PEG 400/Cerium Ammonium Nitrate Combined with Microwave-Assisted Synthesis for Rapid Access to Beta-Amino Ketones. An Easy-to-Use Protocol for Discovering New Hit Compounds
Abstract
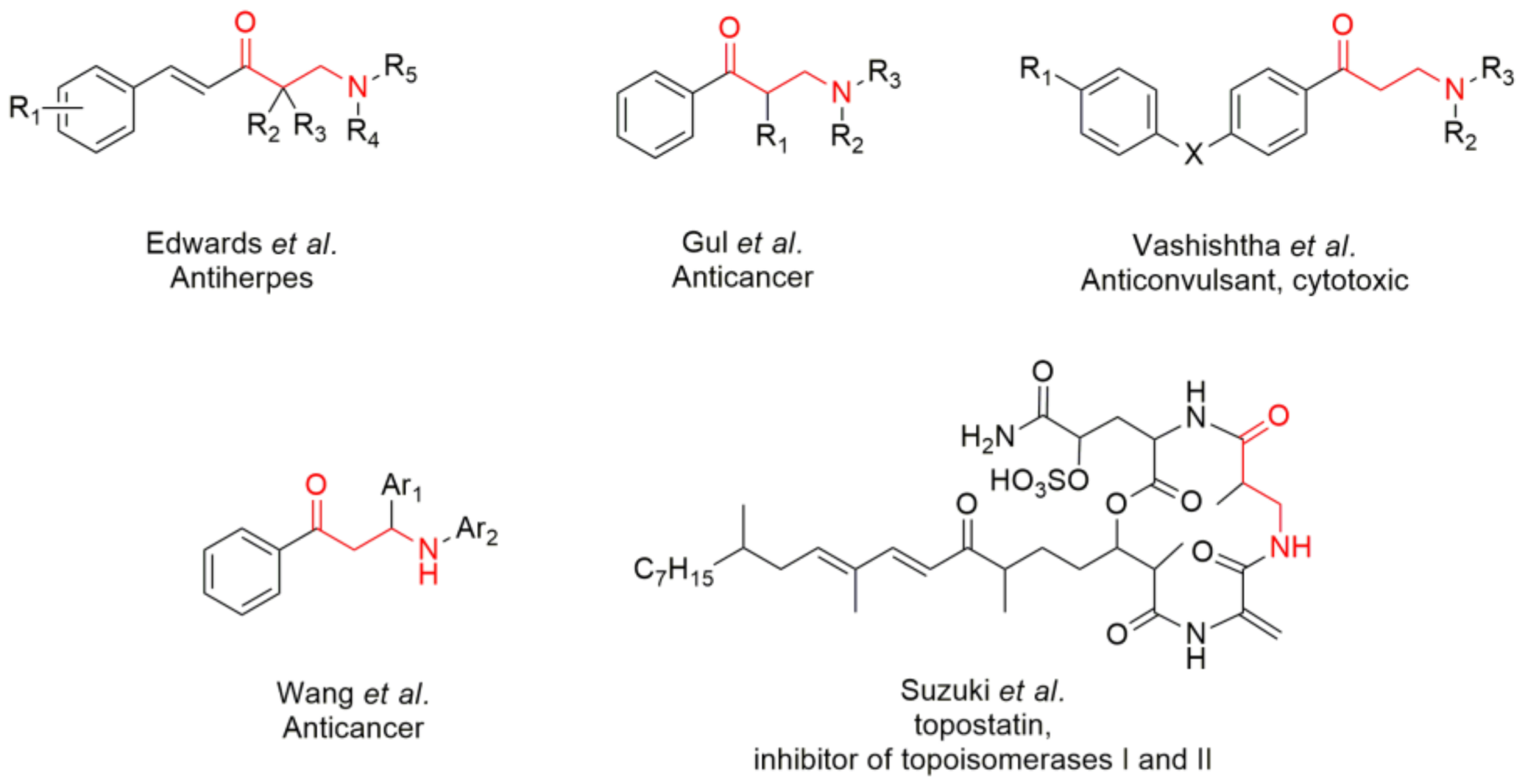
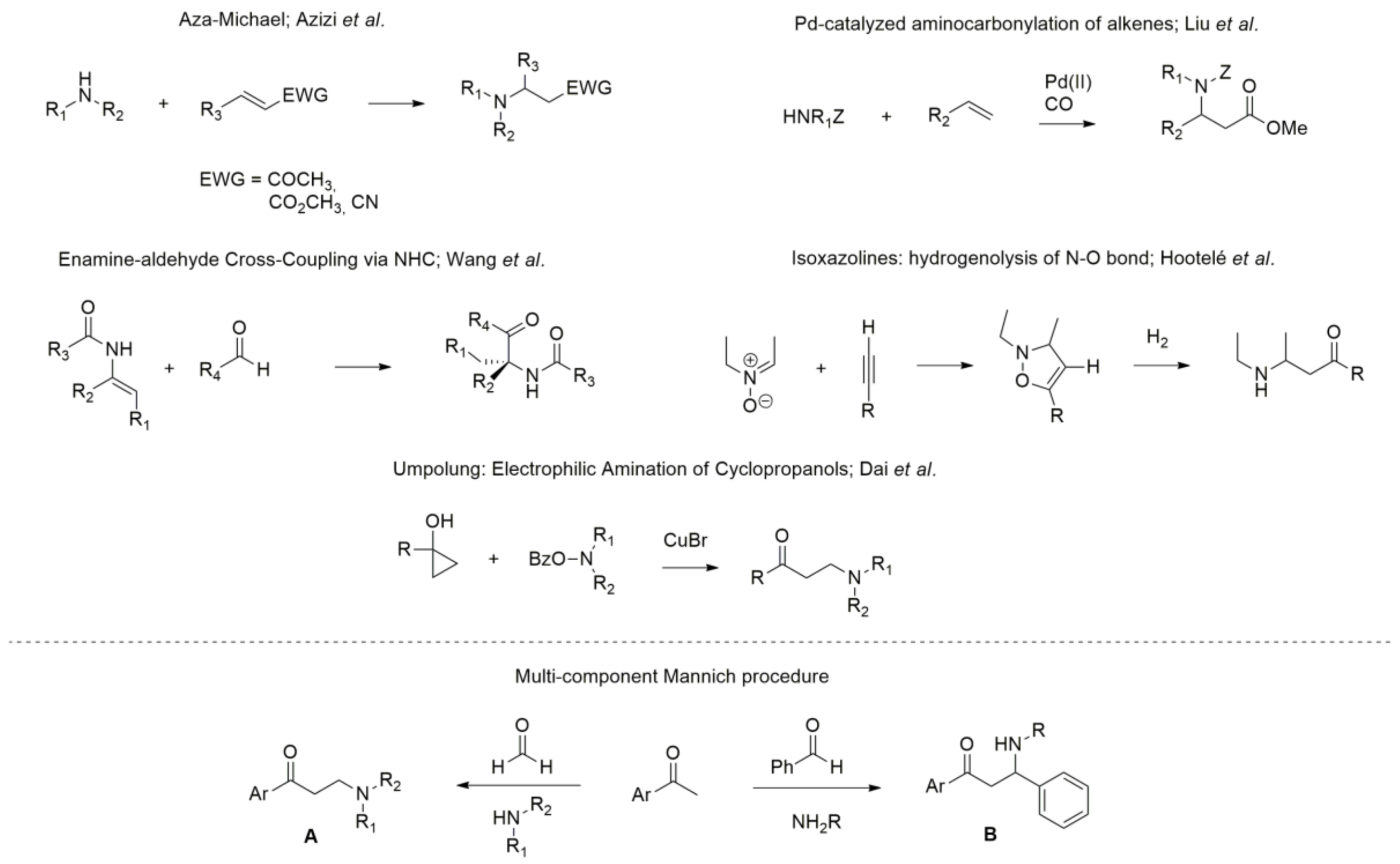
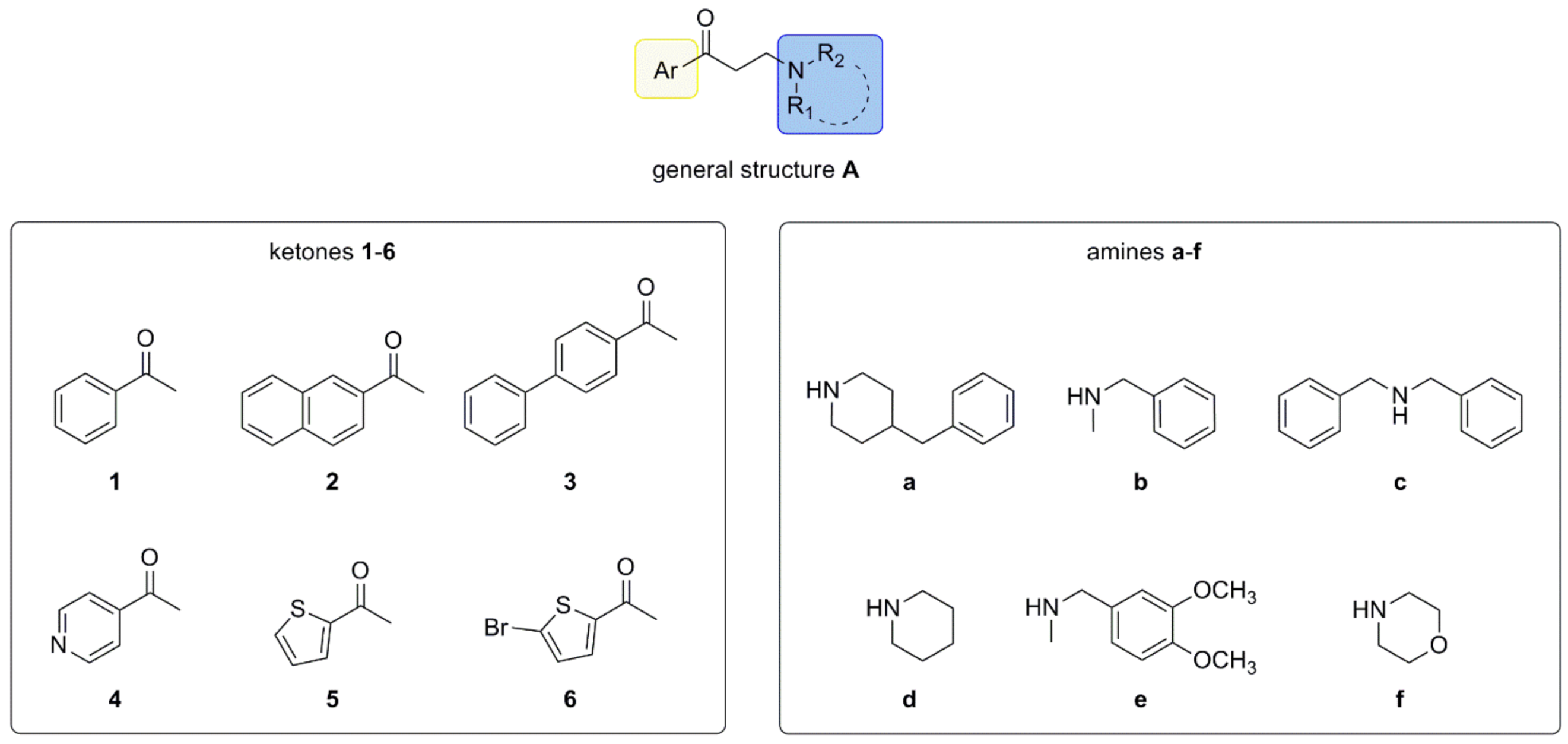
:1. Introduction
2. Results and Discussion
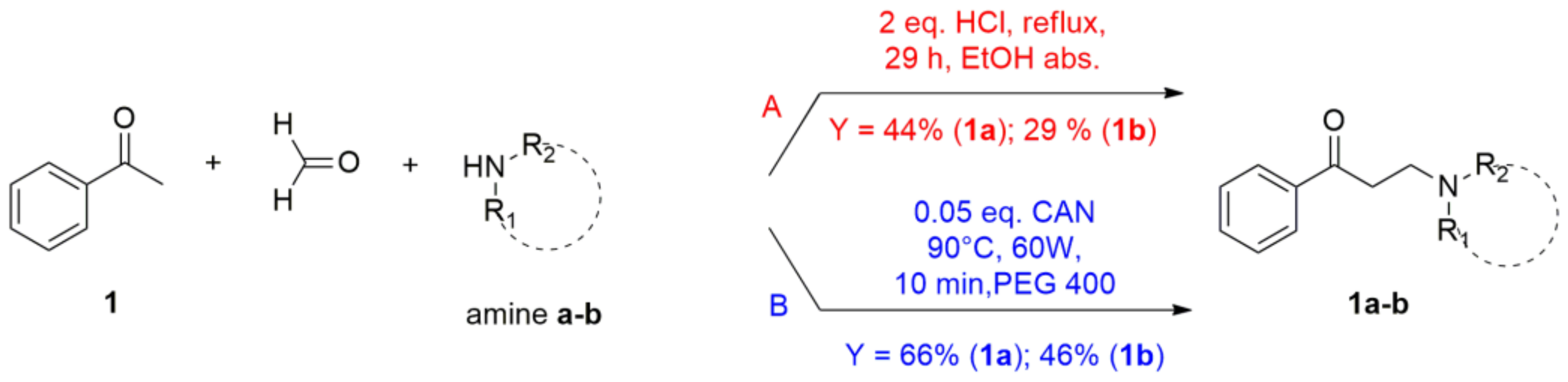
2.1. Setup and Optimization of Synthetic Protocol
2.2. MW-Assisted Library Synthesis
3. Materials and Methods
3.1. General Procedure for the Synthesis of β-Aminoketone 1a and 1b under Conventional Heating (Method A)
3.2. General Procedure for the Synthesis of β-Aminoketones 1a–6f under Microwave Heating (Method B)
3.3. Analytical Data of Prepared Compounds
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Time (Minutes) | % Phosphate Buffer | % Acetonitrile |
|---|---|---|
| 0 | 90 | 10 |
| 3 | 90 | 10 |
| 10 | 60 | 40 |
| 13 | 60 | 40 |
| 20 | 5 | 95 |
| 25 | 90 | 10 |
| 35 | 90 | 10 |
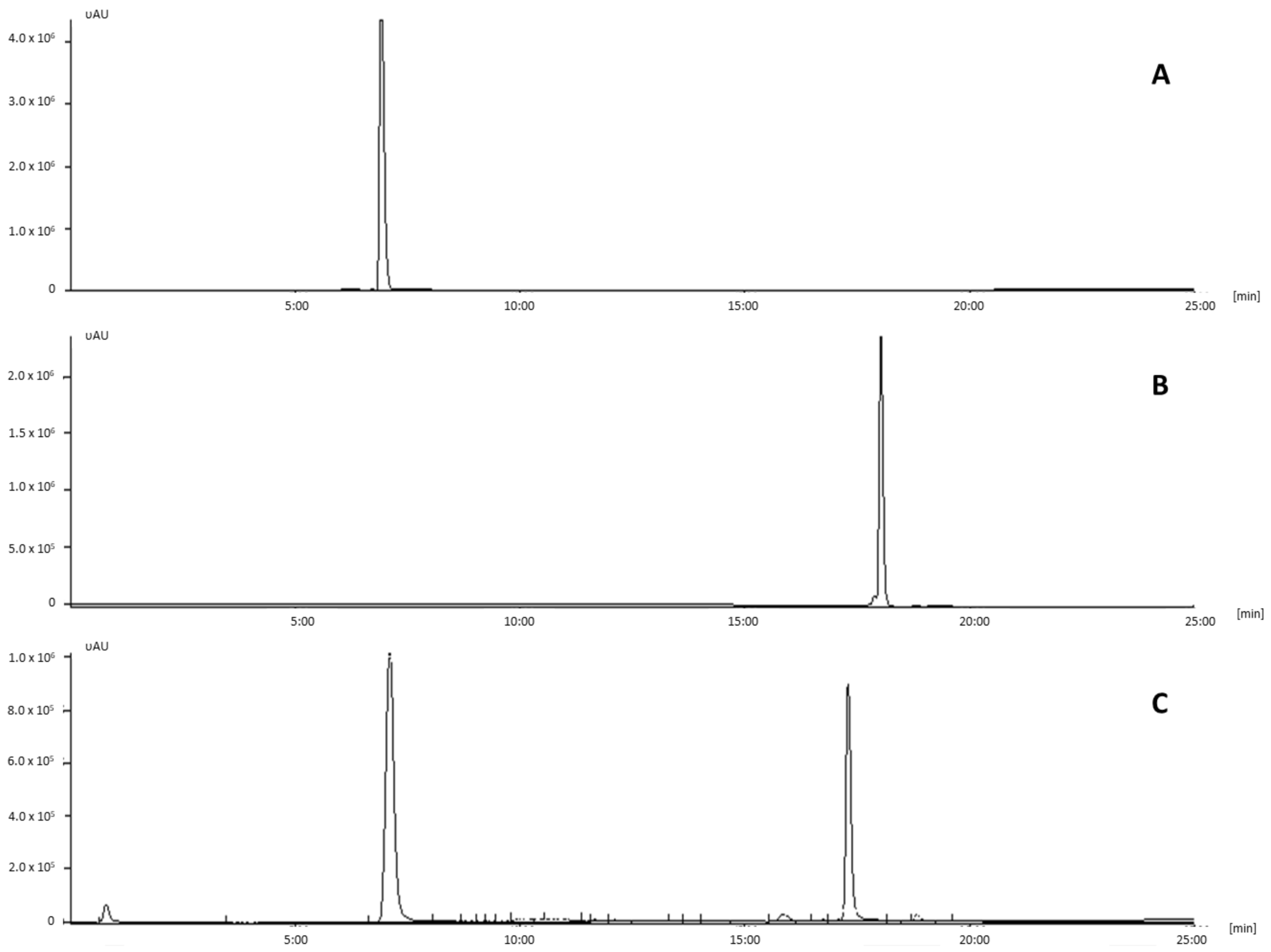
| Time (Minutes) | % Phosphate Buffer | % Acetonitrile |
|---|---|---|
| 0 | 90 | 10 |
| 3 | 90 | 10 |
| 10 | 60 | 40 |
| 13 | 60 | 40 |
| 20 | 5 | 95 |
| 30 | 5 | 95 |
| 35 | 90 | 10 |
| 40 | 90 | 10 |
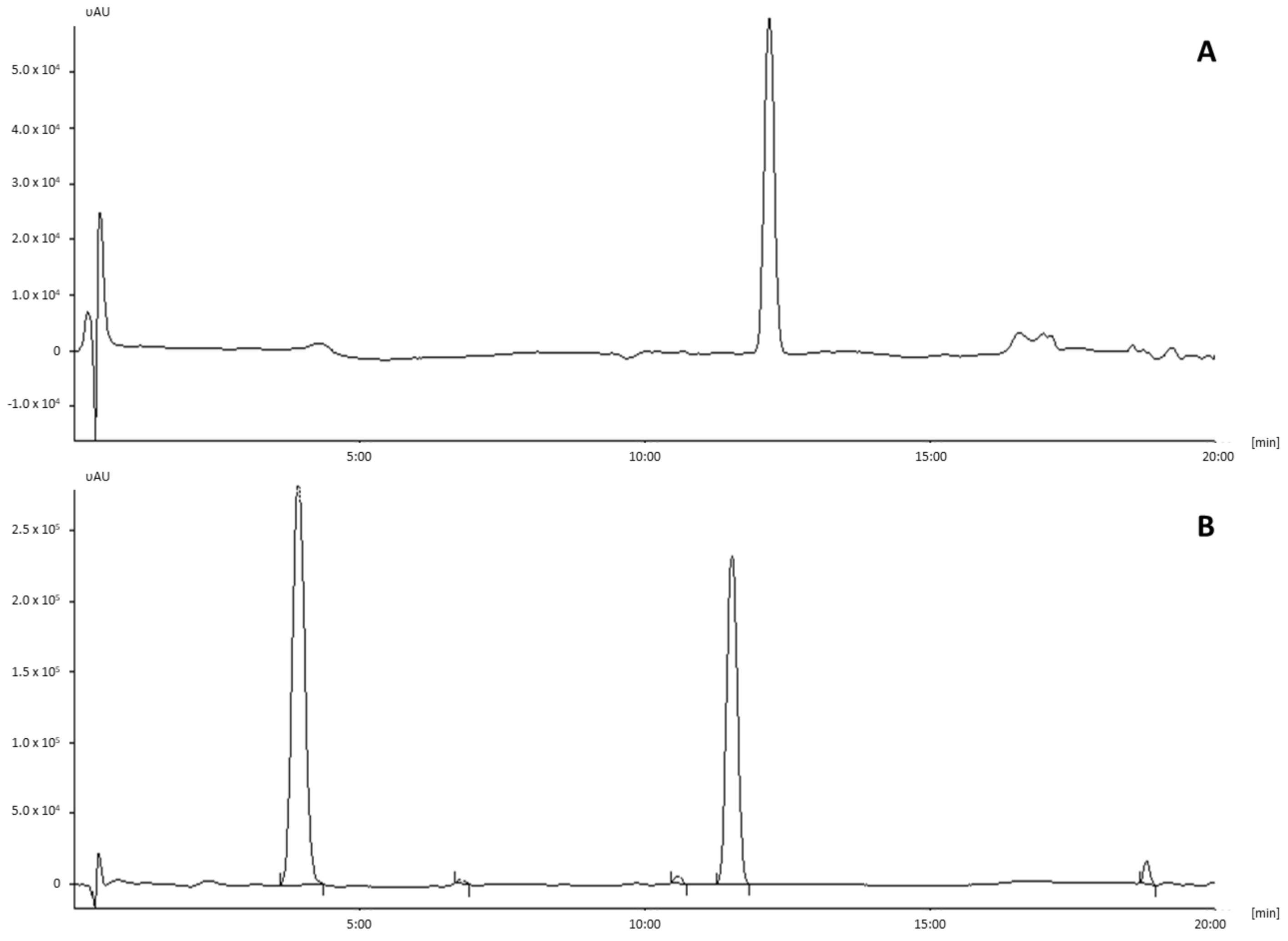
References
- Bala, S.; Sharma, N.; Kajal, A.; Kamboj, S.; Saini, V. Mannich Bases: An Important Pharmacophore in Present Scenario. Int. J. Med. Chem. 2014, 2014, 191072. [Google Scholar] [CrossRef] [PubMed]
- Roman, G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2014, 89, 743–816. [Google Scholar] [CrossRef] [PubMed]
- Kalluraya, B.; Chimbalkar, R.M.; Hegde, J.C. Anticonvulsant activity of nicotinyl/isonicotinyl substituted 1,2,4-triazol-5-thione Mannich bases. Indian J. Heterocycl. Chem. 2005, 15, 15–18. [Google Scholar]
- Köksal, M.; Gökhan, N.; Küpeli, E.; Yesilada, E.; Erdogan, H. Analgesic and antiinflammatory activities of some new Mannich bases of 5-nitro-2-benzoxazolinones. Arch. Pharm. Res. 2007, 30, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Ashok, M.; Holla, B.S.; Poojary, B. Convenient one pot synthesis and antimicrobial evaluation of some new Mannich bases carrying 4-methylthiobenzyl moiety. Eur. J. Med. Chem. 2007, 42, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, S.N.; Sriram, D.; Nath, G.; De Clercq, E. Synthesis, antibacterial, antifungal and anti-HIV activities of norfloxacin Mannich bases. Eur. J. Med. Chem. 2000, 35, 249–255. [Google Scholar] [CrossRef]
- Edwards, M.L.; Ritter, H.W.; Stemerick, D.M.; Stewart, K.T. Mannich bases of 4-phenyl-3-buten-2-one: A new class of antiherpes agent. J. Med. Chem. 1983, 26, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shukla, S.K.; Singh, M. Synthesis and biological activity of sulphadiazine Schiff’s bases of isatin and their N-mannich bases. Asian J. Chem. 2007, 19, 5013–5018. [Google Scholar]
- Malinka, W.; Świątek, P.; Filipek, B.; Sapa, J.; Jezierska, A.; Koll, A. Synthesis, analgesic activity and computational study of new isothiazolopyridines of Mannich base type. Farmaco 2005, 60, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.I.; Vepsalainen, J.; Gul, M.; Erciyas, E.; Hanninen, O. Cytotoxic activities of mono and bis Mannich bases derived from acetophenone against Renca and Jurkat cells. Pharm. Acta Helv. 2000, 74, 393–398. [Google Scholar] [CrossRef]
- Vashishtha, S.C.; Zello, G.A.; Nienaber, K.H.; Balzarini, J.; De Clercq, E.; Stables, J.P.; Dimmock, J.R. Cytotoxic and anticonvulsant aryloxyaryl Mannich bases and related compounds. Eur. J. Med. Chem. 2004, 39, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, Y.; Momekov, G.; Petrov, O.; Karaivanova, M.; Kalcheva, V. Cytotoxic Mannich bases of 6-(3-aryl-2-propenoyl)-2(3H)-benzoxazolones. Eur. J. Med. Chem. 2007, 42, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Y.; Zhou, C.; Liu, Q.; Lu, H.; Ge, Y.; Wang, M. Development of β-amino-carbonyl compounds as androgen receptor antagonists. Acta Pharmacol. Sin. 2014, 35, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Lelais, G.; Seebach, D. β2-amino acids-syntheses, occurrence in natural products, and components of β-peptides1,2. Biopolymers 2004, 76, 206–243. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D.; Beck, A.K.; Bierbaum, D.J. The World of Beta- and Gamma-Peptides Comprised of Homologated Proteinogenic Amino Acids and Other Components. Chem. Biodivers. 2004, 1, 1111–1239. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D.; Matthews, J.L.J. β-Peptides: A surprise at every turn. Chem. Commun. 1997, 21, 2015–2022. [Google Scholar] [CrossRef]
- Suzuki, K.; Nagao, K.; Monnai, Y.; Yagi, A.; Uyeda, M. Topostatin, a Novel Inhibitor of Topoisomerases I and II Produced by Thermomonospora alba Strain No. 1520 III. Inhibitory Properties. J. Antibiot. 1998, 51, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-X.; Qiu, L.-Q.; Yip, C.W.; Chan, A.S.C. A convenient, one-step synthesis of optically active tertiary aminonaphthol and its applications in the highly enantioselective alkenylations of aldehydes. J. Org. Chem. 2003, 68, 1589–1590. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-J.J.; Youssef, D.; Cameron, T.S.; Jha, A. Microwave-assisted synthesis of novel 2-naphthol bis-Mannich Bases. Arkivoc 2008, 2008, 165–177. [Google Scholar] [CrossRef]
- Azizi, N.; Baghi, R.; Ghafuri, H.; Boloutchian, M.; Hashemi, M. Silicon Tetrachloride Catalyzed Aza-Michael Addition of Amines to Conjugated Alkenes under Solvent-Free Conditions. Synlett 2010, 379–382. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, C.; Wang, J. Enantioselective Intermolecular Enamide−Aldehyde Cross-Coupling Catalyzed by Chiral N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2016, 138, 4706–4709. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Dai, M. An Umpolung Strategy for the Synthesis of β-Aminoketones via Copper-Catalyzed Electrophilic Amination of Cyclopropanols. Org. Lett. 2015, 17, 2190–2193. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Qi, X.; Li, M.; Chen, P.; Liu, G. Palladium-Catalyzed Intermolecular Aminocarbonylation of Alkenes: Efficient Access of β-Amino Acid Derivatives. J. Am. Chem. Soc. 2015, 137, 2480–2483. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, V.; Hootelé, C. A new efficient synthesis of β-aminoketones via Δ4-isoxazolines. Tetrahedron Lett. 1988, 29, 5917–5918. [Google Scholar] [CrossRef]
- Tramontini, M. Advances in the chemistry of Mannich bases. Synthesis 1973, 1973, 703–775. [Google Scholar] [CrossRef]
- Arend, M.; Westermann, B.; Risch, N. Modern Variants of the Mannich Reaction. Angew. Chem. Int. Ed. 1998, 37, 1044–1070. [Google Scholar] [CrossRef]
- Filho, J.F.A.; Lemos, B.C.; de Souza, A.S.; Pinheiro, S.; Greco, S.J. Multicomponent Mannich reactions: General aspects, methodologies and applications. Tetrahedron 2017, 73, 6977–7004. [Google Scholar] [CrossRef]
- List, B. The Direct Catalytic Asymmetric Three-Component Mannich Reaction. J. Am. Chem. Soc. 2000, 122, 9336–9337. [Google Scholar] [CrossRef]
- Rossi, D.; Rui, M.; Di Giacomo, M.; Schepmann, D.; Wünsch, B.; Monteleone, S.; Liedl, K.R.; Collina, S. Gaining in pan-affinity towards sigma 1 and sigma 2 receptors. SAR studies on arylalkylamines. Bioorg. Med. Chem. 2017, 25, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Rossi, D.; Pignataro, L.; Bigogno, C.; Canta, A.; Oggioni, N.; Malacrida, A.; Corbo, M.; Cavaletti, G.; Peviani, M.; et al. Toward the identification of neuroprotective agents: G-scale synthesis, pharmacokinetic evaluation and CNS distribution of (R)-RC-33, a promising SIGMA1 receptor agonist. Future Med. Chem. 2016, 8, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Rossi, D.; Marra, A.; Paolillo, M.; Schinelli, S.; Curti, D.; Tesei, A.; Cortesi, M.; Zamagni, A.; Laurini, E.; et al. Synthesis and biological evaluation of new aryl-alkyl(alkenyl)-4-benzylpiperidines, novel Sigma Receptor (SR) modulators, as potential anticancer-agents. Eur. J. Med. Chem. 2016, 124, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Urbano, M.; Pedralia, A.; Serra, M.; Zampieri, D.; Mamolo, M.G.; Laggner, C.; Zanette, C.; Florio, C.; Schepmann, D.; et al. Design, synthesis and SAR analysis of novel selective σ1 ligands (Part 2). Bioorg. Med. Chem. 2010, 18, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Cobos, E.J.; del Pozo, E.; Baeyens, J.M. Irreversible blockade of sigma-1 receptors by haloperidol and its metabolites in guinea pig brain and SH-SY5Y human neuroblastoma cells. J. Neurochem. 2007, 102, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Albayrak, Y.; Hashimoto, K. Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders. Adv. Exp. Med. Biol. 2017, 964, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Blicke, F.F. Mannich Reaction. Org. React. 1942, 1, 303–341. [Google Scholar]
- Azzolina, O.; Collina, S.; Urbano, M.; Fata, E.; Loddo, G.; Linati, L.; Lanza, E.; Barbieri, A. Highly diastereoselective synthesis of enantiopure naphthylaminoalcohols with analgesic properties. Chirality 2006, 18, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, F.; Pilotti, Å.; Luthman, K. Efficient large scale microwave assisted Mannich reactions using substituted acetophenones. Mol. Divers. 2003, 7, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kabalka, G.W.; Zhou, L.L.; Wang, L.; Pagni, R.M. A microwave-enhanced, solventless Mannich condensation of terminal alkynes and secondary amines with para-formaldehyde on cuprous iodide doped alumina. Tetrahedron 2006, 62, 857–867. [Google Scholar] [CrossRef]
- Kidwai, M.; Bhatnagar, D.; Mishra, N.K.; Bansal, V. CAN catalyzed synthesis of b-amino carbonyl compounds via Mannich reaction in PEG. Catal. Commun. 2008, 9, 2547–2549. [Google Scholar] [CrossRef]
- Flynn, G.A.; Lee, S.A.; Faris, M.; Brandt, D.W.; Chakravarty, S. Preparation of Aryl Ketone Derivatives as Intracellular Kinase Inhibitors. U.S. Patent WO 2007136790 A2, 27 November 2007. [Google Scholar]
- Jie, X.; Shang, Y.; Zhang, X.; Su, W. Cu-Catalyzed Sequential Dehydrogenation-Conjugate Addition for β-Functionalization of Saturated Ketones: Scope and Mechanism. J. Am. Chem. Soc. 2016, 138, 5623–5633. [Google Scholar] [CrossRef] [PubMed]
- Istanbullu, H.; Erzurumlu, Y.; Kirmizibayrak, P.B.; Erciyas, E. Evaluation of Alkylating and Intercalating Properties of Mannich Bases for Cytotoxic Activity. Lett. Drug Des. Discov. 2014, 11, 1096–1106. [Google Scholar] [CrossRef]
- Debernardis, J.F.; Kerkman, D.J.; Zinkowski, R.P. Preparation of Substituted (1-aryl-3-piperazin-1′-yl)Propanone Antibiotics, Antimycotics and Antineoplastics. U.S. Patent 6,214,994B1, 10 April 2001. [Google Scholar]
- Yamada, T.; Sakamoto, K.; Watanabe, K.; Nakano, Y. Process for the Preparation of 1-(2-Thienyl)-3-alkylaminopropanols. U.S. Patent WO 2006104249 A1, 5 October 2006. [Google Scholar]
- Mikoshiba, K.; Hamada, K.; Terauchi, A.; Ozaki, S.; Goto, J.I.; Ebisui, E.; Suzuki, A. Transglutaminase-Induced Abnormal Protein Crosslinking Inhibitors Containing Ketone Compounds and Use Thereof. U.S. Patent WO 2011055561 A1, 12 May 2011. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Entry | 1 (eq.) | a (eq.) | % Conversion | % Purity |
|---|---|---|---|---|
| 1 | 1 | 1 | 80.0 | 82.5 |
| 2 | 1 | 1.5 | 76.7 | 77.8 |
| 3 | 1 | 2 | 79.9 | 67.5 |
| 4 | 1 | 2.5 | 78.5 | 60.7 |
| 5 | 1.5 | 1 | 67.1 | 68.9 |
| 6 | 2 | 1 | 72.4 | 97.7 |
| 7 | 2.5 | 1 | 77.2 | 96.1 |
| 8 | 3 | 1 | 67.7 | 95.8 |
| Entry | 1 (eq.) | b (eq.) | % Conversion | % Purity |
|---|---|---|---|---|
| 9 | 1 | 1 | 80.0 | 60.1 |
| 10 | 1 | 2 | 77.2 | 35.1 |
| 11 | 1 | 2.5 | 86.9 | 34.2 |
| 12 | 1 | 3 | 93.4 | 23.5 |
| 13 | 1 | 3.5 | 64.9 | 23.5 |
| 14 | 1.5 | 1 | 81.5 | 69.4 |
| 15 | 2 | 1 | 74.5 | 81.1 |
| 16 | 3 | 1 | 61.6 | 89.6 |
| 17 | 3.5 | 1 | 49.2 | 92.2 |
| Compound | Ar | NR1R2 | Yield % a | Purity % a |
|---|---|---|---|---|
| 1a | phenyl | 4-benzylpiperidine | 72 | 98 |
| 1b | N-benzylmethylamine | 70 | 86 | |
| 1d | piperidine | 58 | 75 | |
| 1e | 3,4-dimethoxy-N-methylbenzylamine | 33 | 66 | |
| 1f | morpholine | - | Traces (5) | |
| 2a | naphtyl | 4-benzylpiperidine | 32 | 69 |
| 2b | N-benzylmethylamine | 34 | 81 | |
| 2d | piperidine | 50 | 75 | |
| 2e | 3,4-dimethoxy-N-methylbenzylamine | 38 | 54 | |
| 2f | morpholine | 46 | 71 | |
| 3a | biphenyl | 4-benzylpiperidine | 75 | 75 |
| 3b | N-benzylmethylamine | 55 | 67 | |
| 3d | piperidine | 50 | 73 | |
| 3e | 3,4-dimethoxy-N-methylbenzylamine | - | Traces (5) | |
| 3f | morpholine | 50 | 61 | |
| 5a | 2-thienyl | 4-benzylpiperidine | 63 | 95 |
| 5b | N-benzylmethylamine | 56 | 85 | |
| 5d | piperidine | 30 | 83 | |
| 5e | 3,4-dimethoxy-N-methylbenzylamine | 25 | 66 | |
| 5f | morpholine | 32 | 77 | |
| 6a | 5-bromo-2-thienyl | 4-benzylpiperidine | 55 | 76 |
| 6b | N-benzylmethylamine | 40 | 37 | |
| 6d | piperidine | 29 | 57 | |
| 6e | 3,4-dimethoxy-N-methylbenzylamine | n.r. | - | |
| 6f | morpholine | 6 | 38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossino, G.; Raimondi, M.V.; Rui, M.; Di Giacomo, M.; Rossi, D.; Collina, S. PEG 400/Cerium Ammonium Nitrate Combined with Microwave-Assisted Synthesis for Rapid Access to Beta-Amino Ketones. An Easy-to-Use Protocol for Discovering New Hit Compounds. Molecules 2018, 23, 775. https://doi.org/10.3390/molecules23040775
Rossino G, Raimondi MV, Rui M, Di Giacomo M, Rossi D, Collina S. PEG 400/Cerium Ammonium Nitrate Combined with Microwave-Assisted Synthesis for Rapid Access to Beta-Amino Ketones. An Easy-to-Use Protocol for Discovering New Hit Compounds. Molecules. 2018; 23(4):775. https://doi.org/10.3390/molecules23040775
Chicago/Turabian StyleRossino, Giacomo, Maria Valeria Raimondi, Marta Rui, Marcello Di Giacomo, Daniela Rossi, and Simona Collina. 2018. "PEG 400/Cerium Ammonium Nitrate Combined with Microwave-Assisted Synthesis for Rapid Access to Beta-Amino Ketones. An Easy-to-Use Protocol for Discovering New Hit Compounds" Molecules 23, no. 4: 775. https://doi.org/10.3390/molecules23040775






