Synergy between Experimental and Theoretical Results of Some Reactions of Annelated 1,3-Azaphospholes
Abstract
:1. Introduction
2. Synthesis of Annelated 1,3-Azaphospholes
2.1. [4+1] Cyclocondensation Method
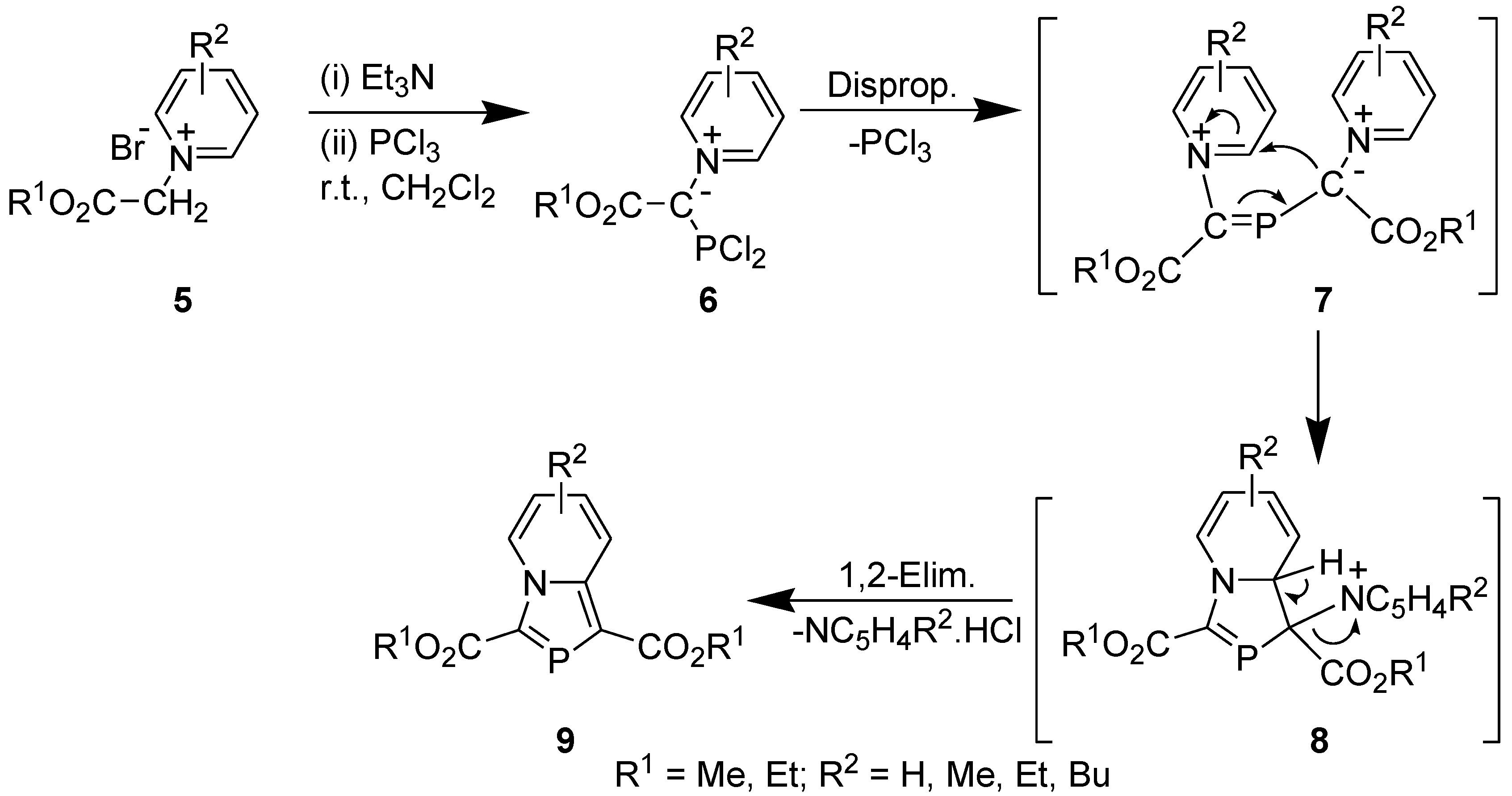
2.2. Synthesis Involving Tandem Pyridinium Ylide generation, Disproportionation, and 1,5-Electrocyclization
3. Reactions Response
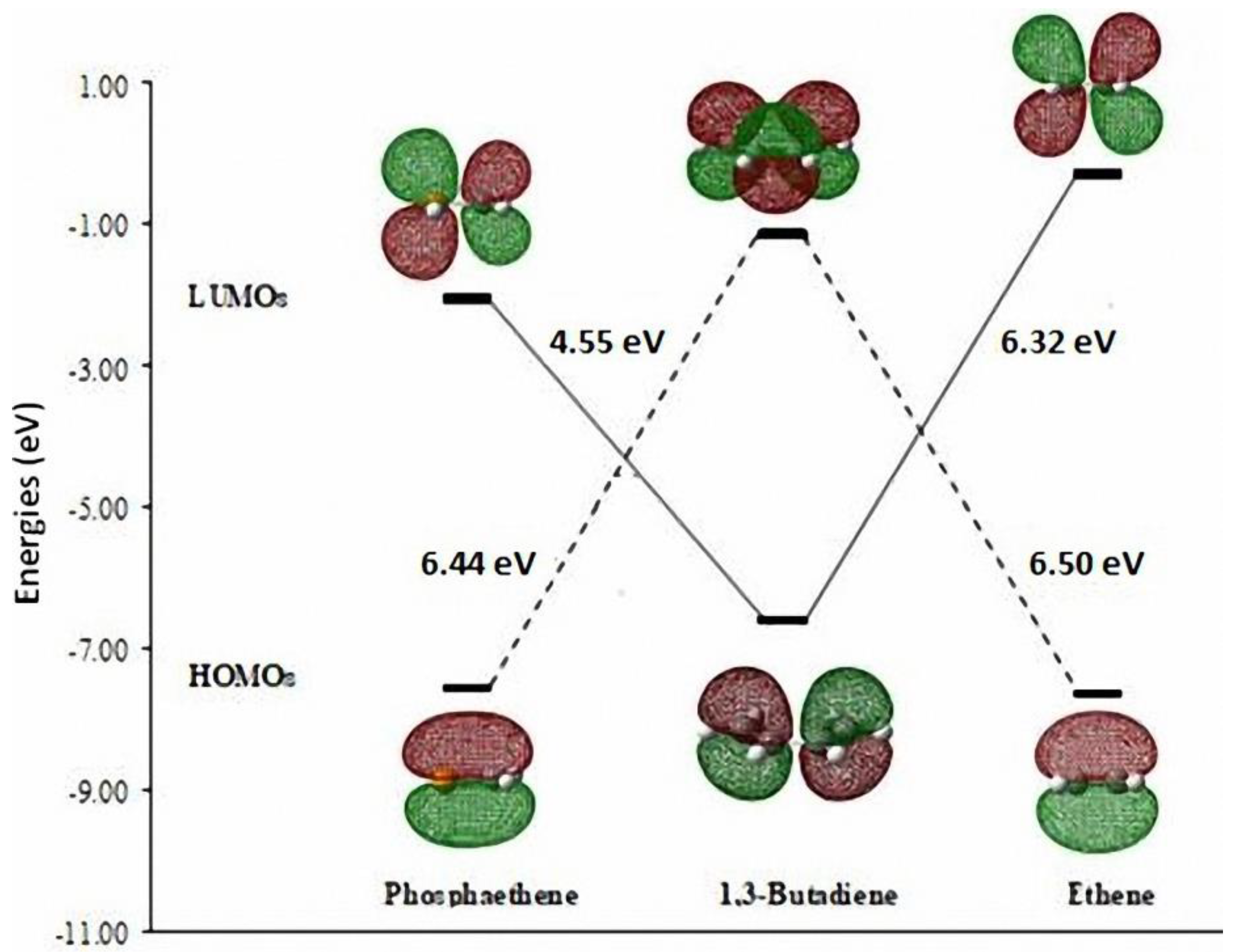
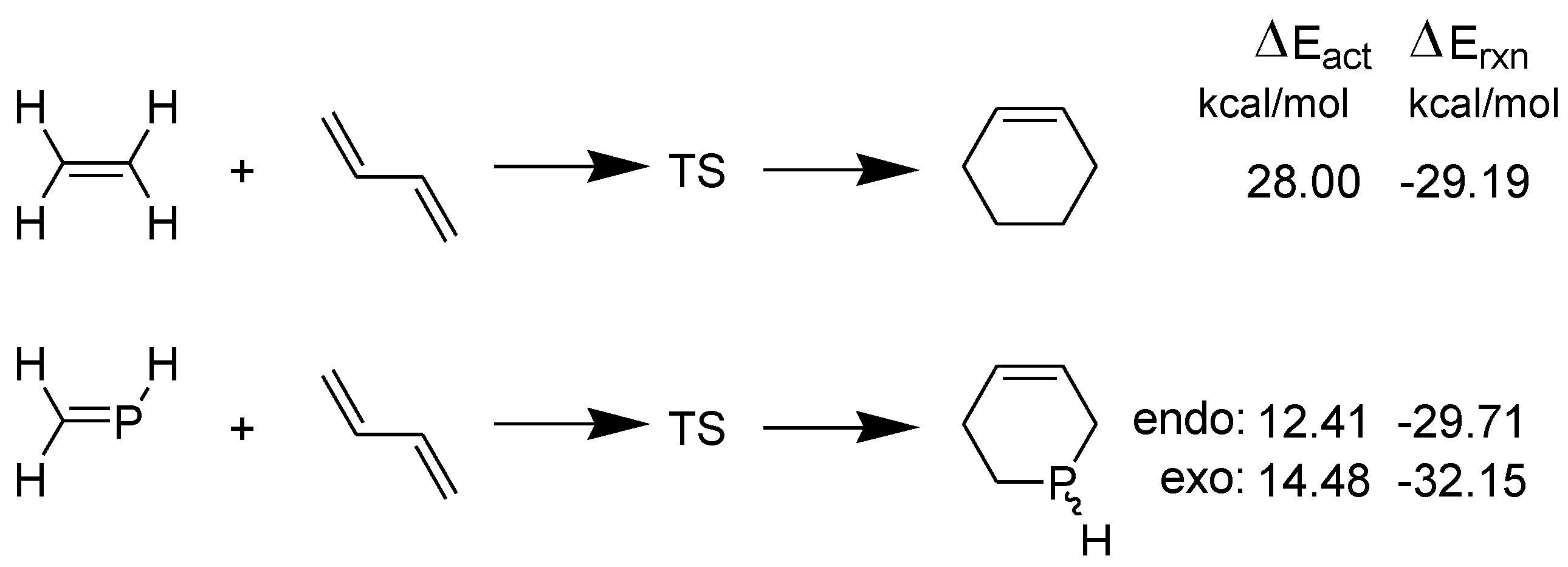
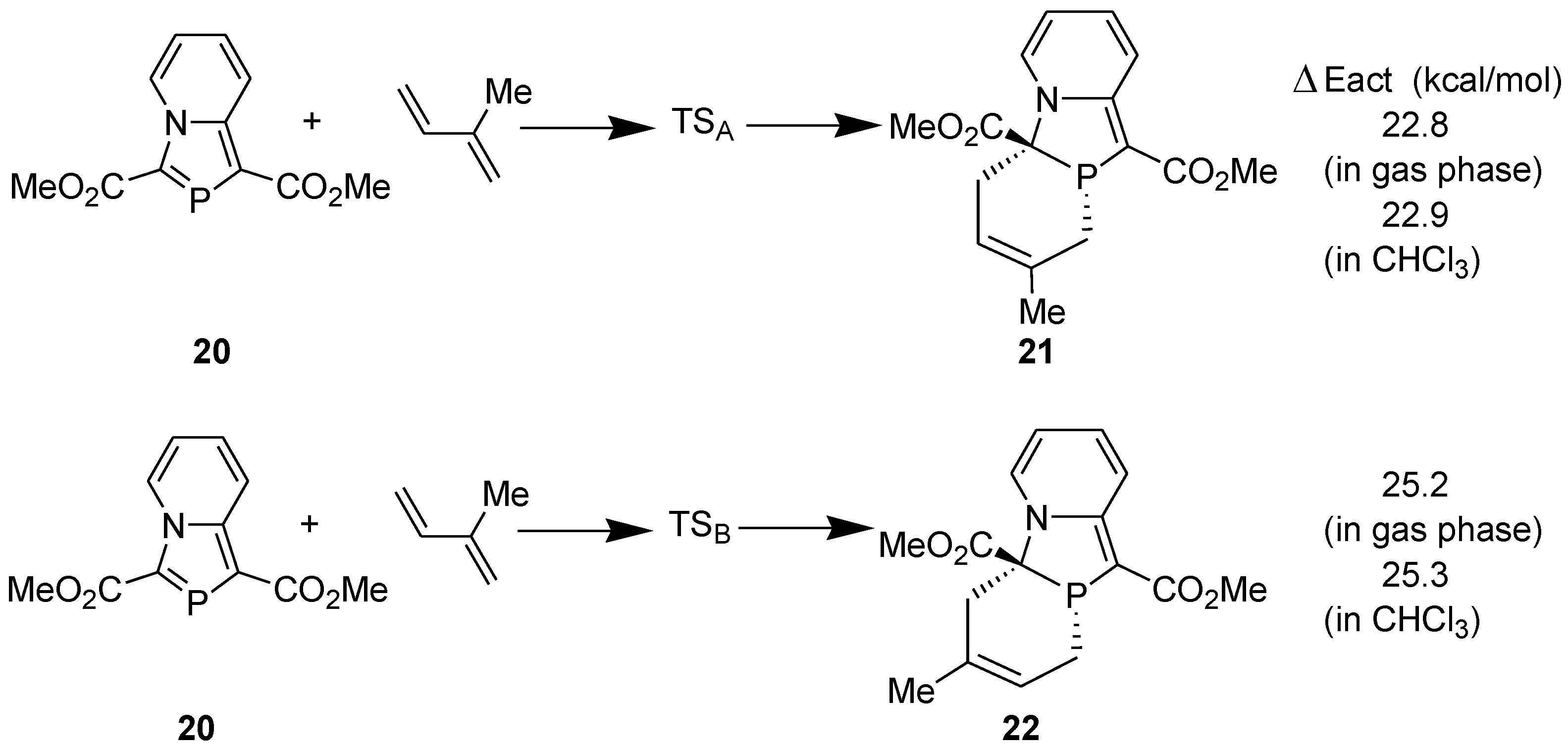
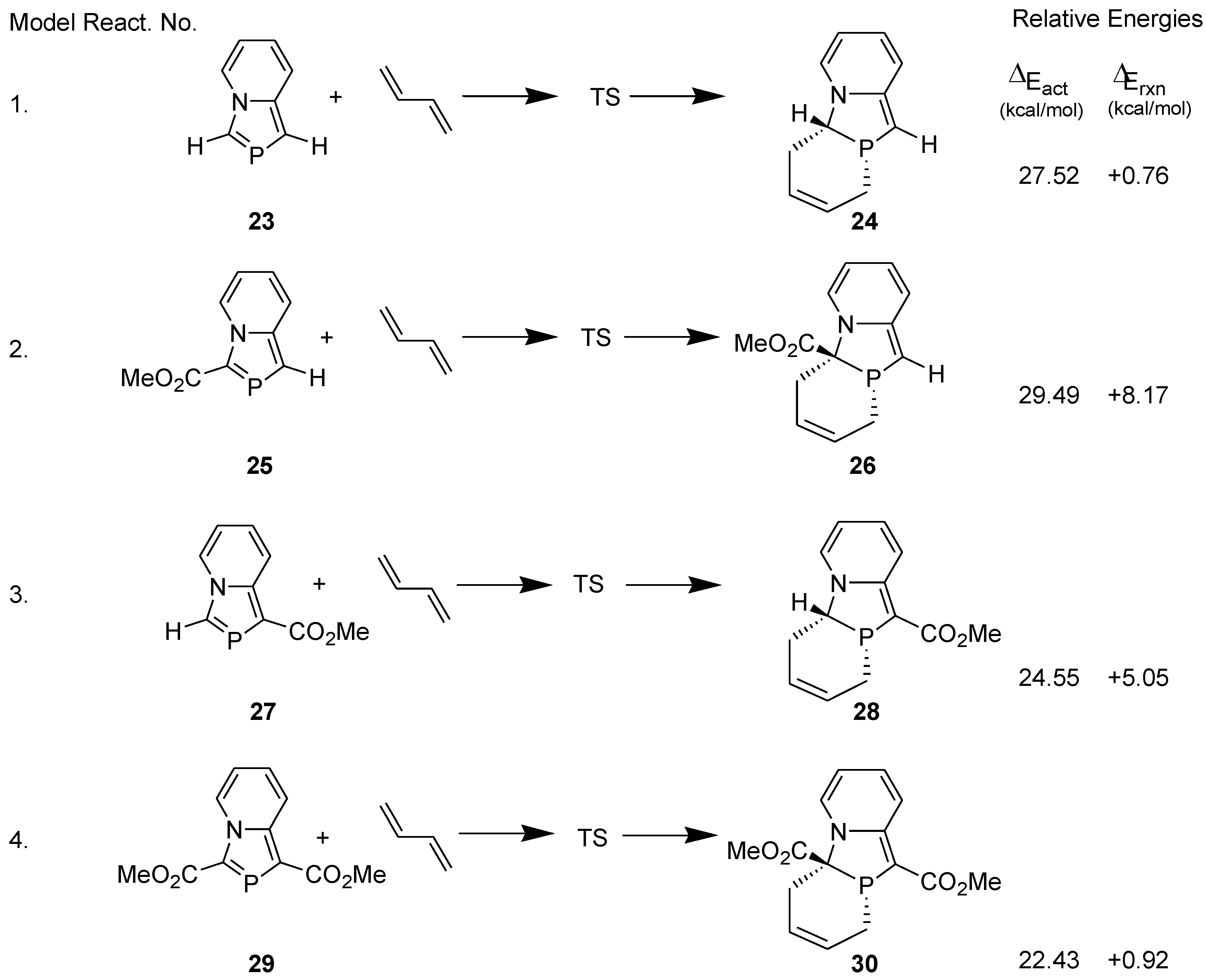
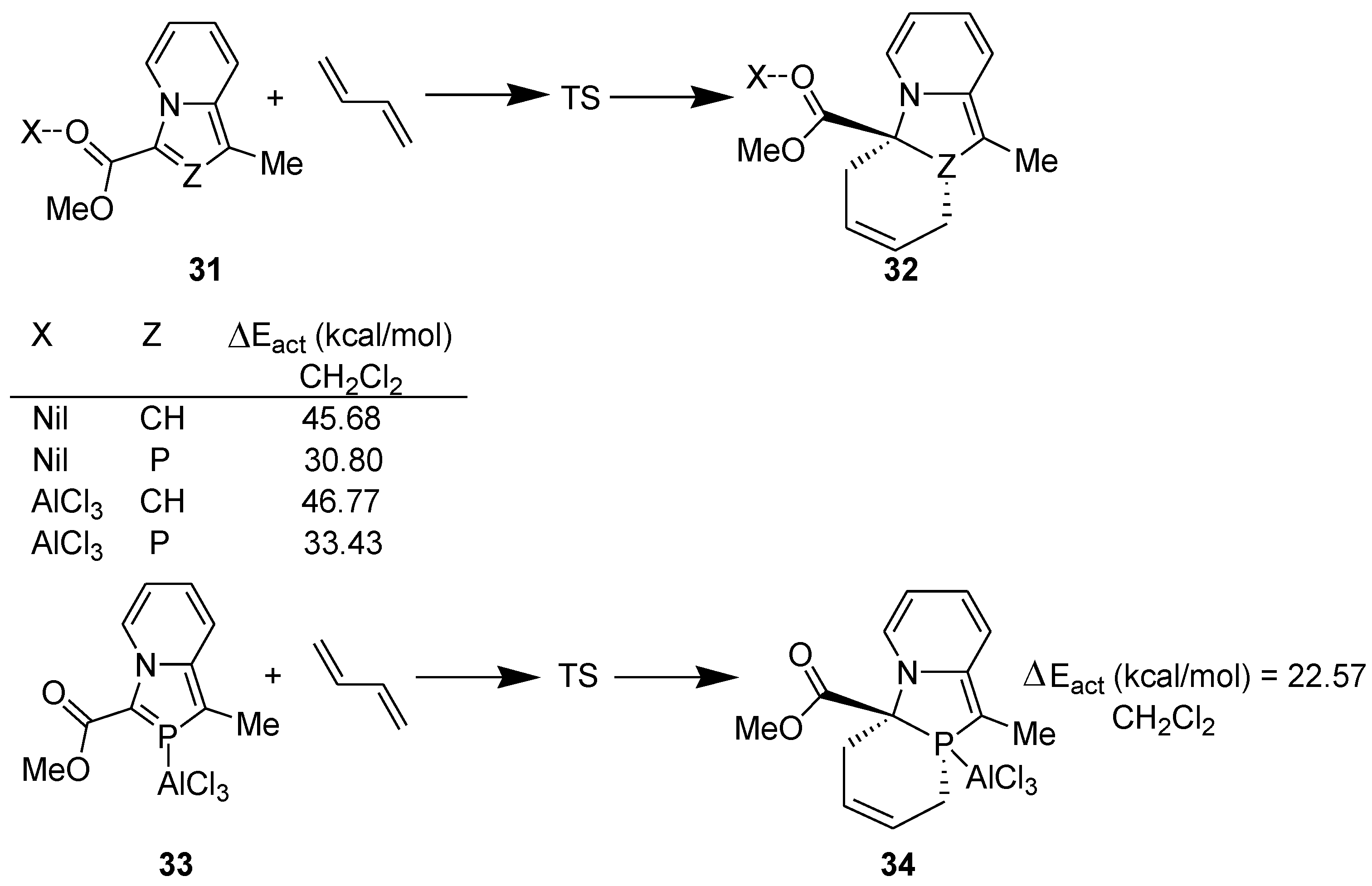
3.1. Diels-Alder Reaction
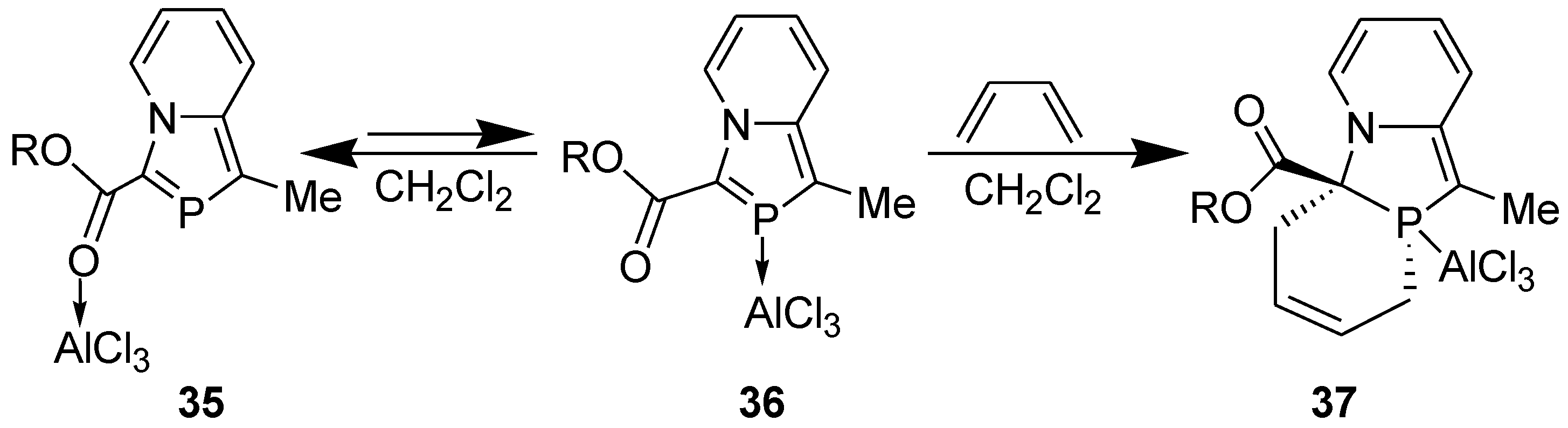
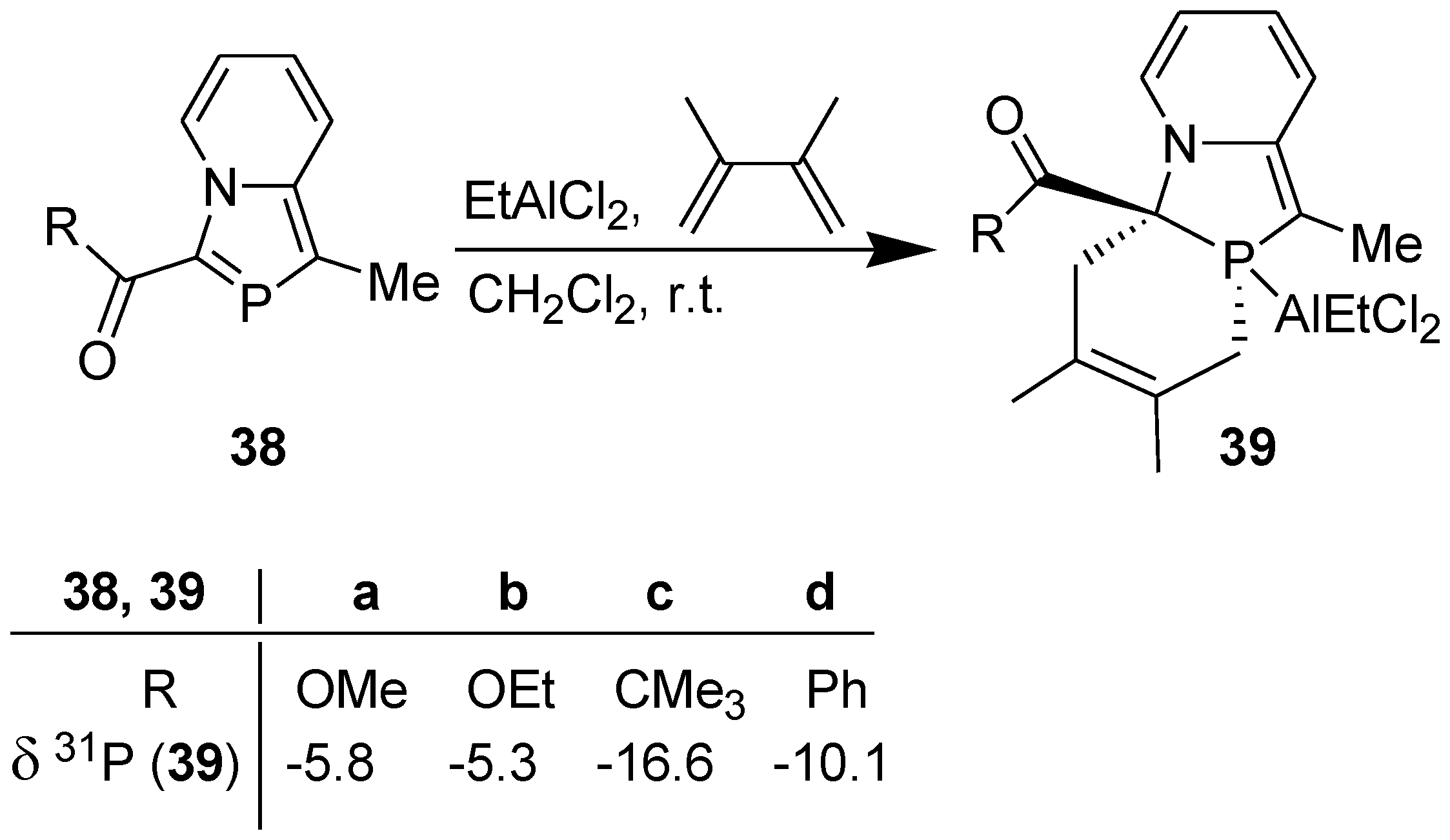
3.2. Lewis Acid Catalyzed Diels-Alder Reaction
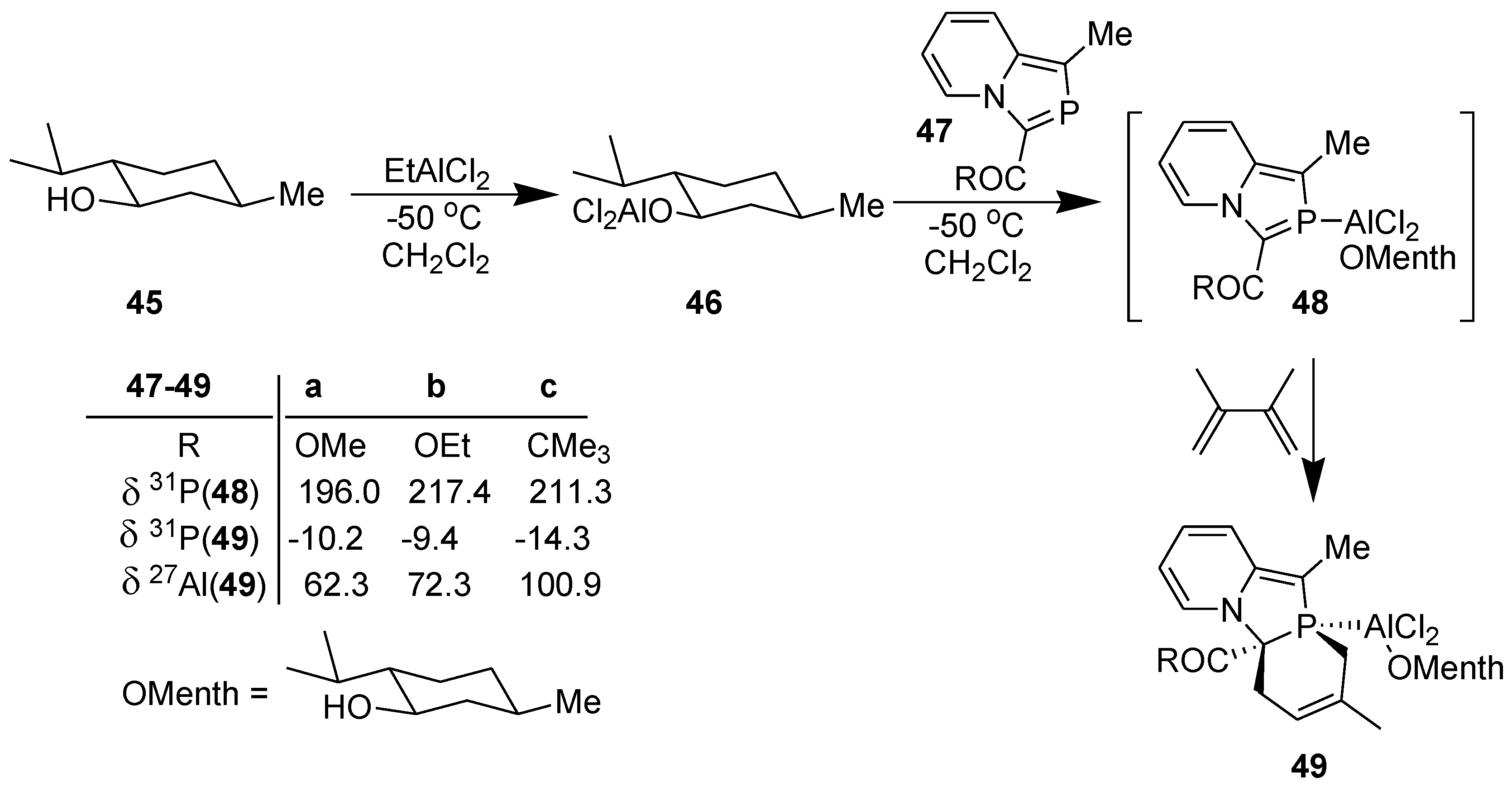
3.3. Asymmetric Diels-Alder Reaction with 2-Phosphaindolizines
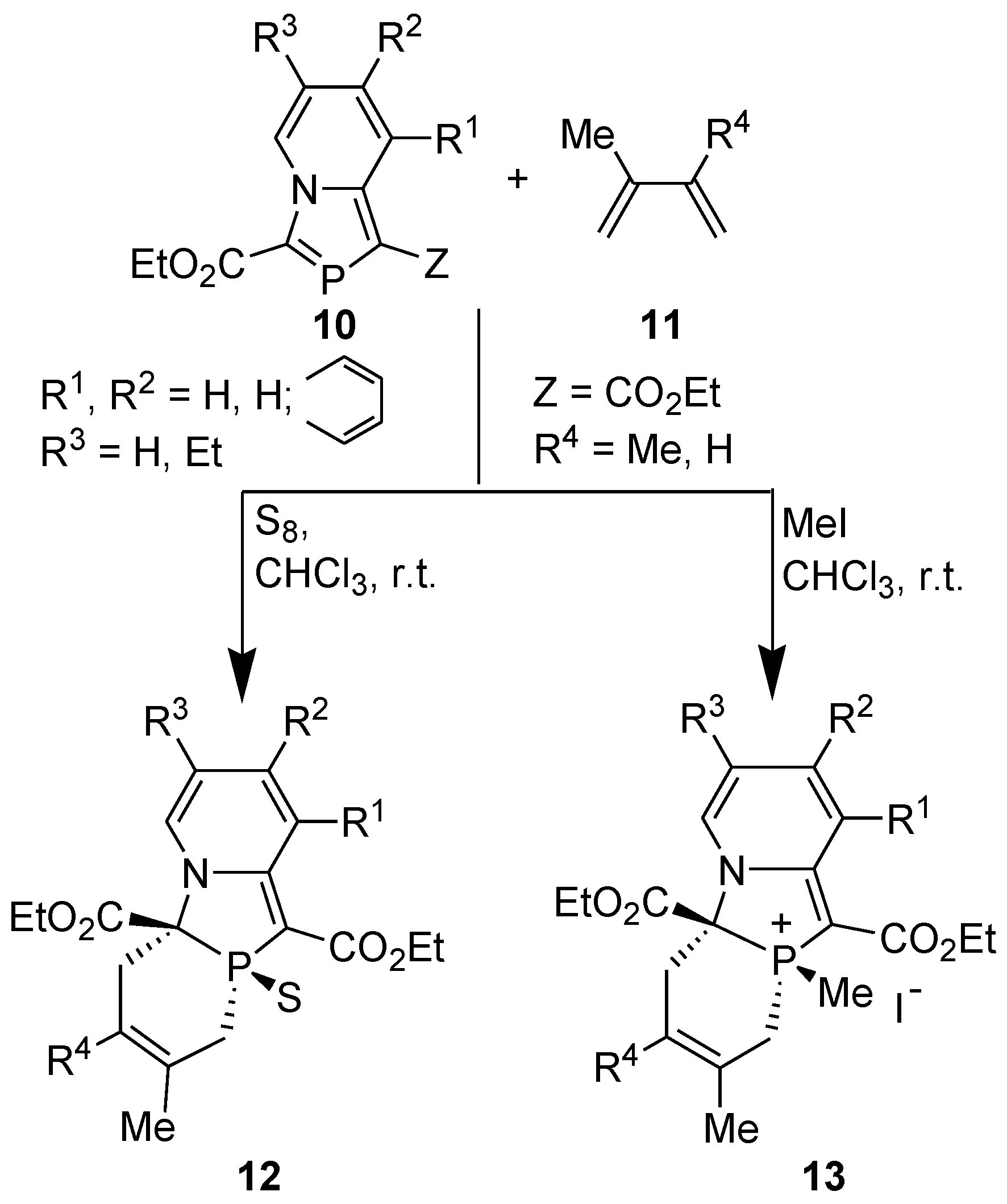
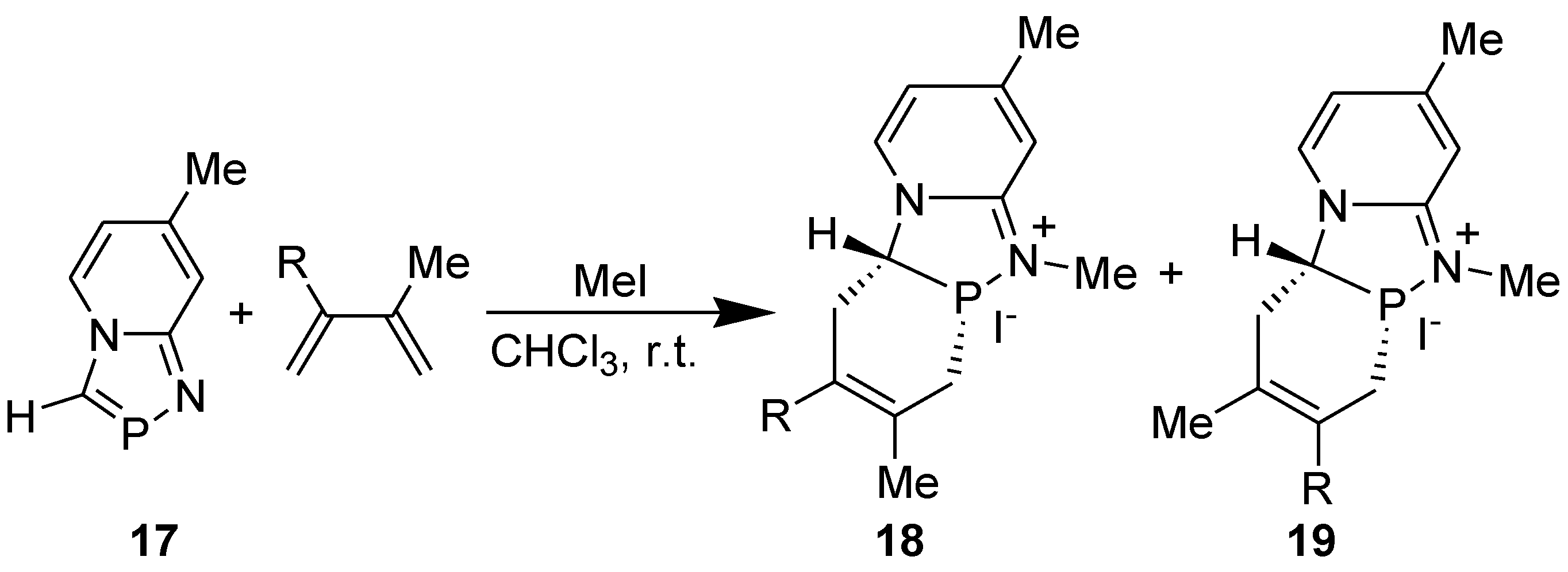
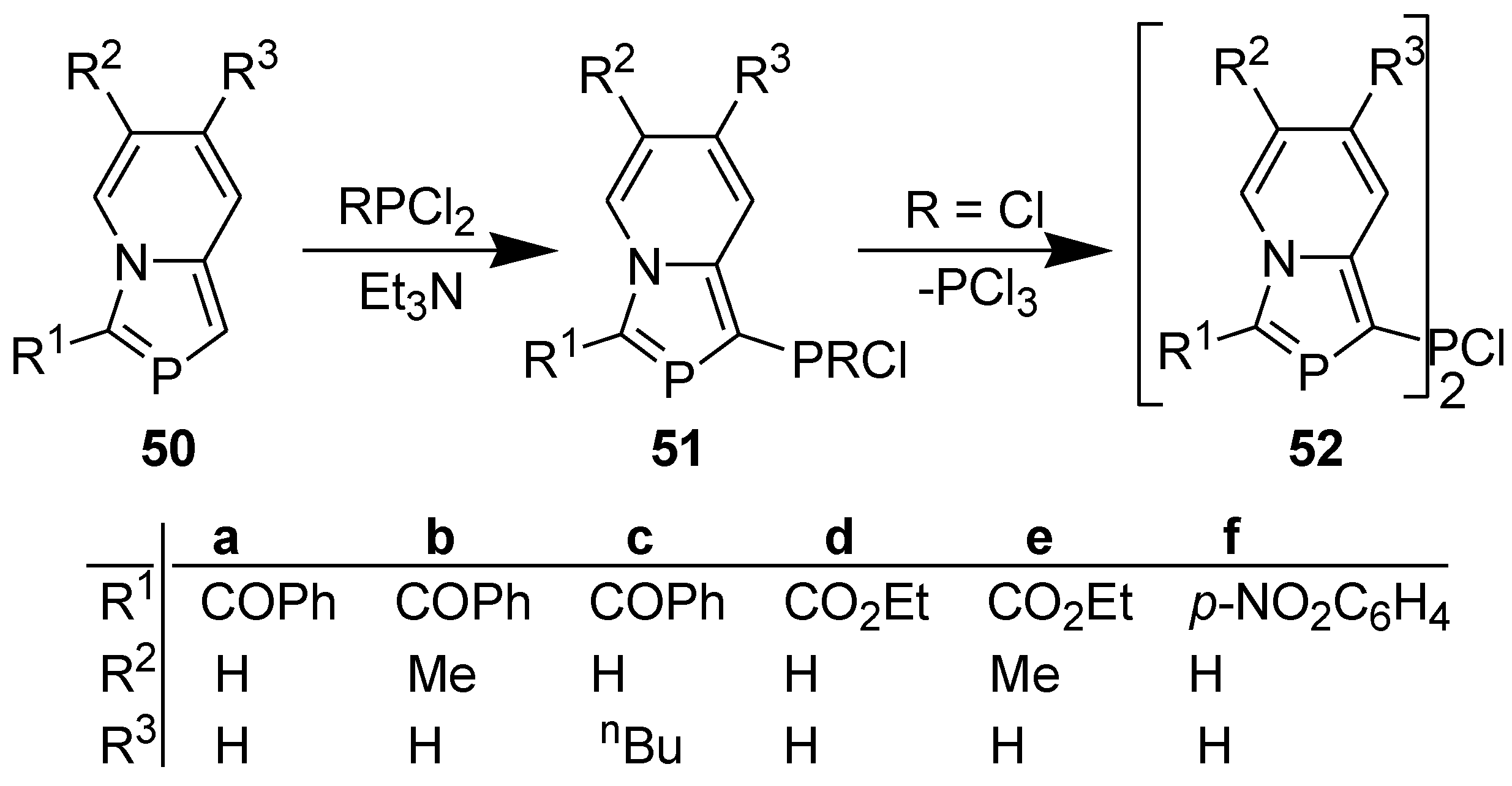
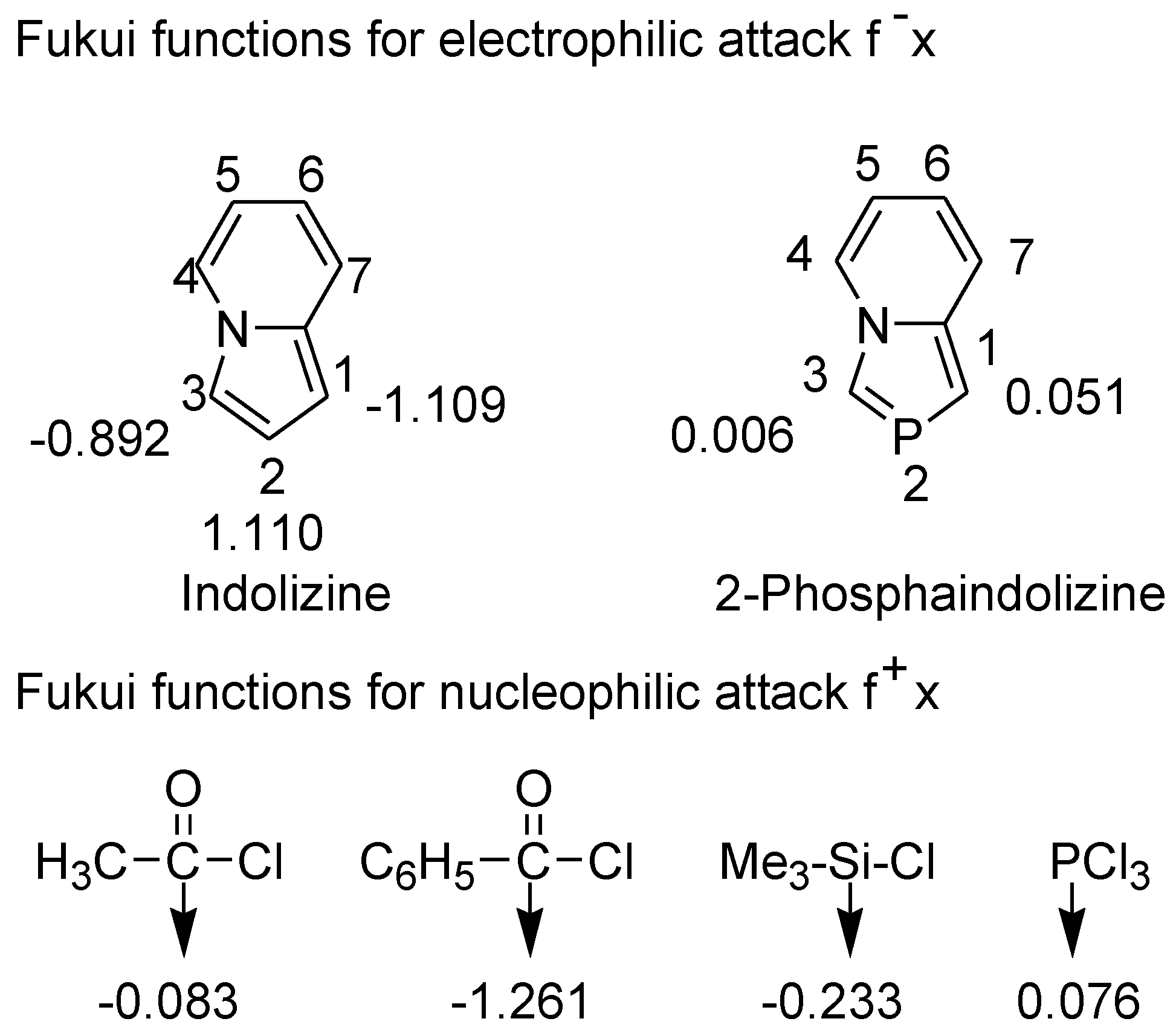
3.4. Electrophilic Substitution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dirac, P.A.M. Quantum mechanics of many-electrons systems. Proc. R. Soc. Lond. Ser. A. 1929, 123, 714–733. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogenous electron gas. Phys. Rev. 1964, 136, B867–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Zangwill, A. A half century of density functional theory. Phys. Today 2015, 68, 34–39. [Google Scholar] [CrossRef]
- Jain, A.; Shin, Y.; Persson, K.A. Computational predictions of energy materials using density functional theory. Nat. Rev. Mater. 2016, 1, 1–13. [Google Scholar] [CrossRef]
- Cohen, A.J.; Mori-Sánchez, P.; Yang, W. Challenges for density functional theory. Chem. Rev. 2012, 112, 289–320. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.O. Density functional theory: Its origins, rise to prominence, and future. Rev. Mod. Phys. 2015, 87, 897–923. [Google Scholar] [CrossRef]
- Tsipis, A.C. DFT flavor of coordination chemistry. Coord. Chem. Rev. 2014, 272, 1–29. [Google Scholar] [CrossRef]
- Cole, D.J.; Hine, N.D.M. Applications of large-scale density functional theory in biology. J. Phys. Condens. Matter 2016, 28, 393001. [Google Scholar] [CrossRef] [PubMed]
- Lupp, D.; Christensen, N.J.; Fristrup, P. Synergy between experimental and theoretical methods in the exploration of homogeneous transition metal catalysis. Dalton Trans. 2014, 43, 11093–11105. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Bligaard, T.; Rossmeisl, J.; Christensen, C.H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lineberger, W.C.; Borden, W.T. The synergy between qualitative theory, quantitative calculations, and direct experiments in understanding, calculating, and measuring the energy differences between the lowest singlet and triplet states of organic diradicals. Phys. Chem. Chem. Phys. 2011, 13, 11792–11813. [Google Scholar] [CrossRef] [PubMed]
- Sierka, M. Synergy between theory and experiment in structure resolution of low-dimensional oxides. Prog. Surf. Sci. 2010, 85, 398–434. [Google Scholar] [CrossRef]
- Mcgrady, J. Guest Editor The themed collection, “The synergy between theory and experiment. Dalton Trans. 2009, 5805–6064. [Google Scholar] [CrossRef]
- Bansal, R.K.; Gupta, N.; Karaghiosoff, K.; Schmidpeter, A.; Spindler, C. 2-Phosphaindolizines. Chem. Ber. 1991, 124, 475–480. [Google Scholar] [CrossRef]
- Bansal, R.K.; Kabra, V.; Gupta, N.; Karaghiosoff, K. Synthesis of some new 2-phosphaindolizines. Indian J. Chem. 1992, 31, 254–256. [Google Scholar]
- Gupta, N.; Jain, C.B.; Heinicke, J.; Bharatiya, N.; Bansal, R.K.; Jones, P.G. 2-Phosphaindolizines. Heteroat. Chem. 1998, 9, 333–339. [Google Scholar] [CrossRef]
- Karaghioshoff, K.; Bansal, R.K.; Gupta, N. 1,4,2-Diazaphospholo[4,5-a]pyridines. Z. Naturforsch. B 1992, 47b, 373–378. [Google Scholar]
- Bansal, R.K.; Pandey, G.; Karaghiosoff, K.; Schmidpeter, A. 1,2,3-Diazaphospholo[1,5-a]pyridines. Synthesis 1995, 173–175. [Google Scholar] [CrossRef]
- Bansal, R.K.; Gandhi, N.; Karaghiosoff, K.; Schmidpeter, A. Synthesis of [1,2,4,3]triazaphospholo[1,5-a]pyridines. Z. Naturforsch. 1995, 50b, 558–562. [Google Scholar] [CrossRef]
- Bansal, R.K.; Mahnot, R.; Sharma, D.C.; Karaghiosoff, K.; Schmidpeter, A. 1,3-Azaphospholo[5,1-b]thiazolines and benzothiazoles. Heteroat. Chem. 1992, 3, 351–357. [Google Scholar] [CrossRef]
- Bansal, R.K.; Jain, C.B.; Gupta, N.; Kabra, V.; Karaghiosoff, K.; Schmidpeter, A. Synthesis and properties of a 5,6-dihydro-1,3-azaphospholo[5,1-b]oxazole. Phosphorus Sulfur Silicon 1994, 86, 139–143. [Google Scholar] [CrossRef]
- Bansal, R.K.; Karaghiosoff, K.; Gandhi, N.; Schmidpeter, A. 2-Substituted cycloiminium salts in azaphosphole synthesis. Synthesis 1995, 361–369. [Google Scholar] [CrossRef]
- Bansal, R.K.; Surana, A.; Gupta, N. 2-Phosphaindolizines via 1,5-electrocyclization. Tetrahedron Lett. 1999, 40, 1565–1568. [Google Scholar] [CrossRef]
- Bansal, R.K.; Gupta, N.; Baweja, M.; Hemrajani, L.; Jain, V.K. Pyridinium dichlorophosphinomethylides. Heteroat. Chem. 2001, 12, 602–609. [Google Scholar] [CrossRef]
- Bansal, R.K.; Hemrajani, L.; Gupta, N. 1,3-Azaphospholo[5,1-a]isoquinolines. Heteroat. Chem. 1999, 10, 598–604. [Google Scholar] [CrossRef]
- Singh, D.; Sinha, P.; Gupta, N.; Bansal, R.K. Synthesis of 1,3-azaphospholo[1,5-f]phenanthridines through 1,5-electrocyclization. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 488–492. [Google Scholar] [CrossRef]
- Appel, R.; Knoll, F.; Ruppert, I. Phospha-alkenes and phospha-alkynes, genesis and properties of the (p-p)π-multiple bond. Angew. Chem. Int. Ed. 1981, 20, 731–744. [Google Scholar] [CrossRef]
- Mathey, F.; Mercier, F.; Charrier, C.; Fischer, J.; Mitschler, A. Dicoordinated 2H-phospholes as transient intermediates in the reactions of tervalent phospholes at high temperature. One-step synthesis of 1-phosphabornadienes and phosphorins from phospholes. J. Am. Chem. Soc. 1981, 103, 4595–4597. [Google Scholar] [CrossRef]
- Wannere, C.S.; Bansal, R.K.; Schleyer, P.v.R. Diels-Alder reaction of phosphaethene with 1,3-dienes: An ab-initio study. J. Org. Chem. 2002, 67, 9162–9174. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.K.; Gupta, N.; Kumawat, S.K.; Dixit, G. Diastereo- and regioselective Diels-Alder reactions of 2-phosphaindolizines. Tetrahedron 2008, 64, 6395–6401. [Google Scholar] [CrossRef]
- Bansal, R.K.; Jain, V.K.; Gupta, N.; Gupta, N.; Hemrajani, L.; Baweja, M.; Jones, P.G. Stereo- and regioselectivity in Diels-Alder reactions of 1,3-azaphospholo[5,1-a]isoquinoline and –[5,1-a]pyridine. Tetrahedron 2002, 58, 1573–1579. [Google Scholar] [CrossRef]
- Bansal, R.K.; Karaghiosoff, K.; Gupta, N.; Gandhi, N.; Kumawat, S.K. Diastereo- and regioselectivity in Diels-Alder reaction of [1,4,2]diazaphospholo[4,5-a]pyridines. Tetrahedron 2005, 61, 10521–10528. [Google Scholar] [CrossRef]
- Karaghiosoff, K.; Cleve, C.; Schmidpeter, A. Chloromethyl-dichlorophosphane: A useful reagent for the synthesis of new heterocycles with dicoordinated phosphorus. Phosphorus Sulfur Silicon Relat. Elem. 1986, 28, 289–296. [Google Scholar] [CrossRef]
- Pellegriner, S.C.; Silva, M.A.; Goodman, J.M. Theoretical evaluation of the origin of the regio- and stereoselectivity in the Diels-Alder reactions of dialkylvinylboranes: Studies on the reactions of vinylborane, dimethylvinylborane, and vinyl-9-BBN with trans-piperylene and isoprene. J. Am. Chem. Soc. 2001, 123, 8832–8837. [Google Scholar] [CrossRef]
- Bansal, R.K.; Gupta, N.; Dixit, G.; Kumawat, S.K. Theoretical investigation of an unusual substituent effect on the dienophilicity of >C=P– Functionality in 2-phosphaindolizines. J. Phys. Org. Chem. 2009, 22, 125–129. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, R.K.; Bansal, R.K.; Hopffgarten, M.V. Catalytic effect of organoaluminium chloride reagents on the dienophilic reactivity of indolizine and 2-phosphaindolizine towards [2+4] cycloaddition: A DFT investigation. Curr. Cat. 2012, 1, 93–99. [Google Scholar] [CrossRef]
- Jangid, R.K.; Gupta, N.; Bansal, R.K.; Hopffgarten, M.V.; Frenking, G. Diels-Alder reaction of 2-phosphaindolizines catalyzed by organoaluminium reagent: Theoretical and experimental results. Tetrahedron Lett. 2011, 52, 1721–1724. [Google Scholar] [CrossRef]
- Alcaraz, J.P.; Mathey, F. Accroissement de la reactivite des phosphorines en tant que dienes et philodienes par complexation du phosphore. Tetrahedron Lett. 1984, 25, 207–210. [Google Scholar] [CrossRef]
- Jangid, R.K.; Sogani, N.; Gupta, N.; Bansal, R.K.; Hopffgarten, M.V.; Frenking, G. Asymmetric Diels-Alder reaction with >C=P– functionality of the 2-phosphaindolizine-η1-P-aluminium(o-menthoxy) dichloride complex: Experimental and theoretical results. Beilstein J. Org. Chem. 2013, 9, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Swinbourne, F.J.; Hunt, J.H.; Klinkert, G. Advances in Indolizine Chemistry in the Series: Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: New York, NY, USA, 1979; Volume 23, pp. 103–170. [Google Scholar]
- Bansal, R.K.; Heinicke, J. Anellated heterophospholes and phospholides and analogies with related non-phosphorus systems. Chem. Rev. 2001, 101, 3549–3578. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, A.A.; Yurchenko, A.A.; Kozlov, E.S.; Shulezhko, V.A.; Pinchuk, A.M. Phosphorylated indolizines. Heteroat. Chem. 1993, 4, 343–360. [Google Scholar] [CrossRef]
- Bansal, R.K.; Gupta, N.; Kabra, V.; Spindler, C.; Karaghiosoff, K.; Schmidpeter, A. Substitution of 2-phosphaindolizines by bromine and by chlorophosphines. Heteroat. Chem. 1992, 3, 359–366. [Google Scholar] [CrossRef]
- Mendez, F.; Gázquez, J.L. Chemical reactivity of enolate ions: The local hard and soft-acids and bases principle viewpoint. J. Am. Chem. Soc. 1994, 116, 9298–9301. [Google Scholar] [CrossRef]
- Kour, M.; Gupta, R.; Bansal, R.K. Experimental and theoretical investigation of the reaction of secondary amines to maleic anhydride. Aust. J. Chem. 2017, 70, 1247–1253. [Google Scholar] [CrossRef]
- Gupta, R.; Paul, B.; Bansal, R.K. Application of Fukui functions for comparing reactivities of indolizine and 2-phosphaindolizine towards electrophilic substitution. 2018; unpublished work. [Google Scholar]

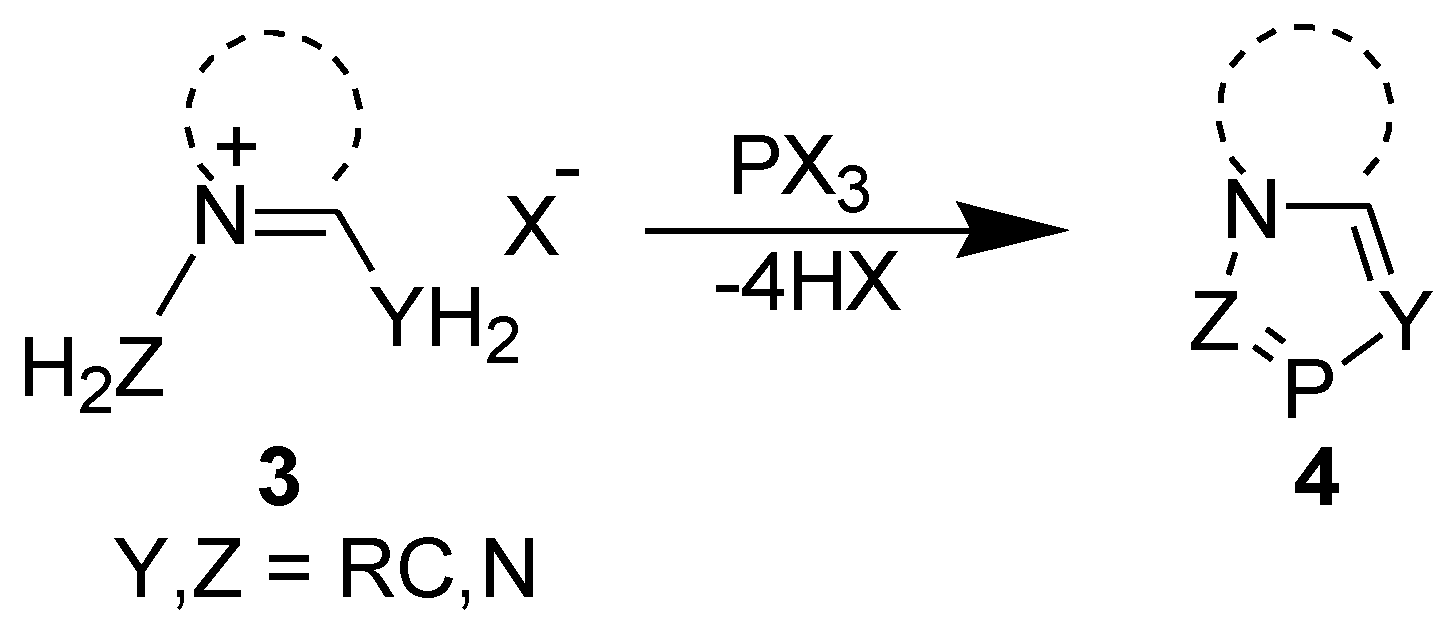



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, R.K.; Gupta, R.; Kour, M. Synergy between Experimental and Theoretical Results of Some Reactions of Annelated 1,3-Azaphospholes. Molecules 2018, 23, 1283. https://doi.org/10.3390/molecules23061283
Bansal RK, Gupta R, Kour M. Synergy between Experimental and Theoretical Results of Some Reactions of Annelated 1,3-Azaphospholes. Molecules. 2018; 23(6):1283. https://doi.org/10.3390/molecules23061283
Chicago/Turabian StyleBansal, Raj K., Raakhi Gupta, and Manjinder Kour. 2018. "Synergy between Experimental and Theoretical Results of Some Reactions of Annelated 1,3-Azaphospholes" Molecules 23, no. 6: 1283. https://doi.org/10.3390/molecules23061283




