Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases
Abstract
:1. Introduction
2. Curcumin
Biological and Pharmacological Properties of Curcumin
3. Therapeutic Potential of Curcumin in Aging-Associated Diseases
4. Recent Advances of Nanocurcumin in Aging-Associated Diseases
5. Clinical Application of Curcumin in Aging-Associated Diseases
6. Future Perspectives and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases requires age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54, S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Ozanne, S.E. Nutrition in early life and age-associated diseases. Ageing Res. Rev. 2017, 39, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banerjee, S.; Wang, Z.; Kong, D.; Majumdar, A.P.N.; Sarkar, F.H. Aging and Inflammation: Etiological culprits of cancer. Curr. Aging Sci. 2009, 2, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The aging process and potential interventions to extend life expectancy. Clin. Interv. Aging 2007, 2, 401–412. [Google Scholar] [PubMed]
- Kithas, P.A.; Supiano, M.A. Hypertension and chronic kidney disease in the elderly. Adv. Chronic Kidney Dis. 2010, 17, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L. Modulating Human Aging and Age-Associated Diseases. Biochim. Biophys. Acta 2009, 1790, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, R.; Pospisil, P.; Kruk, J. Plant-derived antioxidants in disease prevention. Oxid. Med. Cell Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F.I.; Abdollahi, M.; Venkata, K.C.N.; Nabavi, S.M.; Bishayee, A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Perez, A.A.; Rivero, H.C.; Hernandez, M.C.P.; Pagan, A.; Montalban, M.G.; Villora, G.; Cenis, J.L. Silk fibroin nanoparticles: Efficient vehicles for the natural antioxidant quercetin. Int. J. Pharm. 2017, 518, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Surianarayanan, M.; Vijayaraghavan, R.; Mandal, A.B.; MacFarlane, D.R. Curcumin loaded poly(2-hydroxyethyl methacrylate) nanoparticles from gelled ionic liquid–In vitro cytotoxicity and anti-cancer activity in SKOV-3 cells. Eur. J. Pharm. Sci. 2014, 51, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Mahesh, A.; Mahadevan, S.; Mandal, A.B. Synthesis and characterization of curcumin loaded polymer/lipid based nanoparticles and evaluation of their antitumor effects on MCF-7 cells. Biochim. Biophys. Acta 2014, 1840, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and neurodegenerative diseases. Biofactors 2013, 39, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Olszanecki, R.; Jawien, J.; Gajda, M.; Mateuszuk, L.; Gebska, A.; Korabiowska, M.; Chlopicki, S.; Korbut, R. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J. Physiol. Pharmacol. 2005, 56, 627–635. [Google Scholar] [PubMed]
- Wongcharoen, W.; Phrommintikul, A. The protective role of curcumin in cardiovascular diseases. Int. J. Cardiol. 2009, 133, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Ruhul Amin, A.R.M.; Chen, Z.G.; Shin, D.M. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. (Phila) 2013, 6, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Espinoza, Y.; Muriel, P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int. 2009, 29, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-W.; Fu, M.; Gao, S.-H.; Liu, J.-L. Curcumin and Diabetes: A Systematic Review. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C.; Cai, H. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Gehr, T.W.; Ghosh, S. Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin Exerts its Anti-hypertensive Effect by Down-regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, N.; Giannotti, R.; Plateroti, A.M.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical potential in ophthalmology. Planta Med. 2014, 80, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xue, J.; Shen, T.; Mu, S.; Fu, Q. Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway. Int. J. Mol. Med. 2016, 37, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Sharma, A.; Maheshwari, R.K. Beneficial role of curcumin in skin diseases. Adv. Exp. Med. Biol. 2007, 595, 343–357. [Google Scholar] [PubMed]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-Applied curcumin for different diseases therapy. BioMed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Aggarwal, B.B. Turmeric, the golden spice: From traditional medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; ISBN 9781439807132. [Google Scholar]
- Wright, L.E.; Frye, J.B.; Gorti, B.; Timmermann, B.N.; Funk, J.L. Bioactivity of Turmeric-Derived Curcuminoids and Related Metabolites in Breast Cancer. Curr. Pharm. Des. 2013, 19, 6218–6225. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Singh, I.D.; Singh, S.; Kishore, M.; Jha, P.C. Curcumin–Pharmacological Actions and its Role in Oral Submucous Fibrosis: A Review. J. Clin. Diagn. Res. 2015, 9, ZE01–ZE03. [Google Scholar] [CrossRef] [PubMed]
- Ucisik, M.H.; Kupcu, S.; Schuster, B.; Sleytr, U.B. Characterization of CurcuEmulsomes: Nanoformulation for enhanced solubility and delivery of curcumin. J. Nanobiotechnol. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iriti, M.; Vitalini, S.; Fico, G.; Faoro, F. Neuroprotective Herbs and Foods from Different Traditional Medicines and Diets. Molecules 2010, 15, 3517–3555. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Teow, S.-Y.; Liew, K.; Ali, S.A.; Khoo, A.S.; Peh, S.-C. Antibacterial action of curcumin against Staphylococcus aureus: A brief review. J. Trop. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Alwi, I.; Santoso, T.; Suyono, S.; Sutrisna, B.; Suyatna, F.D.; Kresno, S.B.; Ernie, S. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med. Indones. 2008, 40, 201–210. [Google Scholar] [PubMed]
- Liu, Y. Curcumin: An Ingredient that Reduces Platelet Aggregation and Hyperlipidemia, and Enhances Antioxidant and Immune Functions. ACS Symp. Ser. 1997, 660, 199–205. [Google Scholar]
- Kim, D.C.; Ku, S.K.; Bae, J.S. Anticoagulant activities of curcumin and its derivative. BMP Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef]
- Wang, N.P.; Wang, Z.F.; Tootle, S.; Philip, T.; Zhao, Z.Q. Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br. J. Pharmacol. 2012, 167, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Suzuki, K.; Veeraveedu, P.T.; Arumugam, S.; Lakshmanan, A.P.; Sone, H.; Watanabe, K. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov. Today 2013, 18, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Al-Suhaimi, E.A.; Wahid, F.; Shehzad, O.; Shehzad, A. Therapeutic potential of curcumin for multiple sclerosis. Neurol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, S.W.; Lee, G.H.; Choi, M.K.; Jung, H.W.; Kim, Y.J.; Kwon, H.J.; Chae, H.J. Turmeric extract and its active compound, curcumin, protect against chronic CCl4-induced liver damage by enhancing antioxidation. BMC Complement. Altern. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.; Kumar, A.P.; Sethi, G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Hao, J.-K.; Xie, T.; Mukhtar, N.J.; Zhang, W.; Malik, T.H.; Lu, C.-W.; Zhou, D.-D. Curcumin, a Potential Therapeutic Candidate for Anterior Segment Eye Diseases: A Review. Front. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Beevers, C.S.; Huang, S. Pharmacological and clinical properties of curcumin. Botanics 2011. [Google Scholar] [CrossRef]
- Zhao, J.-F.; Ching, L.-C.; Huang, Y.-C.; Chen, C.-Y.; Chiang, A.-N.; Kou, Y.R.; Shyue, S.-K.; Lee, T.-S. Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis. Mol. Nutr. Food Res. 2012, 56, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.A.; Thippeswamy, N.B. Inhibition of human low density lipoprotein oxidation by active principles from spices. Mol. Cell. Biochem. 2002, 229, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Kempaiah, R.K.; Srinivasan, K. Integrity of erythrocytes of hypercholesterolemic rats during spices treatment. Mol. Cell Biochem. 2002, 236, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Tuorkey, M.J. Curcumin a potent cancer preventive agent: Mechanisms of cancer cell killing. Interv. Med. Appl. Sci. 2014, 6, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Y.; Li, C.; Wang, X.; Zhu, R.; Liu, C.; Liu, H.; Wang, L.; Ma, R.; Fu, M.; et al. Recent Advances of Curcumin in the Prevention and Treatment of Renal Fibrosis. BioMed. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Hekmatdoost, A.; Mirmiran, P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int. J Endocrinol. Metab. 2014. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Taghibiglou, C. The mechanisms of action of curcumin in Alzheimer’s disease. J. Alzheimers Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Bu, J.; Zhu, Y.; Xiao, X.; Liang, Z.; Zhang, R. Curcumin improves bone microarchitecture in glucocorticoid-induced secondary osteoporosis mice through the activation of microRNA-365 via regulating MMP-9. Int. J. Clin. Exp. Pathol. 2015, 8, 15684–15695. [Google Scholar] [PubMed]
- Kumar, K.; Rai, A.K. Curcumin: A yellow magical spice of kitchen for treatment of rheumatoid arthritis. Int. Res. J. Pharm. 2011, 2, 29–31. [Google Scholar]
- Shin, S.-K.; Ha, T.-Y.; McGregor, R.A.; Choi, M.-S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011, 55, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Scapagnini, G.; Barbagallo, M. Curcumin, inflammation, ageing and age-related diseases. Immun. Ageing 2010. [Google Scholar] [CrossRef] [PubMed]
- Sundar, D.S.; Antoniraj, M.G.; Kumar, C.S.; Mohapatra, S.S.; Houreld, N.N.; Ruckmani, K. Recent Trends of Biocompatible and Biodegradable Nanoparticles in Drug Delivery: A Review. Curr. Med. Chem. 2016, 23, 3730–3751. [Google Scholar] [CrossRef] [PubMed]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Nanocurcumin: A promising therapeutic advancement over native curcumin. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 331–368. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Akhter, S.; Mohsin, N.; Abdel-Wahab, B.A.; Ahmad, J.; Warsi, M.H.; Rahman, M.; Mallick, N.; Ahmad, F.J. Transformation of curcumin from food additive to multifunctional medicine: Nanotechnology bridging the gap. Curr. Drug Discov. Technol. 2014, 11, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Ameruoso, A.; Palomba, R.; Palange, A.L.; Cervadoro, A.; Lee, A.; Mascolo, D.D.; Decuzzi, P. Ameliorating Amyloid-β Fibrils Triggered Inflammation via Curcumin-Loaded Polymeric Nanoconstructs. Front. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wang, Y.X.; Chow, A.H.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, R.; Ranjani, J.; Rajendhran, J.; Mayandi, J.; Annaraj, J. Enhancing the anti-gastric cancer activity of curcumin with biocompatible and pH sensitive PMMA-AA/ZnO nanoparticles. Mater. Sci. Eng. C 2018, 82, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Khan, S.; Maher, D.M.; Ebeling, M.C.; Sundram, V.; Chauhan, N.; Ganju, A.; Balakrishna, S.; Gupta, B.K.; Zafar, N.; et al. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials 2014, 35, 8635–8648. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Kumar, S.; Kumar, R.; Gaharwar, U.S.; Rajamani, P. PLGA-CTAB curcumin nanoparticles: Fabrication, characterization and molecular basis of anticancer activity in triple negative breast cancer cell lines (MDA-MB-231 cells). Biomed. Pharmacother. 2017, 94, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yi, J.; Zhang, Y.; Yokoyama, W. Fabrication of curcumin-loaded bovine serum albumin (BSA)-dextran nanoparticles and the cellular antioxidant activity. Food Chem. 2018, 239, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Zhu, R.; Liu, Q.; Fei, J.; Wang, S. Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1b transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials 2015, 53, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Zhang, W.; Bao, C.; Xie, Z. Relief of oxidative stress and cardiomyocyte apoptosis by using curcumin nanoparticles. Colloids Surf. B 2017, 153, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, A.K.; Perumal, Y.; Pavurala, N.; Chopra, K.; Mazumder, S. Preparation, characterization and anti-inflammatory effects of curcumin loaded carboxymethyl cellulose acetate butyrate nanoparticles on adjuvant induced arthritis in rats. J. Drug Deliv. Sci. Technol. 2017, 41, 269–279. [Google Scholar] [CrossRef]
- Ahn, J.; Jeong, J.; Lee, H.; Sung, M.-J.; Jung, C.H.; Lee, H.; Hur, J.; Park, J.H.; Jang, Y.J.; Ha, T.Y. Poly(lactic-co-glycolic acid) Nanoparticles Potentiate the Protective Effect of Curcumin against Bone Loss in Ovariectomized Rats. J. Biomed. Nanotechnol. 2017, 13, 688–698. [Google Scholar] [CrossRef]
- Li, M.; Xin, M.; Guo, C.; Lin, G.; Wu, X. New nanomicelle curcumin formulation for ocular delivery: Improved stability, solubility, and ocular anti-inflammatory treatment. Drug Dev. Ind. Pharm. 2017, 43, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Liu, Y.K.; Tsai, N.M.; Hsieh, J.H.; Chen, C.H.; Lin, C.M.; Liao, K.W. A Lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomedicine 2012, 8, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Montalban, M.G.; Coburn, J.M.; Lozano-Perez, A.A.; Cenis, J.L.; Villora, G.; Kaplan, D.L. Production of curcumin-loaded silk fibroin nanoparticles for cancer therapy. Nanomaterials 2018. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Wada, H.; Suzuki, H.; Sasaki, H.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Katanasaka, Y.; Shimatsu, A.; Kimura, T.; et al. A Novel Drug Delivery System of Oral Curcumin Markedly Improves Efficacy of Treatment for Heart Failure after Myocardial Infarction in Rats. Biol. Pharm. Bull. 2012, 35, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Young, N.A.; Wu, L.-C.; Gardner, M.; Hampton, J.; Bruss, M.; Jarjour, W. AB0185 Nano-Emulsified Curcumin (NEC), a Patented Anti-Inflammatory Nutraceutical Compound Developed at Ohio State, Reduces Renal Pathology in an Animal Model of Lupus Nephritis. Ann. Rheum. Dis. 2015, 74, 952–953. [Google Scholar] [CrossRef]
- Chen, X.; Sun, J.; Li, H.; Wang, H.; Lin, Y.; Hu, Y.; Zheng, D. Curcumin-Loaded Nanoparticles Protect Against Rhabdomyolysis-Induced Acute Kidney Injury. Cell. Physiol. Biochem. 2017, 43, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.L.; Baum, L. Highly Stabilized Curcumin Nanoparticles Tested in an in Vitro Blood–Brain Barrier Model and in Alzheimer’s Disease Tg2576 Mice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Grama, C.N.; Suryanarayana, P.; Patil, M.A.; Raghu, G.; Balakrishna, N.; Ravi Kumar, M.N.V.; Reddy, G.B. Efficacy of Biodegradable Curcumin Nanoparticles in Delaying Cataract in Diabetic Rat Model. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.-K.; Moon, H.-J.; Kim, H.-J.; Lee, S.J.; Lee, J.B.; Bae, M.S.; Yi, J.K.; Hwang, Y.S.; Bang, J.B.; et al. Inhibition of Osteoclast Differentiation by Gold Nanoparticles Functionalized with Cyclodextrin Curcumin Complexes. ACS Nano 2014, 8, 12049–12062. [Google Scholar] [CrossRef] [PubMed]
- Effects of Short-Term Curcumin and Multi-Polyphenol Supplementation on the Anti-Inflammatory Properties of HDL (PSI). Available online: https://clinicaltrials.gov/ct2/show/NCT02998918 (accessed on 25 January 2018).
- Curcumin in Preventing Colorectal Cancer in Patients Undergoing Colorectal Endoscopy or Colorectal Surgery. Available online: https://clinicaltrials.gov/ct2/show/record/NCT00973869 (accessed on 25 January 2018).
- The Effect of Diet on Chronic Inflammation and Related Disorders Following Spinal Cord Injury. Available online: https://clinicaltrials.gov/ct2/show/record/NCT02099890 (accessed on 25 January 2018).
- Micro-Particle Curcumin for the Treatment of Chronic Kidney Disease (MPAC-CKD). Available online: https://clinicaltrials.gov/ct2/show/NCT02369549 (accessed on 25 January 2018).
- Effects of Curcumin Supplementation on Lipid Profile and Inflammatory Markers of Patients with Type 2 Diabetes. Available online: https://clinicaltrials.gov/ct2/show/NCT02529969 (accessed on 25 January 2018).
- Safety and Efficacy of Anti-Oxidants and Anti-Inflammatory Agents in Glaucoma and Diabetic Retinopathy. Available online: https://clinicaltrials.gov/ct2/show/NCT02984813 (accessed on 25 January 2018).
- A Pilot Study of Curcumin and Ginkgo for Treating Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00164749 (accessed on 25 January 2018).
- Curcumin in Rheumatoid Arthritis. Available online: https://clinicaltrials.gov/ct2/show/NCT00752154 (accessed on 25 January 2018).
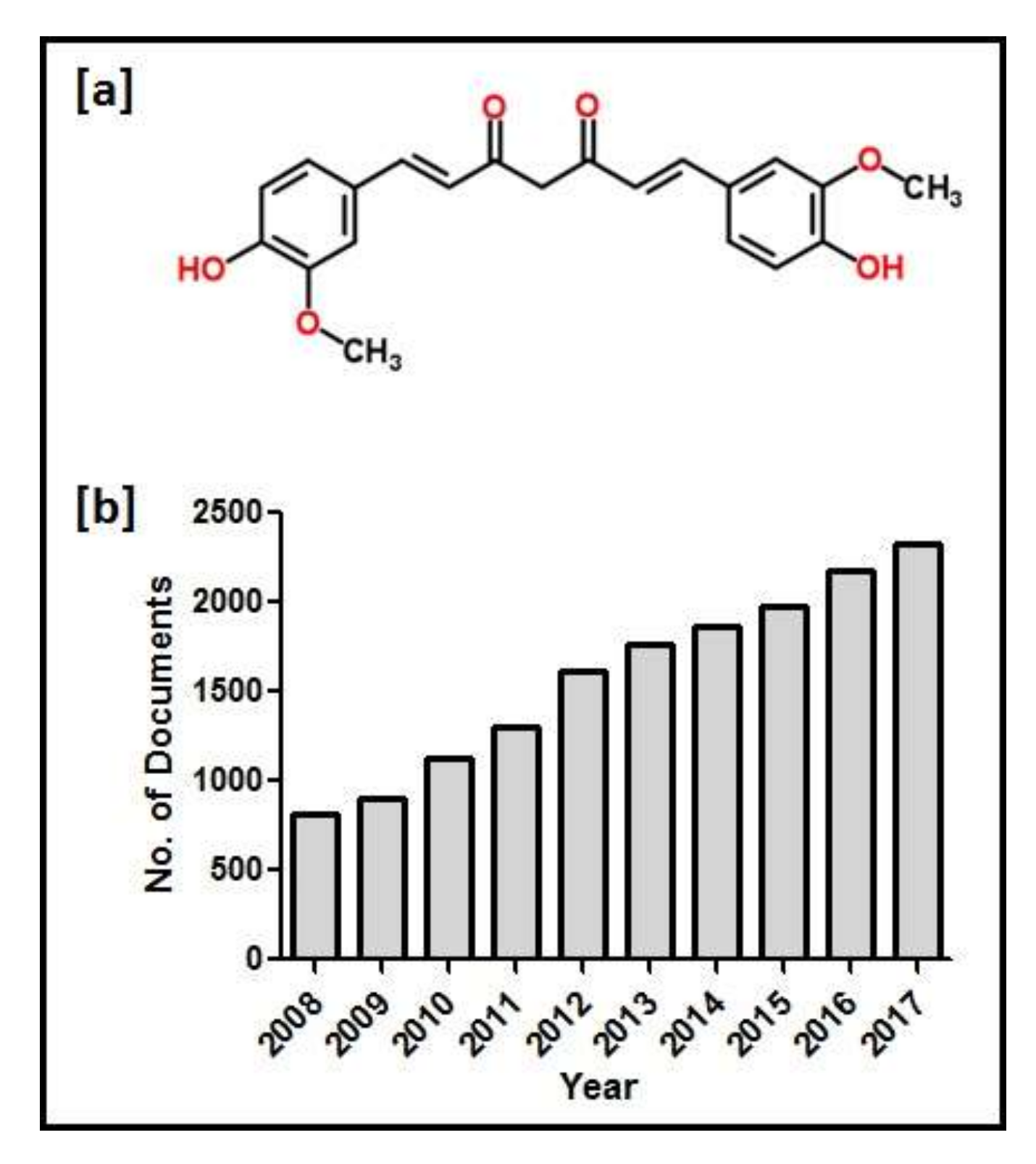
| Aging-Associated Diseases | Mechanism of Action | References |
|---|---|---|
| Atherosclerosis | Reduces cholesterol accumulation; downregulates SR-A and upregulates ABCA1 by a proteasome and LXRα dependent pathway; inhibition of low density lipoprotein oxidation; stabilization of cell membrane cholesterol. | [50,51,52] |
| Cancer | Induction of apoptotic signal; suppression of anti-apoptotic proteins; modulates miRNAs, Wnt/β-Catenin signals, protein kinases, and proteasome activation; inhibition of NF-κB; reverses the multidrug resistance of cancer cells. | [53] |
| Cardiovascular diseases | Anti-oxidant (inhibits eNOS, and iNOS); inhibits sarcoplasmic Ca2+-ATPase; membrane stabilizing effect; prevents Adriamycin-induced cardiomyopathy; prevents diabetic cardiovascular complications; inhibits p300 and NF-κB; decreases serum cholesterol levels. | [16] |
| Chronic inflammation | Alleviates oxidative stress; suppress pro-inflammatory pathways; TNF blocker. | [23] |
| Chronic kidney diseases | Reduces inflammatory molecules MCP-1, NF-κB, TNF-α, IL-1β, COX-2 and cav-1; induces the expression of anti-inflammatory factors such as HO-1, M6PRBP1, and NEDD4. | [54] |
| Diabetes | Reduces oxidative stress; reduction of blood glucose levels; stimulates insulin production. | [55] |
| Hypertension | Reduces AT1R mediated vasoconstriction. | [25] |
| Neurodegenerative diseases | Lowers cholesterol levels; inhibits amyloid-β; modulates microglia; Acetylcholinesterase and Tau inhibition; copper binding; anti-oxidant properties. | [56] |
| Ocular diseases | Neuroprotection in glaucoma; anti-inflammation activities in anterior uveitis and dry eye; anti-allergy in conjunctivitis; anti-proliferation activities and promotes apoptosis in pterygium; inhibits neovascularization; anti-oxidative stress in cataracts; protects epithelial barriers and promotes wound healing in corneal diseases. | [48] |
| Osteoporosis | Suppresses the expression of MMP-9; protective effect against bone deterioration. | [57] |
| Rheumatoid arthritis | Anti-inflammatory activity; inhibits enzymes involved in inflammation (COX-2); downregulates the activation of transcription factor NF-κB and expression of other inflammatory intermediate molecules (TNF-α, adhesion molecules, MMPs, COX-2, and 5-LOX) associated with arthritis. | [58] |
| Aging-Associated Diseases | Therapeutic Potential of Curcumin | Outcomes | References |
|---|---|---|---|
| Atherosclerosis | Anti-atherogenic effect of curcumin via different mechanisms. | The effect of curcumin was studied and compared with the drug lovastatin, and the long-term treatment with curcumin lowered plasma and hepatic cholesterol, and suppressed early atherosclerotic lesions. | [59] |
| Cancer | Curcumin interferes with multiple cell signaling pathways which include cell cycle, apoptosis, proliferation, angiogenesis, invasion, metastasis and inflammation. | The anticancer activity of curcumin was reported against different cancers, and it plays a significant role in different cell signaling pathway and numerous molecular targets. The in vitro, in vivo and clinical studies revealed the therapeutic value of curcumin for the treatment of “old-age“ diseases like cancer. | [60] |
| Cardiovascular diseases | The inflammatory effects of curcumin may play a key role in the prevention of cardiovascular diseases. | Curcumin showed the possibility of preventing atrial arrhythmias and some ventricular arrhythmias. | [16] |
| Chronic inflammation | Curcumin can suppress both acute and chronic inflammation by scavenging reactive oxygen species and enhancing antioxidant defences. | Curcumin shows very good antioxidant properties, and it plays a key role in the prevention and treatment of chronic inflammation. | [23,61] |
| Chronic kidney diseases | Curcumin increases the expression of intestinal alkaline phosphatase and tight junction proteins. | The study shows the potential anti-inflammatory effects of curcumin and their positive effects for the treatment of chronic kidney diseases. | [24] |
| Diabetes | Curcumin reduces glycemia and hyperlipidemia in rodent models, and favourably affects some leading aspects of diabetes which include insulin resistance, hyperglycemia and islet apoptosis and necrosis. | Curcumin is actively involved in the prevention and treatment of diabetes. The study showed that curcumin and their complexes can successfully be used for the treatment of various disorders associated with diabetes such as liver disorders, adipose tissue dysfunction, diabetic neuropathy, diabetic nephropathy, diabetic vascular disease, and other complications associated with diabetes. | [20] |
| Hypertension | Curcumin shows beneficial effects on hypertension; it prevents the development of hypertension by regulating AT1 receptor expression. | The study was performed using curcumin in an Ang II-induced hypertensive model, and it showed that curcumin aids in the downregulation of the AT1 receptor in A10 cells and it subsequently prevents hypertension. | [25] |
| Neurodegenerative diseases | Curcumin has reported to be an effective neuroprotective agent and it may prevent aging associated changes in cellular proteins. | Protein homeostasis plays an important role in aging-associated diseases. The study performed in an invertebrate model (Caenorhabditis elegans) showed that the drug curcumin aids to maintain protein homeostasis and increases the life span of the model organism. Several animal model studies showed that curcumin prevents or delays various neurodegenerative diseases. | [14] |
| Ocular diseases | Curcumin exhibits potential therapeutic activity against several ocular diseases. | Curcumin showed beneficial effects in the prevention and treatment of several ocular diseases. The dosage of curcumin, up to 8g/day for three months, does not produce any dose-limiting toxicity in pharmacological studies. Clinical data proved that the few weeks of curcumin treatment reduced the signs and symptoms of eye discomfort and is safe in the treatment of humans. | [26] |
| Osteoporosis | Curcumin may be a potential candidate for the treatment of osteoporosis. | The protective effects of curcumin were studied against dexamethasone induced osteoporosis in a rat model. The results proved that curcumin effectively prevented glucocorticoid- induced osteoporosis. | [27] |
| Rheumatoid arthritis | Curcumin possess various pharmacological activities including antiarthritic effects. | The effect of curcumin was studied in an adjuvant-induced arthritis rat model, and it showed similar therapeutic effects for the treatment of rheumatoid arthritis compared with the drug methotrexate. | [22] |
| Aging-Associated Diseases | Detail of Nanoparticles | Size (nm) | Outcomes | References |
|---|---|---|---|---|
| Cancer | Curcumin loaded cationic liposome- polyethylene glycol (PEG) and poly (ethylene imine) complex | 270 nm | Curcumin liposomes showed five-fold cytotoxic activity on curcumin-sensitive cells and twenty-fold against curcumin-resistant cells compared to native curcumin, and achieved 45 ± 0.2% curcumin encapsulation efficiency in the liposome complex. In vivo studies showed that the administration of curcumin liposomes inhibited 60–90% of tumor growth. | [77] |
| Cancer | Curcumin loaded silk fibroin nanoparticles | 155–170 nm | Curcumin loaded silk fibroin nanoparticles were synthesized using both physical adsorption (drug loading content of 6.63 ± 0.09% and encapsulation efficiency of 53.75 ± 0.81%) and co-precipitation (drug loading content of 2.47 ± 0.11% and encapsulation efficiency of 48.84 ± 2.67%). The synthesized material showed excellent antitumor activity against both Hep3B and Kelly cells. | [78] |
| Cardiovascular diseases | Colloidal nanoparticles (Curcumin with gum ghatti solution) | 190 nm | A small amount of synthesized colloidal curcumin nanoparticles could be more therapeutically effective for heart failure than native curcumin. | [79] |
| Chronic inflammation | Nano-emulsified curcumin (NEC) | - | NEC was orally supplied to NZM2410 mice (lupus nephritis model) and kidney function was monitored by testing blood urea nitrogen. Results suggested that NEC has a good therapeutic potential in the treatment of chronic inflammation and other autoimmune diseases. | [80] |
| Chronic kidney diseases | Curcumin nanoparticles | 80–100 nm | The in vitro and in vivo study suggested that the curcumin nanoparticles enhanced the treatment efficacy of Rhabdomyolysis induced acute kidney injury than free curcumin. The release study was performed using dialysis. The initial 20 h of dialysis showed that upto 40% of curcumin nanoparticles were released and 80% of free curcumin was released. Nanoparticulate curcumin achieved prolonged release profile than free curcumin. | [81] |
| Neurodegenerative diseases | Nanocurcumin (Curcumin loaded p(PEG-poly-lactic acid) micelles | 80 nm | There was improved bioavailability of nanocurcumin in the brains of Tg2576 mice as compared to free curcumin, and nanocurcumin showed positive effects in the treatment of Alzheimer’s disease. The entrapment efficiency of curcumin was almost 100% and the loading efficiency of curcumin was 37.6%. | [82] |
| Ocular diseases | Curcumin encapsulated PLGA nanoparticles | 282.50 ± 5.72 nm | The study demonstrated the potential of curcumin encapsulated nanoparticles in managing diabetic cataracts in a streptozotocin induced diabetic rat cataract model. The enhanced performance of nanocurcumin was observed in different biochemical pathways than free curcumin. It may be due to the improved oral bioavailability of curcumin. | [83] |
| Osteoporosis | Gold nanoparticles functionalized with cyclodextrin curcumin complexes | 36.3 nm | Loading efficiency of curcumin was around 38.95% and the curcumin loaded nanoparticle complex showed an effective intracellular uptake and acts as a potential therapeutic agent in the treatment of bone diseases associated with excessive bone resorption. | [84] |
| Rheumatoid arthritis | Curcumin nanoemulsion (curcumin, solutol-HS 15, soybean oil) | 150 nm | In vivo rat study showed that the synthesized curcumin nanoemulsion acts as an effective antiarthritic agent. The oral route of curcumin nanoemulsion showed threefold increase of AUC (area under the curve) and Cmax value than the suspension (intravenous (iv) route) and it significantly enhanced the drug absorption than free curcumin. Overall, the in vivo rat study suggested that the nanoform of curcumin aids to convert the therapy route from iv to oral administration for the effective treatment of RA therapy | [22] |
| Keywords Used | No. of Studies Found |
|---|---|
| Atherosclerosis-curcumin | 1 |
| Cancer-curcumin | 57 |
| Cardiovascular diseases-curcumin | 9 |
| Chronic inflammation-curcumin | 2 |
| Chronic kidney diseases-curcumin | 5 |
| Diabetes-curcumin | 11 |
| Hypertension-curcumin | 1 |
| Neurodegenerative diseases-curcumin | 6 |
| Ocular diseases-curcumin | 4 |
| Osteoporosis-curcumin | 0 |
| Rheumatoid arthritis-curcumin | 2 |
| ClinicalTrials.gov Identifier | Ages (Years) | Disease | Doses of Curcumin | Phase | Reference |
|---|---|---|---|---|---|
| NCT02998918 | 18 to 60 (Adult) | Inflammation Atherosclerosis Cardiovascular Disease | 500 mg of curcumin phytosome twice daily for 1 week | 2 | [85] |
| NCT00973869 | 18 and older (Adult, Senior) | Colorectal cancer | Oral curcumin once daily for 14–28 days | 1 | [86] |
| NCT02099890 | 18 and older (Adult, Senior) | Neuropathic pain; Depression; Cognitive impairment; Somatic neuropathy; Autonomic dysfunction | InflanNox capsule (400 mg curcumin) taken 3 times daily along with other anti-inflammatory supplements | 3 | [87] |
| NCT02369549 | 18 and older (Adult, Senior) | Chronic kidney disease | Three 30 mg capsules of micro-particle curcumin daily in the morning | 3 | [88] |
| NCT02529969 | 40 to 65 (Adult) | Non-insulin dependent diabetes | 500 mg curcumin capsule | 2 | [89] |
| NCT02984813 | 18 and older (Adult, Senior) | Open-angle glaucoma diabetic retinopathy | Two pills daily (one contains curcumin and other contains active compounds) for 3 months | 1 | [90] |
| NCT00164749 | 50 and older (Adult, Senior) | Alzheimer’s disease | Two different dosages (1 g/day and 4 g/day) along with ginkgo extract | 2 | [91] |
| NCT00752154 | 18 and older (Adult, Senior) | Rheumatoid arthritis | Curcumin (Longvida™) 4 capsules approximately 2 g/day for 2 weeks, then the dose will be increased to 4 capsules twice a day (4 g/day) | Early phase 1 | [92] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundar Dhilip Kumar, S.; Houreld, N.N.; Abrahamse, H. Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules 2018, 23, 835. https://doi.org/10.3390/molecules23040835
Sundar Dhilip Kumar S, Houreld NN, Abrahamse H. Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules. 2018; 23(4):835. https://doi.org/10.3390/molecules23040835
Chicago/Turabian StyleSundar Dhilip Kumar, Sathish, Nicolette Nadene Houreld, and Heidi Abrahamse. 2018. "Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases" Molecules 23, no. 4: 835. https://doi.org/10.3390/molecules23040835






