5.2 | 36.3 ± 2.9 | 4.2 |
| 7a | 353.0 ± 17.1 | 233.8 ± 9.6 | 47.2 ± 3.8 | 5.0 |
| 7b | 121.1 ± 6.9 | 183.9 ± 8.0 | 13.2 ± 1.1 | 13.9 |
| 7c | 192.0 ± 10.8 | 242.2 ± 11.5 | 40.3 ± 2.5 | 6.0 |
| 7d | 234.0 ± 15.4 | 250.3 ± 9.8 | 23.5 ± 1.00 | 10.7 |
| Compound | Bax (pg/mg of Total Protein) | Bcl-2 (ng/mg of Total Protein) | Bax/Bcl-2 | Caspase-3 (ng/mg of Total Protein) |
|---|---|---|---|---|
| 4m | 274.8 ± 13.5 (11.1) * | 2.1 ± 0.1 (0.33) * | 130.5 | 0.19 ± 0.01 (6.4) * |
| 7b | 180.0 ± 7.3 (7.3) * | 2.8 ± 0.1 (0.45) * | 64.1 | 0.15 ± 0.01 (4.9) * |
| Control | 24.7 ± 1.7 | 6.3 ± 0.2 | 3.9 | 0.03 ± 0.01 |
| Compound | AlogP98 a | PSA_2D b | Solubility c | Solubility Level d | Absorption Level e | CYP2D6 f |
|---|---|---|---|---|---|---|
| 4a | 4.476 | 62.087 | −6.305 | 1 | 0 | 0 |
| 4b | 4.682 | 62.087 | −6.605 | 1 | 0 | 0 |
| 4c | 5.141 | 62.087 | −7.005 | 1 | 0 | 0 |
| 4d | 5.225 | 62.087 | −7.079 | 1 | 0 | 0 |
| 4e | 4.46 | 71.017 | −6.292 | 1 | 0 | 0 |
| 4f | 4.962 | 62.087 | −6.765 | 1 | 0 | 0 |
| 4g | 5.449 | 62.087 | −7.226 | 1 | 0 | 0 |
| 4h | 5.449 | 62.087 | −7.197 | 1 | 0 | 0 |
| 4i | 5.654 | 62.087 | −7.484 | 1 | 1 | 0 |
| 4j | 6.113 | 62.087 | −7.885 | 1 | 1 | 0 |
| 4k | 6.197 | 62.087 | −7.958 | 1 | 1 | 0 |
| 4l | 5.432 | 71.017 | −7.159 | 1 | 0 | 0 |
| 4m | 5.935 | 62.087 | −7.645 | 1 | 1 | 0 |
| 4n | 6.421 | 62.087 | −8.093 | 0 | 1 | 0 |
| 7a | 3.581 | 64.021 | −6.005 | 1 | 0 | 0 |
| 7b | 2.9 | 72.951 | −5.327 | 2 | 0 | 0 |
| 7c | 3.403 | 64.021 | −5.771 | 2 | 0 | 0 |
| 7d | 3.889 | 64.021 | −6.263 | 1 | 0 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Naggar, M.; Eldehna, W.M.; Almahli, H.; Elgez, A.; Fares, M.; Elaasser, M.M.; Abdel-Aziz, H.A. Novel Thiazolidinone/Thiazolo[3,2-a]Benzimidazolone-Isatin Conjugates as Apoptotic Anti-proliferative Agents Towards Breast Cancer: One-Pot Synthesis and In Vitro Biological Evaluation. Molecules 2018, 23, 1420. https://doi.org/10.3390/molecules23061420
El-Naggar M, Eldehna WM, Almahli H, Elgez A, Fares M, Elaasser MM, Abdel-Aziz HA. Novel Thiazolidinone/Thiazolo[3,2-a]Benzimidazolone-Isatin Conjugates as Apoptotic Anti-proliferative Agents Towards Breast Cancer: One-Pot Synthesis and In Vitro Biological Evaluation. Molecules. 2018; 23(6):1420. https://doi.org/10.3390/molecules23061420
Chicago/Turabian StyleEl-Naggar, Mohamed, Wagdy M. Eldehna, Hadia Almahli, Amr Elgez, Mohamed Fares, Mahmoud M. Elaasser, and Hatem A. Abdel-Aziz. 2018. "Novel Thiazolidinone/Thiazolo[3,2-a]Benzimidazolone-Isatin Conjugates as Apoptotic Anti-proliferative Agents Towards Breast Cancer: One-Pot Synthesis and In Vitro Biological Evaluation" Molecules 23, no. 6: 1420. https://doi.org/10.3390/molecules23061420






