Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes
Abstract
:1. Introduction
2. Physiochemical Properties of Cyclodextrins
3. How Much Solubilization Is Needed?
4. The Effect of the Guest Molecule on the Cyclodextrin Solubility
5. Excipients and Cyclodextrin Solubility
6. Conclusions and Directions
Funding
Conflicts of Interest
Abbreviations
| CD | Cyclodextrin |
| CE | Complexation efficiency |
| CMβCD | Carboxymethyl-βCD |
| DMβCD | Dimethyl-βCD |
| DMαCD | Dimethyl-αCD |
| DMγCD | Dimethyl-γCD |
| DS | Degree of substitution |
| G1βCD | Glucosyl-βCD |
| G2βCD | Maltosyl-βCD |
| GUGβCD | Glucoronyl-glucosyl-βCD |
| HEβCD | Hydroxyethyl-βCD |
| HPMC | Hydroxypropyl methylcellulose |
| HPαCD | 2-hydroxypropyl-αCD |
| HPγCD | Hydroxypropyl-γCD |
| Na CMC | Sodium carboxymethylcellulose |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinyl pyrrolidone |
| SBEγCD | Sulfobutylether-γCD |
| SUG | Sugammadex |
| TMβCD | Trimethyl-βCD |
| TMαCD | Trimethyl-αCD |
| TMγCD | Trimethyl-γCD |
| αCD | α-Cyclodextrin |
| βCD | β-Cyclodextrin |
| γCD | γ-Cyclodextrin |
References
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Muankaew, C.; Loftsson, T. Cyclodextrin-based formulations: A non-invasive platform for targeted drug delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- FDA, U.S.F.D.U.S. Inactive Ingredient Search for Approved Drug Prodructs. Availabe online: https://www.accessdata.fda.gov/scripts/cder/iig/getiigWEB.cfm (accessed on 1 April 2018 ).
- Arima, H.; Motoyama, K.; Higashi, T. Potential use of cyclodextrins as drug carriers and active pharmaceutical ingredients. Chem. Pharm. Bull. 2017, 65, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-D.; Tang, G.-P.; Chu, P.K. Cyclodextrin-based host–guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Stella, V.J.; Rao, V.M.; Zannou, E.A.; Zia, V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Del. Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Madden, D.E.; Carr, D.; Loftsson, T. The effect of parenterally administered cyclodextrins on the pharmacokinetics of coadministered drugs. J. Pharm. Sci. 2012, 101, 4402–4408. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef] [PubMed]
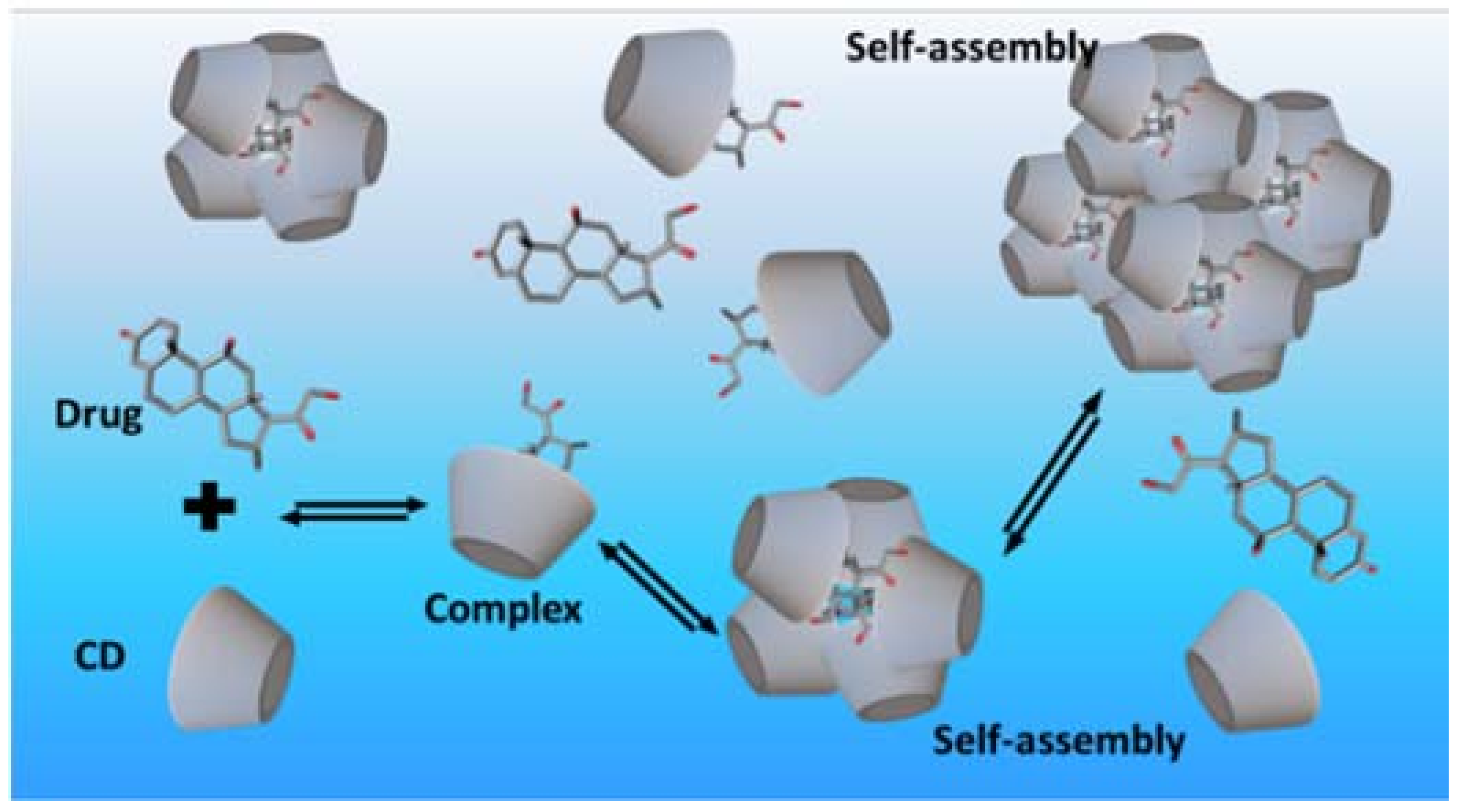
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Del. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernas, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Cyclodextrins and the biopharmaceutics classification system of drugs. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 63–67. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E.; Konráðsdóttir, F. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm. Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Nakatani, H.; Hiromi, K. In vitro action of human and porcine α-amylases on cyclomalto-oligosaccharides. Carbohydr. Res. 1990, 204, 207–213. [Google Scholar] [CrossRef]
- Lumholdt, L.R.; Holm, R.; Jørgensen, E.B.; Larsen, K.L. In vitro investigations of α-amylase mediated hydrolysis of cyclodextrins in the presence of ibuprofen, flurbiprofen, or benzo[a]pyrene. Carbohydr. Res. 2012, 362, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Gaidamauskas, E.; Norkus, E.; Butkus, E.; Crans, D.C.; Grincienė, G. Deprotonation of β-cyclodextrin in alkaline solutions. Carbohydr. Res. 2009, 344, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster Marcus, E. Cyclodextrins as functional excipients: Methods to enhance complexation efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.W.; Nicolis, I.; Keller, N.; Dalbiez, J.P. Aggregation of cyclodextrins: An explanation of the abnormal solubility of β-cyclodextrin. J. Incl. Phenom. Mol. Recognit. Chem. 1992, 13, 139–143. [Google Scholar] [CrossRef]
- Sabadini, E.; Cosgrove, T.; Egídio, F.D.C. Solubility of cyclomaltooligosaccharides (cyclodextrins) in H2O and D2O: A comparative study. Carbohydr. Res. 2006, 341, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.J.; Chen, J.Y.-J.; Jansson, J.L.M.; Widmalm, G.; Maliniak, A. Molecular properties related to the anomalous solubility of β-cyclodextrin. J. Phys. Chem. B 2004, 108, 4236–4238. [Google Scholar] [CrossRef]
- Cai, W.; Sun, T.; Shao, X.; Chipot, C. Can the anomalous aqueous solubility of β-cyclodextrin be explained by its hydration free energy alone? Phys. Chem. Chem. Phys. 2008, 10, 3236–3243. [Google Scholar] [CrossRef] [PubMed]
- Duchěne, D.; Wouessidjewe, D. Pharmaceutical uses of cyclodextrins and derivatives. Drug Dev. Ind. Pharm. 1990, 16, 2487–2499. [Google Scholar] [CrossRef]
- Szejtli, J. Highly soluble β-cyclodextrin derivatives. Starch-Stärke 1984, 36, 429–432. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wenz, G. Influence of intramolecular hydrogen bonds on the binding potential of methylated β-cyclodextrin derivatives. Beilstein J. Org. Chem. 2012, 8, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.C.D.; Martins, T.E.A.; Veiga, F.; Ferraz, H.G. Cyclodextrins and ternary complexes: Technology to improve solubility of poorly soluble drugs. Braz. J. Pharm. Sci. 2011, 47, 665–681. [Google Scholar] [CrossRef]
- Hanna, K.; de Brauer, C.; Germain, P. Cyclodextrin-enhanced solubilization of pentachlorophenol in water. J. Environ. Manag. 2004, 71, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tavornvipas, S.; Arima, H.; Hirayama, F.; Uekama, K.; Ishiguro, T.; Oka, M.; Hamayasu, K.; Hashimoto, H. Some pharmaceutical properties of a new branched cyclodextrin, 6-O-α-(4-O-α-d-Glucuronyl)-d-glucosylβ-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 391–394. [Google Scholar] [CrossRef]
- Hirayama, F.; Mieda, S.; Miyamoto, Y.; Arima, H.; Uekama, K. Heptakis(2,6-di-O-methyl-3-O-acetyl)-β-cyclodextrin: A water-soluble cyclodextrin derivative with low hemolytic activity. J. Pharm. Sci. 1999, 88, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Tongiani, S.; Ozeki, T.; Stella, V.J. Sulfobutyl ether-alkyl ether mixed cyclodextrin derivatives with enhanced inclusion ability. J. Pharm. Sci. 2009, 98, 4769–4780. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, É.; Szemán, J.; Csabai, K.; Malanga, M.; Szente, L. Methyl-beta-cyclodextrins: The role of number and types of substituents in solubilizing power. J. Pharm. Sci. 2014, 103, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Yong, C.W.; Washington, C.; Smith, W. Structural behaviour of 2-hydroxypropyl-β-cyclodextrin in water: Molecular dynamics simulation studies. Pharm. Res. 2008, 25, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Terekhova, I.V.; Kumeev, R.S.; Al’per, G.A. The interaction of caffeine with substituted cyclodextrins in water. Russ. J. Phys. Chem. A 2007, 81, 1071–1075. [Google Scholar] [CrossRef]
- Müller, B.W.; Brauns, U. Hydroxypropyl-β cyclodextrin derivatives: Influence of average degree of substitution on complexing ability and surface activity. J. Pharm. Sci. 1986, 75, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, L.; Bricout, H.; Tilloy, S.; Monflier, E. Biphasic aqueous organometallic catalysis promoted by cyclodextrins: Can surface tension measurements explain the efficiency of chemically modified cyclodextrins? J. Colloid Interface Sci. 2007, 307, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Másson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Fenyvesi, É. Cyclodextrin-lipid complexes: Cavity size matters. Struct. Chem. 2017, 28, 479–492. [Google Scholar] [CrossRef]
- Legrand, F.-X.; Sauthier, M.; Flahaut, C.; Hachani, J.; Elfakir, C.; Fourmentin, S.; Tilloy, S.; Monflier, E. Aqueous hydroformylation reaction mediated by randomly methylated β-cyclodextrin: How substitution degree influences catalytic activity and selectivity. J. Mol. Catal. A Chem. 2009, 303, 72–77. [Google Scholar] [CrossRef]
- Azarbayjani, A.F.; Lin, H.; Yap, C.W.; Chan, Y.W.; Chan, S.Y. Surface tension and wettability in transdermal delivery: A study on the in-vitro permeation of haloperidol with cyclodextrin across human epidermis. J. Pharm. Pharmacol. 2010, 62, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.W.R.; Faulkner, J.; Han, S.M. Use of hydroxypropyl- and hydroxyethyl-derivatized β-cyclodextrins for the thin-layer chromatographic separation of enantiomers and diastereomers. J. Chromatogr. A 1988, 452, 323–330. [Google Scholar] [CrossRef]
- Francotte, E.; Brandel, L.; Jung, M. Influence of the degree of substitution of cyclodextrin sulfobutyl ether derivatives on enantioselective separations by electrokinetic chromatography. J. Chromatogr. A 1997, 792, 379–384. [Google Scholar] [CrossRef]
- Okada, Y.; Matsuda, K.; Hara, K.; Hamayasu, K.; Hashimoto, H.; Koizumi, K. Properties and the inclusion behavior of 6-O-α-d-Galactosyl- and 6-O-α-d-Mannosyl-cyclodextrins. Chem. Pharm. Bull. 1999, 47, 1564–1568. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Physical chemical analysis of percutaneous absorption process from creams and ointments. J. Soc. Cosmet. Chem. 1960, 11, 85–97. [Google Scholar]
- Schaefer, H.; Schalla, W.; Zesch, A.; Stüttgen, G. Skin Permeability; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Gallaher, D.; Plank, D. α-Cyclodextrin as a food ingredient to reduce fat absorption. Agro Food Ind. Hi-Tech 2015, 26, 5–7. [Google Scholar]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Magnúsdóttir, A.; Másson, M.; Sigurjónsdóttir, J.F. Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 2002, 91, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Xu, X.; Liu, B.; Xu, X.; Sun, L.; Li, H.; Zhang, J. Simultaneous high-throughput determination of interaction kinetics for drugs and cyclodextrins by high performance affinity chromatography with mass spectrometry detection. Anal. Chim. Acta 2016, 909, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ge, J.; Guo, T.; Yang, S.; He, Z.; York, P.; Sun, L.; Xu, X.; Zhang, J. Determination of the kinetic rate constant of cyclodextrin supramolecular systems by high performance affinity chromatography. J. Chromatogr. A 2013, 1305, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ge, J.; Zhang, J.; Guo, T.; Chi, L.; He, Z.; Xu, X.; York, P.; Sun, L.; Li, H. Multianalyte determination of the kinetic rate constants of drug–cyclodextrin supermolecules by high performance affinity chromatography. J. Chromatogr. A 2014, 1359, 287–295. [Google Scholar] [CrossRef] [PubMed]
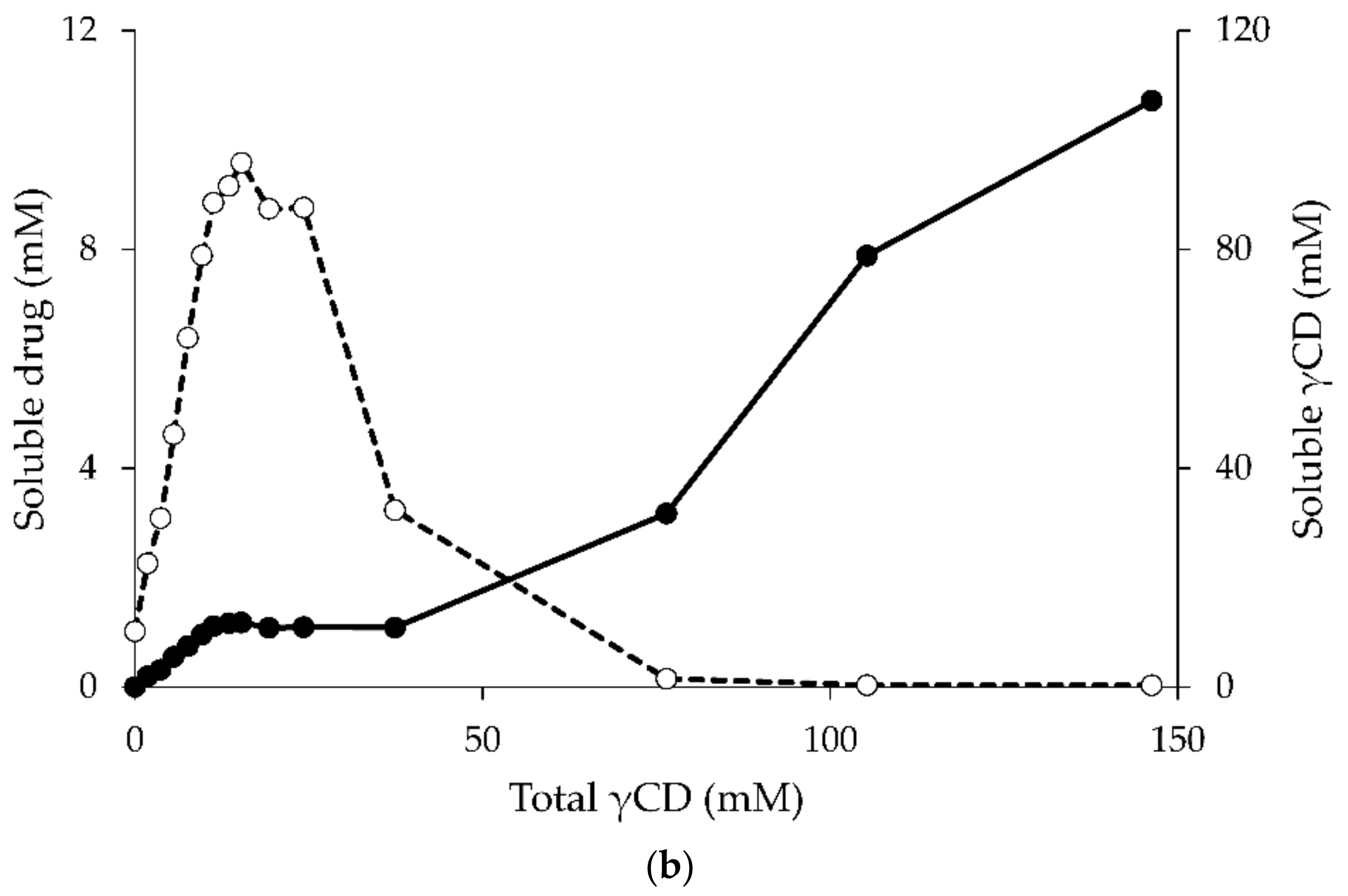
- Jansook, P.; Moya-Ortega, M.D.; Loftsson, T. Effect of self-aggregation of γ-cyclodextrin on drug solubilization. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 229–236. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. A new approach for quantitative determination of γ-cyclodextrin in aqueous solutions: Application in aggregate determinations and solubility in hydrocortisone/γ-cyclodextrin inclusion complex. J. Pharm. Sci. 2015, 104, 3925–3933. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Do, T.T.; Van den Mooter, G.; Loftsson, T. Inclusion complexes of p-hydroxybenzoic acid esters and γ-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 111–122. [Google Scholar] [CrossRef]
- Schönbeck, C.; Madsen, T.L.; Peters, G.H.; Holm, R.; Loftsson, T. Soluble 1:1 complexes and insoluble 3:2 complexes—Understanding the phase-solubility diagram of hydrocortisone and γ-cyclodextrin. Int. J. Pharm. 2017, 531, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Lehner, S.J.; Müller, B.W.; Seydel, J.K. Effect of hydroxypropyl-β-cyclodextrin on the antimicrobial action of preservatives. J. Pharm. Pharmacol. 1994, 46, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Stefánsdóttir, Ó.; Friôriksdóttir, H.; Guômundsson, Ö. Interactions between preservatives and 2-hydroxypropyl-β-cyclodextrin. Drug Dev. Ind. Pharm. 1992, 18, 1477–1484. [Google Scholar] [CrossRef]
- Holm, R.; Olesen, N.E.; Alexandersen, S.D.; Dahlgaard, B.N.; Westh, P.; Mu, H. Thermodynamic investigation of the interaction between cyclodextrins and preservatives—Application and verification in a mathematical model to determine the needed preservative surplus in aqueous cyclodextrin formulations. Eur. J. Pharm. Sci. 2016, 87, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Increasing the cyclodextrin complexation of drugs and drug biovailability through addition of water-soluble polymers. Pharmazie 1998, 53, 733–740. [Google Scholar] [PubMed]
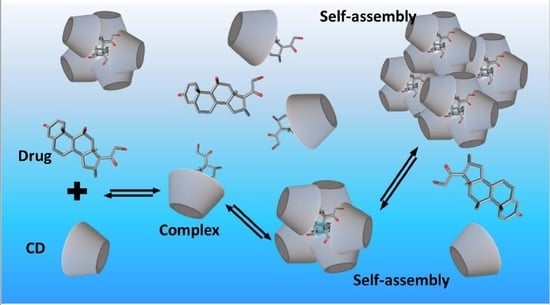
- Jansook, P.; Kurkov, S.V.; Loftsson, T. Cyclodextrins as solubilizers: Formation of complex aggregates. J. Pharm. Sci. 2010, 99, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Messner, M.; Kurkov, S.V.; Jansook, P.; Loftsson, T. Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 2010, 387, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, J.; Järvinen, K.; Taipale, H.; Jarho, P.; Loftsson, T.; Järvinen, T. Coadministration of a water-soluble polymer increases the usefulness of cyclodextrins in solid oral dosage forms. In Proceedings of the Ninth International Symposium on Cyclodextrins, Dordrecht, The Netherlands, 31 May–3 June 1998; pp. 261–264. [Google Scholar]
- Jansook, P.; Loftsson, T. CDs as solubilizers: Effects of excipients and competing drugs. Int. J. Pharm. 2009, 379, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oksanen, D.A.; Massefski, J.W.; Blake, J.F.; Duffy, E.M.; Chrunyk, B. Inclusion complexation of ziprasidone mesylate with β-cyclodextrin sulfobutyl ether. J. Pharm. Sci. 1998, 87, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Omari, M.M.A.; Zughul, M.B.; Davies, J.E.D.; Badwan, A.A. Factors contributing to solubility synergism of some basic drugs with β-cyclodextrin in ternary molecular complexes. J. Incl. Phenom. Macrocycl. Chem. 2006, 54, 159–164. [Google Scholar] [CrossRef]
- Loftsson, T.; Matthíasson, K.; Másson, M. The effects of organic salts on the cyclodextrin solubilization of drugs. Int. J. Pharm. 2003, 262, 101–107. [Google Scholar] [CrossRef]
- He, Y.; Li, P.; Yalkowsky, S.H. Solubilization of fluasterone in cosolvent/cyclodextrin combinations. Int. J. Pharm. 2003, 264, 25–34. [Google Scholar] [CrossRef]
- Soltani, N.; Shaynafar, A.; Djozan, D.; Jouyban, A. Solubility of three basic drugs in propylene glycol + water mixtures in the presence of β-cyclodextrin. J. Drug Deliv. Sci. Technol. 2013, 23, 187–190. [Google Scholar] [CrossRef]
- Müller, B.W.; Albers, E. Effect of hydrotropic substances on the complexation of sparingly soluble drugs with cyclodextrin dervatives and the influence of cyclodextrin complexation on the pharmacokinetics of the drugs. J. Pharm. Sci. 1991, 80, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Sigurðardóttir, A.M. The effect of polyvinylpyrrolidone and hydroxypropyl methylcellulose on HPβCD complexation of hydrocortisone and its permeability through hairless mouse skin. Eur. J. Pharm. Sci. 1994, 2, 297–301. [Google Scholar] [CrossRef]
- Loftsson, T.; Frikdriksdóttir, H.; Sigurkdardóttir, A.M.; Ueda, H. The effect of water-soluble polymers on drug-cyclodextrin complexation. Int. J. Pharm. 1994, 110, 169–177. [Google Scholar] [CrossRef]
- Loftsson, T.; Másson, M. The effects of water-soluble polymers on cyclodextrins and cyclodextrin solubilization of drugs. J. Drug Deliv. Sci. Technol. 2004, 14, 35–43. [Google Scholar] [CrossRef]
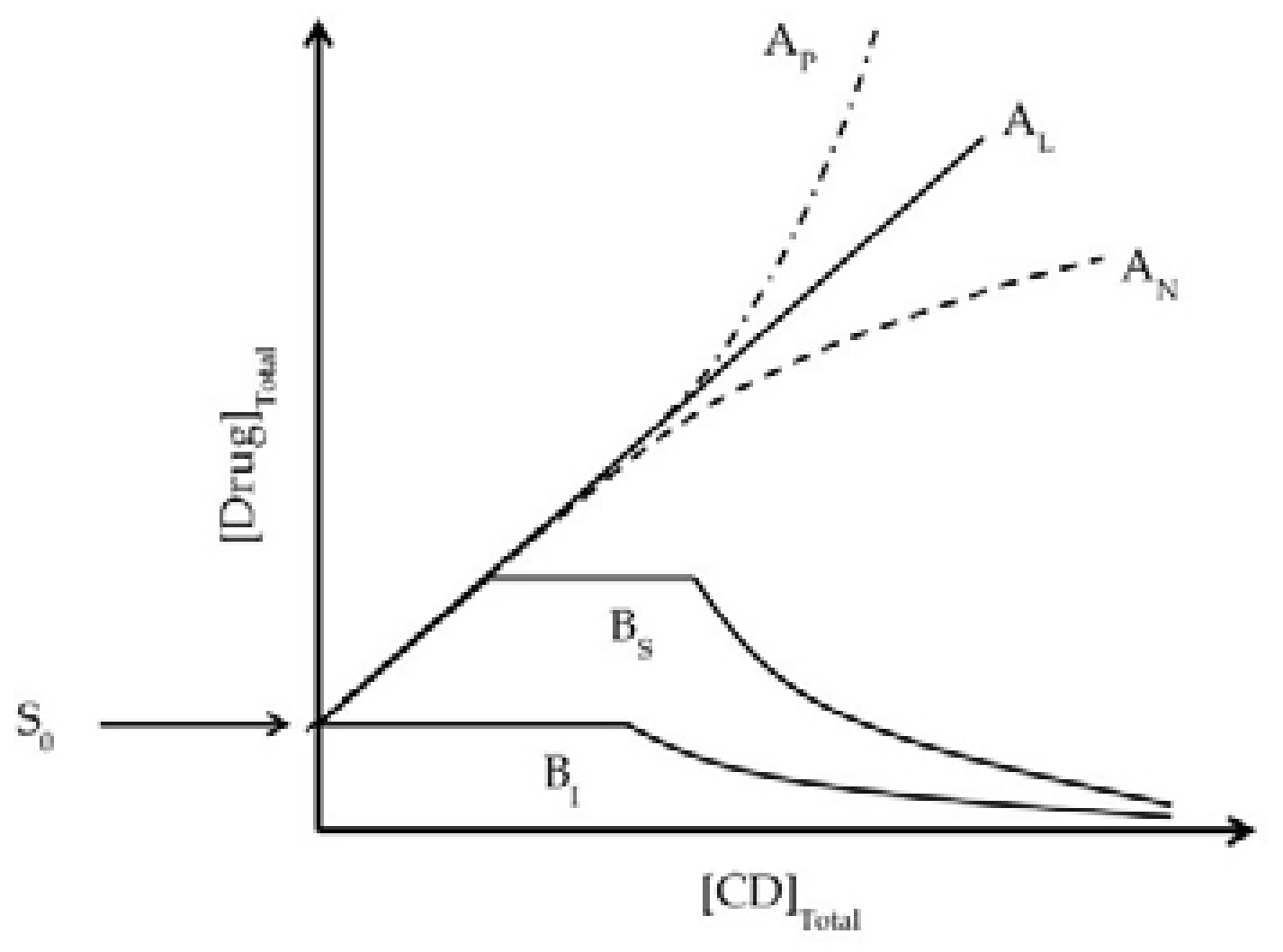
- Yamakawa, T.; Nishimura, S. Liquid formulation of a novel non-fluorinated topical quinolone, T-3912, utilizing the synergic solubilizing effect of the combined use of magnesium ions and hydroxypropyl-β-cyclodextrin. J. Control. Release 2003, 86, 101–113. [Google Scholar] [CrossRef]
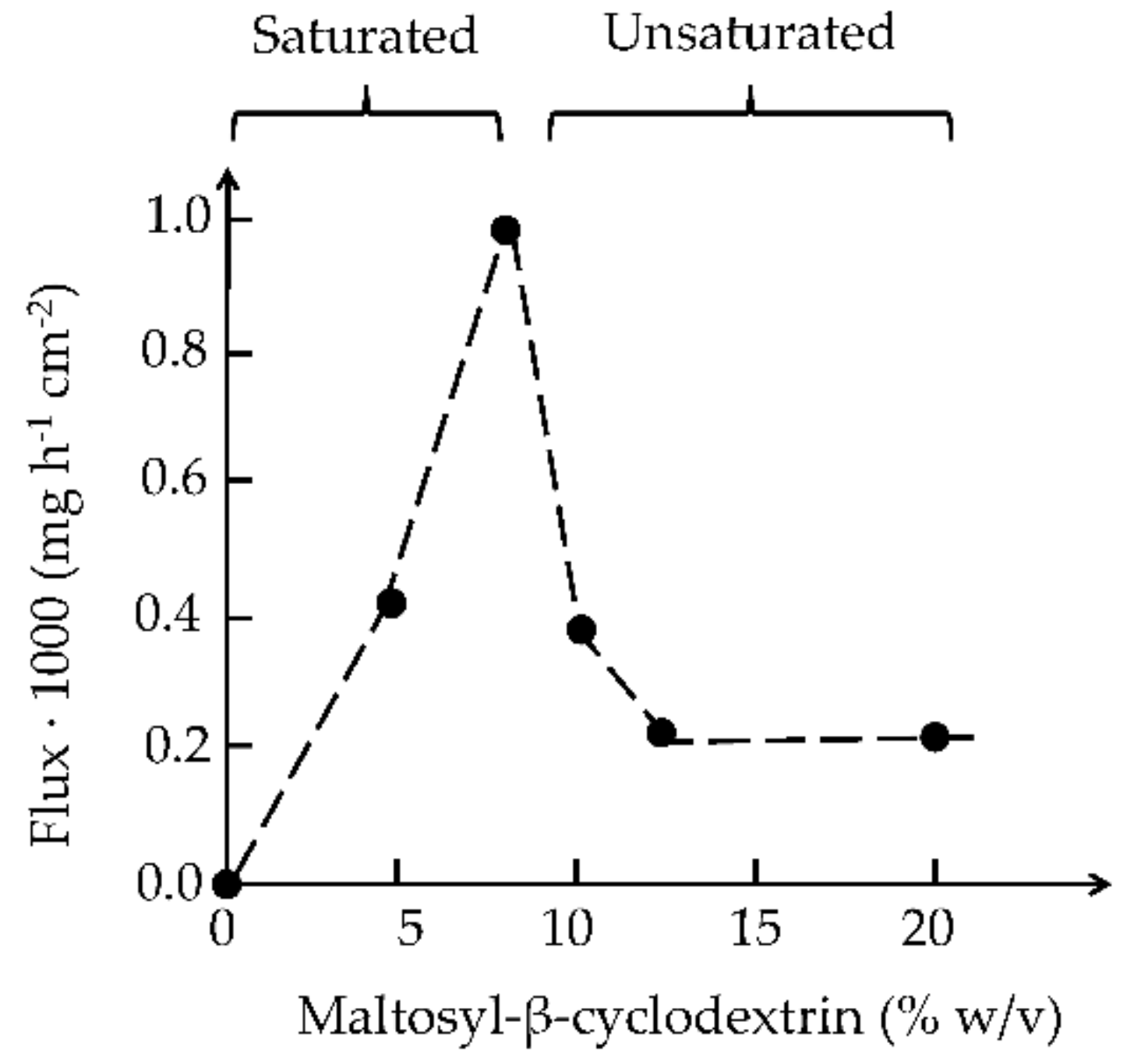

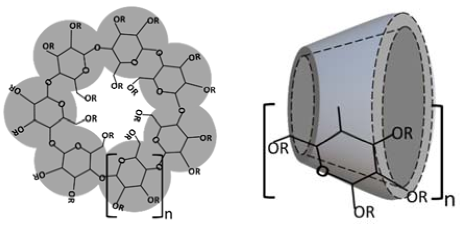
| Cyclodextrin | n | R = H or | Abbreviation | Synonyms | Pharmacopoeia Monographs 1 |
|---|---|---|---|---|---|
| α-Cyclodextrin | 0 | αCD | alfadex | Ph.Eur., USP-NF, JPC | |
| β-Cyclodextrin | 1 | βCD | betadex | Ph.Eur., USP-NF, JPC | |
| 2-Hydroxypropyl-β-cyclodextrin | 1 | -CH2CHOHCH3 | HPβCD | hydroxypropylbetadex | Ph.Eur., USP-NF |
| Sulfobutylether β-cyclodextrin sodium | 1 | -(CH2)4SO3− Na+ | SBEβCD | betadex sulfobutyl ether sodium | USP-NF |
| γ-Cyclodextrin | 2 | γCD | gammadex | Ph.Eur., USP-NF, JPC |
| Types | Substituent | 1 DS | Inner Cavity Diameter (Å) | Hydrogen Donors | Hydrogen Acceptors | Solubility (mg/mL, 25 °C) | Log Po/w | Surface Tension (mN/m) | References |
|---|---|---|---|---|---|---|---|---|---|
| Naural CD | |||||||||
| αCD | H | 0 | 4.7–5.3 | 18 | 30 | 145 | −13 | 71 | [1,42] |
| βCD | H | 0 | 6.0–6.5 | 21 | 35 | 18.5 | −14 | 71 | [1] |
| γCD | H | 0 | 7.5–8.3 | 24 | 40 | 232 | −17 | 71 | [1] |
| Modified CD | |||||||||
| HPαCD | -CH2-CHOH-CH3 | 3.6 | 4.5–5.3 | 18 | 36 | - | - | - | [43] |
| CMβCD | -CH2-CO2H | 3–5 | - | 21 | 49 | 50 | −4.9 | - | [32] |
| DMβCD | -CH3 | 12–16 | 5.8–6.5 | 7 | 35 | 570 | - | 62 | [34] |
| RMβCD | -CH3 | 9.7–13.6 | - | 9 | 35 | >500 | −6 | 57.5–54.1 | [1,44,45] |
| TMβCD | -CH3 | 21 | 4–7 | 0 | 35 | 310 | - | 56 | [34,46] |
| HEβCD | -CH2-CH2OH | 3.6 | - | 21 | 42 | >2000 | - | - | [26,47] |
| HPβCD | -CH2-CHOH-CH3 | 2.8–10.5 | 6.0 | 25 | 39 | >1200 | −11 | 54.8–57.5 | [1,47] |
| SBEβCD | (CH2)4-SO3Na | 6.2–6.9 | - | 21 | 35 | >1200 | <−10 | 71 | [1] |
| HPγCD | -CH2-CHOH-CH3 | 3.0–5.4 | 8.0 | 24 | 45 | 800 | −13 | - | [1] |
| SBEγCD | (CH2)4-SO3Na | 4–8 | - | - | - | - | - | - | [43,48] |
| SUG | -SCH2CH2CO2Na | 8 | 7.5–8.3 | 24 | 48 | Very soluble | −16 | 72.2 | [1] |
| Branched CD | |||||||||
| G1βCD | glucosyl | 1 | 6.0–6.5 | 24 | 40 | 970 | −9 | 71 | [26,49] |
| G2βCD | maltosyl | 1 | - | 27 | 45 | >1500 | −9 | 72 | [33] |
| GUGβCD | glucoronylglucosyl | 1 | - | - | - | >2000 | - | 73 | [33] |
| Formulation | Amount of CD | Comments |
|---|---|---|
| Parenteral solutions | Sufficient to solubilize the drug dose in, for example, 10 mL of water. | Significant excess CD (as much as two to three times what is needed to solubilize the drug) is frequently included in parenteral formulations, especially those that are given intravenously. This will not affect the drug pharmacokinetics since the drug is almost instantaneously released from the complex upon dilution in the blood circulation. |
| Solid oral dosage forms (e.g., tablets and capsules) | Sufficient to increase aqueous solubility the drug dose to prevent dissolution controlled absorption. | The formulation bulk usually limits the amount of CD that can be included in solid dosage forms. For example, if βCD (molecular weight 1135 Da) is used in a solid dosage form containing 100 mg of a drug with molecular weight 250 Da the formulation bulk will be increased by over five-fold. |
| Oral solutions | Sufficient to dissolve the drug dose in the aqueous vehicle. | Excess of CD (e.g., ≥20) should be used to prevent drug precipitation upon storage and usage of the formulation. Due to formulation dilution in the gastrointestinal tract some excess CD will not hamper the drug release. However, large excess (over 50 to 100) can hamper the drug release. |
| Topical solutions with limited dilution upon administration (e.g., eye drops) | Sufficient to dissolve the drug dose in the aqueous vehicle. | Only a small excess of CD (e.g., 10 to 20%) should be used to prevent drug precipitation upon storage and usage of the formulation. Excess amounts of CD (e.g., more than 10%) can reduce topical bioavailability of the drug. |
| Excipients | Examples | Effect on Complexation Enhancement | Some Brief Observations | References |
|---|---|---|---|---|
| Acids, bases, inorganic/organic salts | hydrochloride, citrate, aspartate, mesylate, maleate, tartrate, phosphate, acetate | Increase intrinsic solubility of drugs (S0) and/or the apparent stability constant (K1:1) resulting in increased CE | Salt formation of ziprasidone mesylate enhance the CE of drug up to 100 and 240 times in aqueous HPβCD and SBEβCD solutions when compared with the free base of drug | [71] |
| Ternary complex of terfenadine/βCD/inorganic acid (phosphate, citrate) induce the synergistic effect of CD solubilization | [72] | |||
| The addition of sodium acetate into the complexing medium containing βCD could increase hydrocortisone solubility up to 220% | [73] | |||
| Enhance S0 but in most cases decrease K1:1 | K1:1 of fluasterone/HPβCD complex decreases with increasing ethanol concentration but the drug solubility increased at high ethanol concentration (>40% v/v) | [74] | ||
| Cosolvents | ethanol, propylene glycol (PG) | Ternary complex of diazepam/PG/βCD increased the diazepam solubility than that of the mixture of PG and water | [75] | |
| Hamper complexation by the competitive effect | At higher concentrations of PG, the methyltestosterone solubility in presence of HPβCD decreased possibly due to the complex dissociation | [76] | ||
| Water-soluble polymers | HPMC, Na CMC, PVA, PVP | Formation of ternary complex (drug/CD/polymer) that can increase K1:1 | Polymers and CDs can form water-soluble complexes with poorly water-soluble drugs, for example, acetazolamide, carbamazepine hydrocortisone, naproxen, pregnenolone, tropicamide, etc. have been reviewedSynergistic solubilization effect is possible through micellar-like solubilization or stabilization of self-assembled CD and/or CD complex aggregates | [20,77,78,79] |
| Metal ions | Mg2+ | Enhance CE by increasing S0 via formation of drug/CD/metal ion complexes | Synergistic solubilization of quinolone was obtained when the addition of Mg2+ to the drug/HPβCD complexes | [80] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. https://doi.org/10.3390/molecules23051161
Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules. 2018; 23(5):1161. https://doi.org/10.3390/molecules23051161
Chicago/Turabian StyleSaokham, Phennapha, Chutimon Muankaew, Phatsawee Jansook, and Thorsteinn Loftsson. 2018. "Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes" Molecules 23, no. 5: 1161. https://doi.org/10.3390/molecules23051161
APA StyleSaokham, P., Muankaew, C., Jansook, P., & Loftsson, T. (2018). Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules, 23(5), 1161. https://doi.org/10.3390/molecules23051161