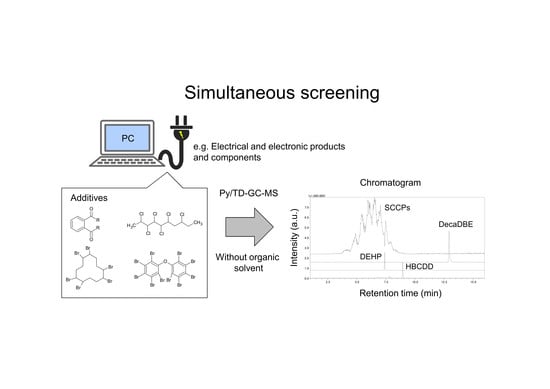
Simultaneous Screening of Major Flame Retardants and Plasticizers in Polymer Materials Using Pyrolyzer/Thermal Desorption Gas Chromatography Mass Spectrometry (Py/TD–GC–MS)
Abstract
:1. Introduction
2. Results
2.1. MS Quantification
2.2. Method Optimization
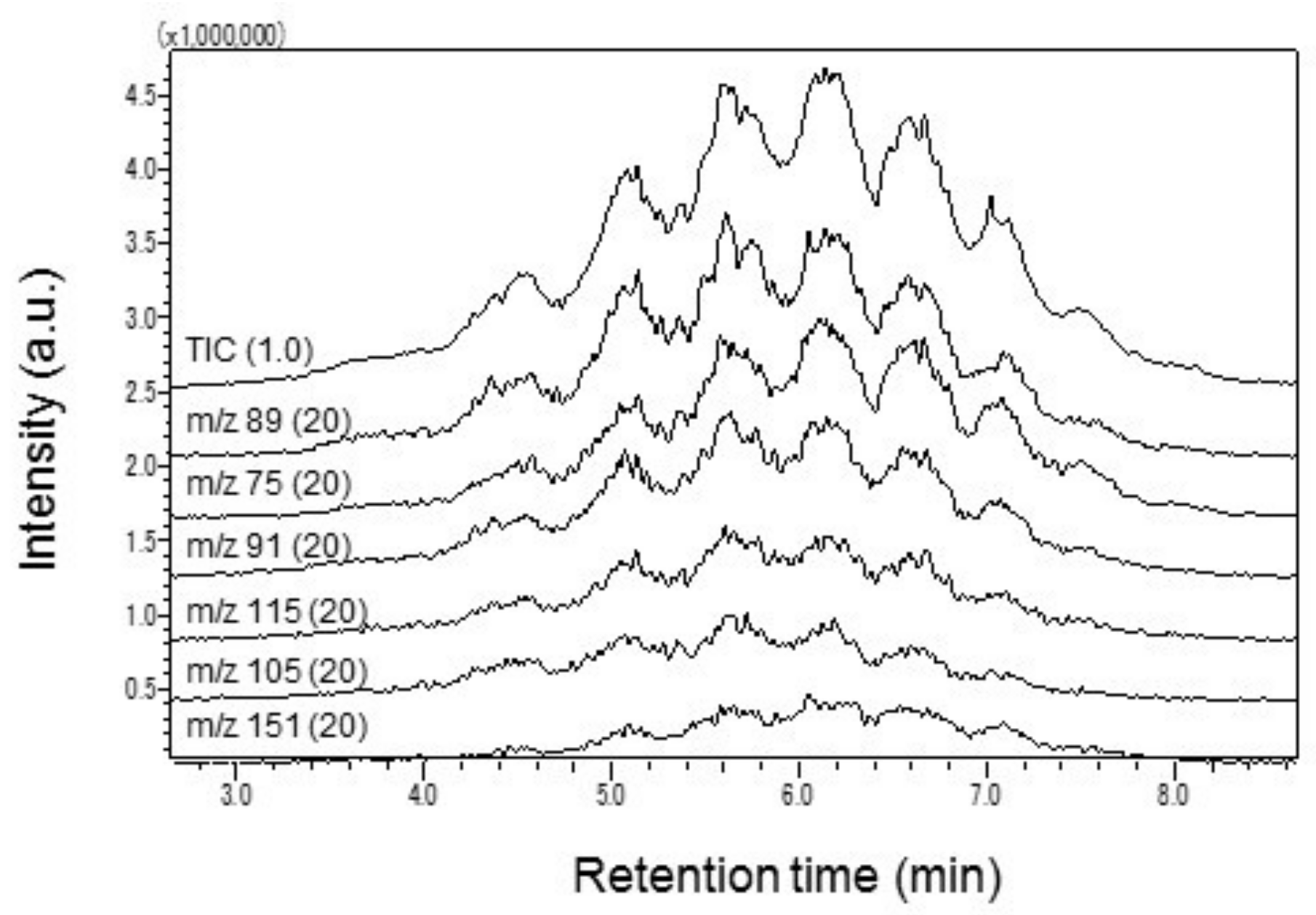
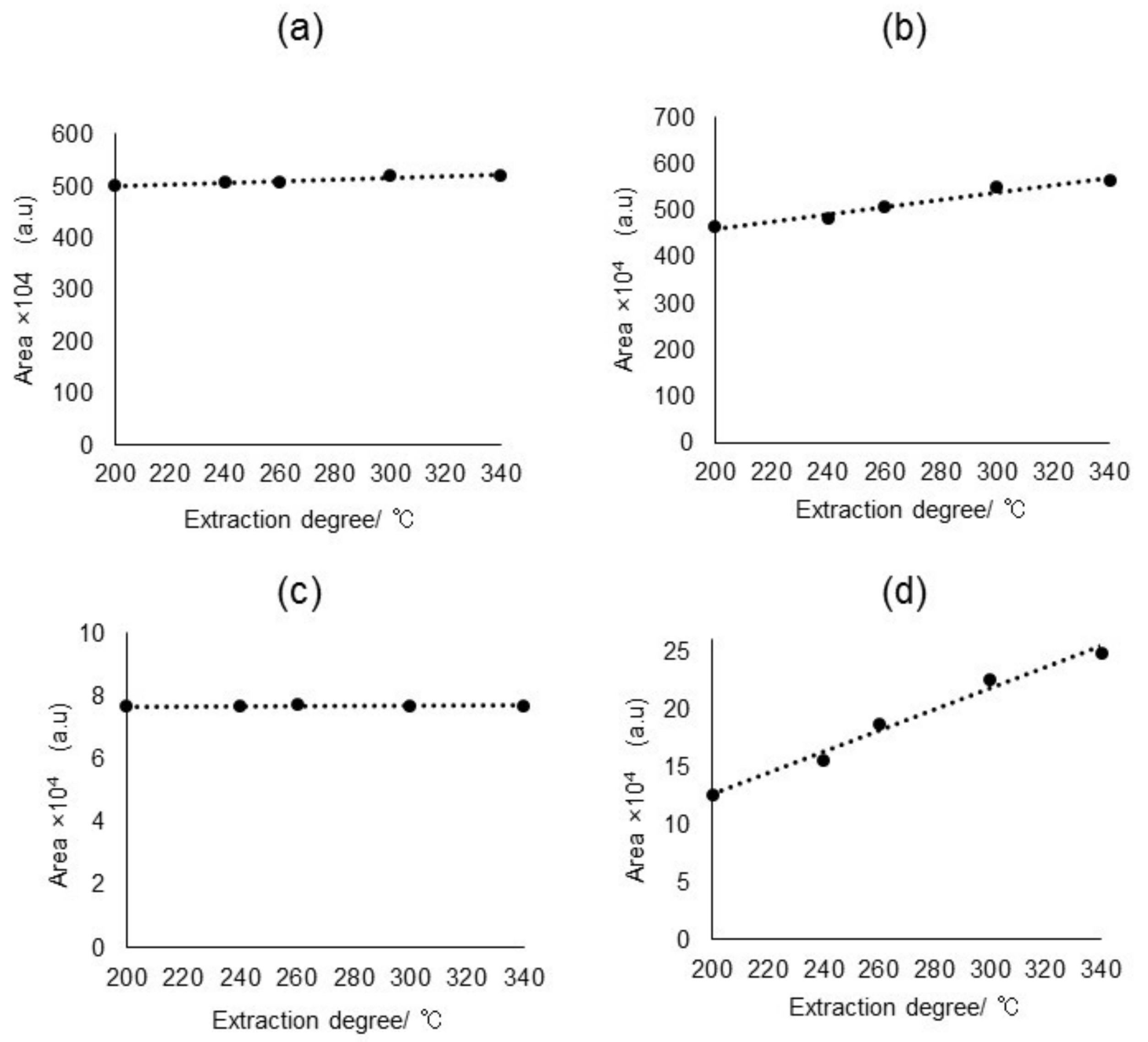
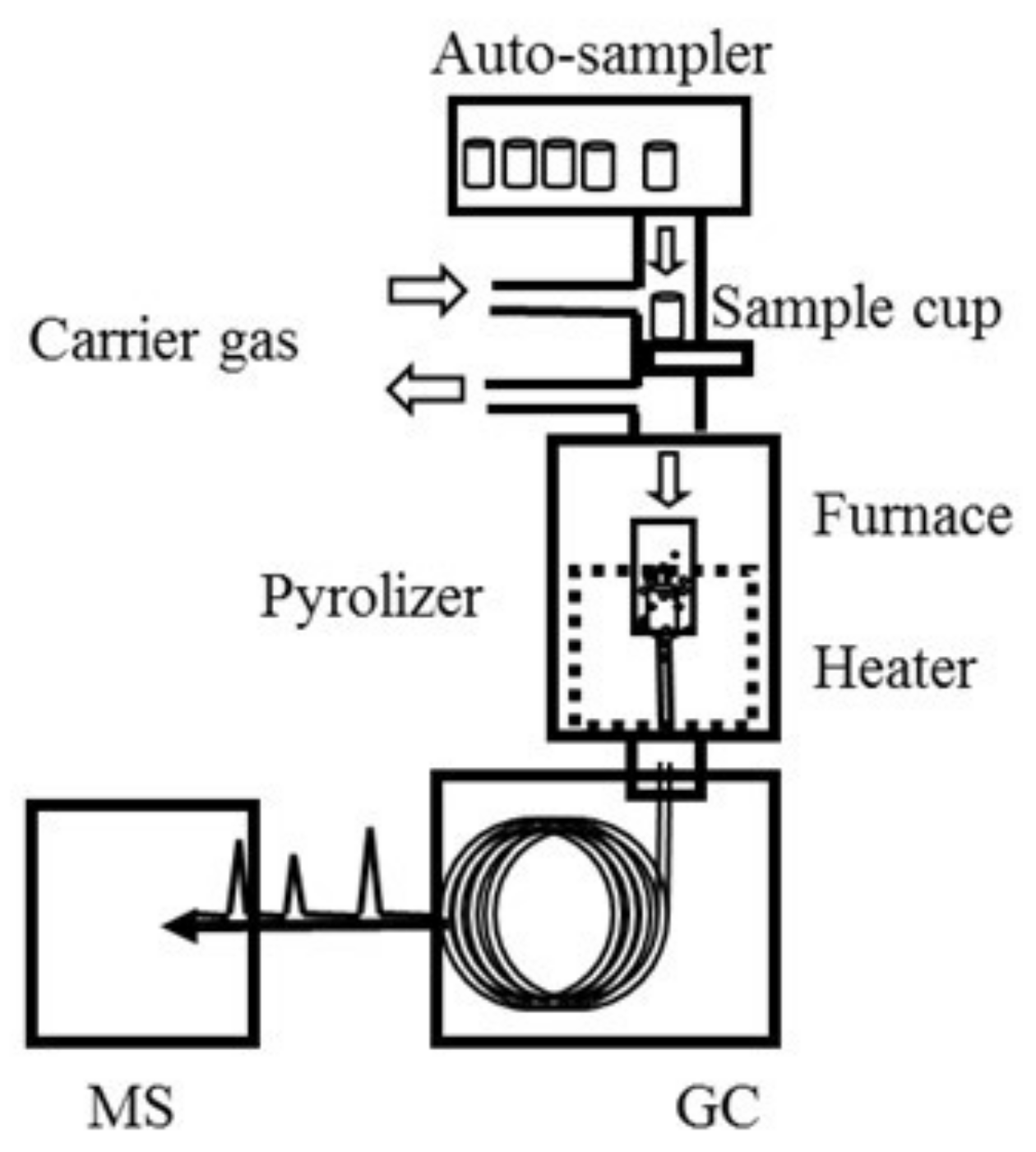
2.2.1. Py/TD Process
2.2.2. GC Conditions
2.3. Calibration Curves and Detection Limits
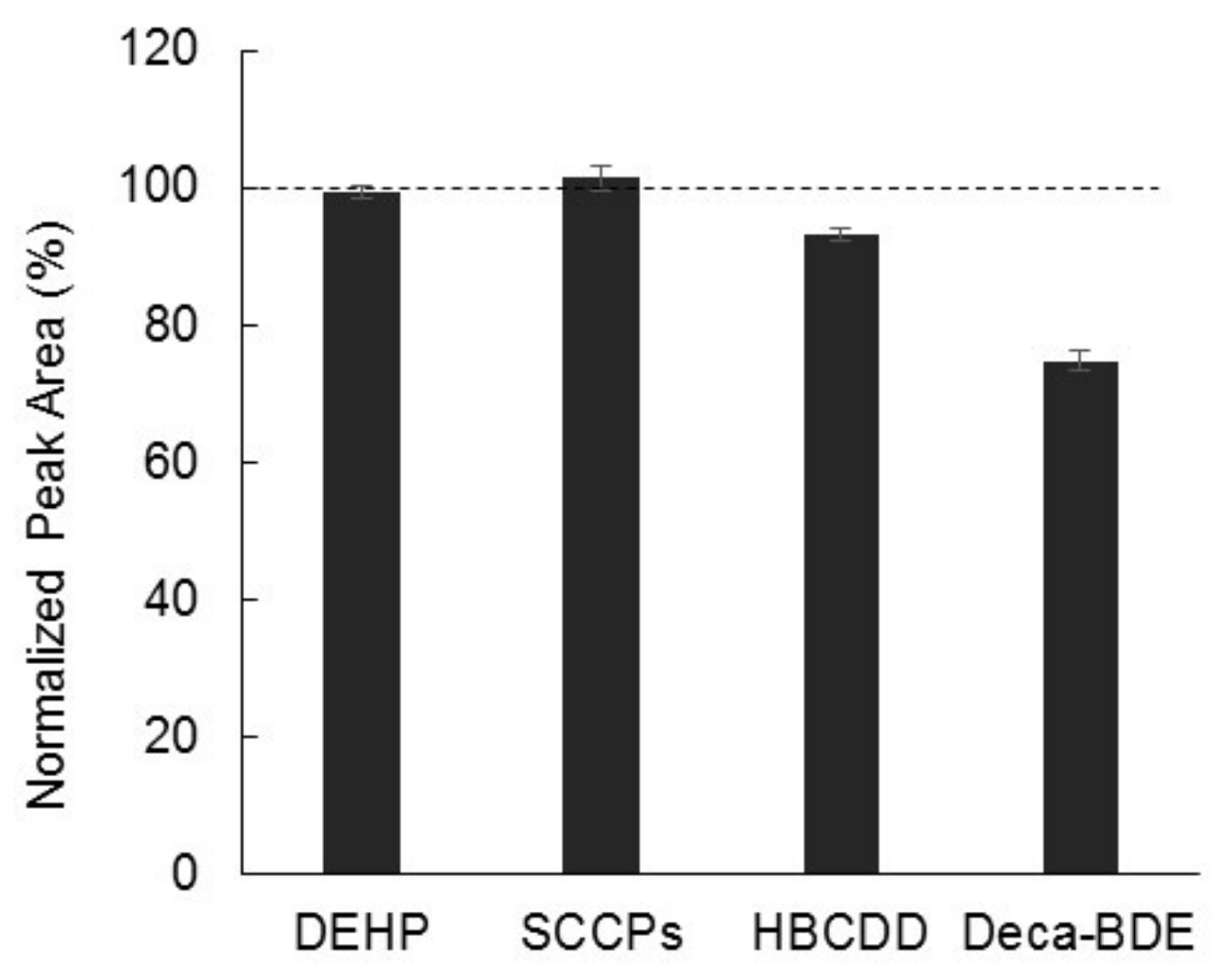
2.4. Analysis of Complex Samples
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Preparation of Mixture Samples for Simultaneous Analysis
4.3. Instrument Setup
4.4. Py/TD-GC–MS Parameter Settings
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koch, H.M.; Preuss, R.; Angerer, J.D. Di (2-ethylhexyl) phthalate (DEHP): Human metabolism and internal exposure—An update and latest results. Int. J. Androl. 2006, 29, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.E.-S.; Legler, J. Overview of the mammalian and environmental toxicity of chlorinated paraffins. In Chlorinated Paraffins; Springer: Berlin, Germany, 2010; pp. 135–154. [Google Scholar]
- Covaci, A.; Gerecke, A.C.; Law, R.J.; Voorspoels, S.; Kohler, M.; Heeb, N.V.; Leslie, H.; Allchin, C.R.; De Boer, J. Hexabromocyclododecanes (HBCDs) in the environment and humans: A review. Environ. Sci. Technol. 2006, 40, 3679–3688. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.D.; Berger, M.L.; Weijs, L.; Covaci, A. Tissue-specific accumulation of polybrominated diphenyl ethers (PBDEs) including deca-BDE and hexabromocyclododecanes (HBCDs) in harbor seals from the Northwest Atlantic. Environ. Int. 2012, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- The European Parliament and The Council of The European Union. Regulation (EC) no 850/2004 of the European Parliament and of the Council of 29 April 2004 on Persistent Organic Pollutants and Amending Directive 79/117/eec. Off. J. Eur. Union 2004, 158, 7–49. [Google Scholar]
- The European Parliament and The Council of The European Union. Regulation (EC) no 1907/2006 of the European Parliament and of the Council of 18 December 2006, Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Off. J. Eur. Union 2007, 136, 3–280. [Google Scholar]
- The European Parliament and The Council of The European Union. Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the Restriction of the use of Certain Hazardous Substances in Electrical and Electronic Equipment. Off. J. Eur. Union 2011, 174, 88–110. [Google Scholar]
- The European Commission. Commission Delegated Directive (EU) 2015/863 of 11 March 2015, Amending Annex II to Directive 2011/65/EU of the European Parliament and of the Council as Regards the List of Restricted Substances. Off. J. Eur. Union 2015, 137, 10–12. [Google Scholar]
- Stringer, R.; Labunska, I.; Santillo, D.; Johnston, P.; Siddorn, J.; Stephenson, A. Concentrations of phthalate esters and identification of other additives in PVC children’s toys. Environ. Sci. Pollut. Res. 2000, 7, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lovekamp-Swan, T.; Davis, B.J. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003, 111, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Darnerud, P.O. Toxic effects of brominated flame retardants in man and in wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef]
- World Health Organization International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC Press: Lyon, France, 1990; Volume 48, pp. 55–72. [Google Scholar]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Bart, J.C. Additives in Polymers: Industrial Analysis and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- International Electrotechnical Commission. IEC 62321-8, Determination of Certain Substances in Electrotechnical Products—Part 8: Phthalates in Polymers by Gas Chromatography-Mass Spectrometry (GC-MS), Gas Chromatography-Mass Spectrometry Using a Pyrolyzer/Thermal Desorption Accessory (Py/TD-GC-MS); IEC Central Office: Geneva, Switzerland, 2017; pp. 1–70. [Google Scholar]
- Maruyama, F.; Fujimaki, S.; Sakamoto, Y.; Kudo, Y.; Miyagawa, H. Screening of phthalates in polymer materials by pyrolysis GC/MS. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2015, 31, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Sobeih, K.L.; Baron, M.; Gonzalez-Rodriguez, J. Recent trends and developments in pyrolysis-gas chromatography. J. Chromatogr. A 2008, 1186, 51–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuzawa, T.; Watanabe, C.; Freeman, R.R.; Tsuge, S. Rapid and simple determination of phthalates in plastic toys by a thermal desorption-GC/MS method. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2009, 25, 1057–1058. [Google Scholar] [CrossRef]
- Rial-Otero, R.; Galesio, M.; Capelo, J.-L.; Simal-Gándara, J. A review of synthetic polymer characterization by pyrolysis–GC–MS. Chromatographia 2009, 70, 339–348. [Google Scholar] [CrossRef]
- Tsuge, S.; Ohtani, H.; Watanabe, C. Pyrolysis-GC/MS Data Book of Synthetic Polymers: Pyrograms, Thermograms and MS of Pyrolyzates; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Jansson, K.D.; Zawodny, C.P.; Wampler, T.P. Determination of polymer additives using analytical pyrolysis. J. Anal. Appl. Pyrolysis 2007, 79, 353–361. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, Y.M.; Moon, H.M.; Hosaka, A.; Watanabe, C.; Teramae, N.; Choe, E.K.; Myung, S.W. Comparative study of thermal desorption and solvent extraction-gas chromatography-mass spectrometric analysis for the quantification of phthalates in polymers. J. Chromatogr. A 2016, 1451, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Gomez, A.; de Boer, J.; Leonards, P.E. Novel analytical methods for flame retardants and plasticizers based on gas chromatography, comprehensive two-dimensional gas chromatography, and direct probe coupled to atmospheric pressure chemical ionization-high resolution time-of-flight-mass spectrometry. Anal. Chem. 2013, 85, 9572–9580. [Google Scholar] [PubMed]
- Kim, Y.M.; Kim, J.W.; Moon, H.M.; Lee, M.J.; Hosaka, A.; Watanabe, A.; Teramae, N.; Park, Y.K.; Myung, S.W. Rapid quantification of N-methyl-2-pyrrolidone in polymer matrices by thermal desorption-GC/MS. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2017, 33, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Bart, J. Polymer/additive analysis by flash pyrolysis techniques. J. Anal. Appl. Pyrolysis 2001, 58, 3–28. [Google Scholar] [CrossRef]
- The European Commission. Commission Regulation (EU) no 519/2012 of 19 June 2012, Amending Regulation (EC) no 850/2004 of the European Parliament and of the Council on Persistent Organic Pollutants as regards Annex I. Off. J. Eur. Union 2012, 159, 1–4. [Google Scholar]
- The European Commission. Commission Regulation (EU) 2015/2030 of 13 November 2015, Amending Regulation (EC) no 850/2004 of the European Parliament and of the Council on Persistent Organic Pollutants as Regards Annex I. Off. J. Eur. Union 2015, 298, 1–3. [Google Scholar]
- Fiedler, H. Short-chain chlorinated paraffins: Production, use and international regulations. In Chlorinated Paraffins; Springer: Berlin, Germany, 2010; pp. 1–40. [Google Scholar]
- Tomy, G.T. Analysis of chlorinated paraffins in environmental matrices: The ultimate challenge for the analytical chemist. In Chlorinated Paraffins; Springer: Berlin, Germany, 2009; pp. 83–106. [Google Scholar]
- Larsen, E.R.; Ecker, E.L. Thermal stability of fire retardants:* III, decomposition of pentabromochlorocyclohexane and hexabromocyclododecane under processing conditions. J. Fire Sci. 1988, 6, 139–159. [Google Scholar] [CrossRef]
- Takasuga, T.M.H.; Nouda, C.; Harada, K.; Koizumi, A. Analysis of short-chain chlorinated paraffins (SCCPs) by GC-HRMS (NCI) with GC-HRToF-MS applied food sample. In Proceedings of the 31st International Symposium on Halogenated Persistent Organic Pollutants (DIOXIN 2011), Brussels, Belgium, 21–25 August 2011; Volume 73, pp. 981–984. [Google Scholar]
- Castells, P.; Santos, F.J.; Galceran, M.T. Evaluation of three ionisation modes for the analysis of chlorinated paraffins by gas chromatography/ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Junk, S.A.; Meisch, H.U. Determination of chlorinated paraffins by GC-MS. Fresenius’ J. Anal. Chem. 1994, 347, 361–364. [Google Scholar] [CrossRef]
- Zencak, Z.; Reth, M.; Oehme, M. Determination of total polychlorinated n-alkane concentration in biota by electron ionization-MS/MS. Anal. Chem. 2004, 76, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Eric, R.L.; Ernest, L.E. Thermal stability of fire retardants: I, hexabromocyclododecane (hbcd. J. Fire Sci. 1986, 4, 261–275. [Google Scholar]
- Pellizzato, F.; Ricci, M.; Held, A.; Emons, H. Analysis of short-chain chlorinated paraffins: A discussion paper. J. Environ. Monit. JEM 2007, 9, 924–930. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds not available from the authors. |



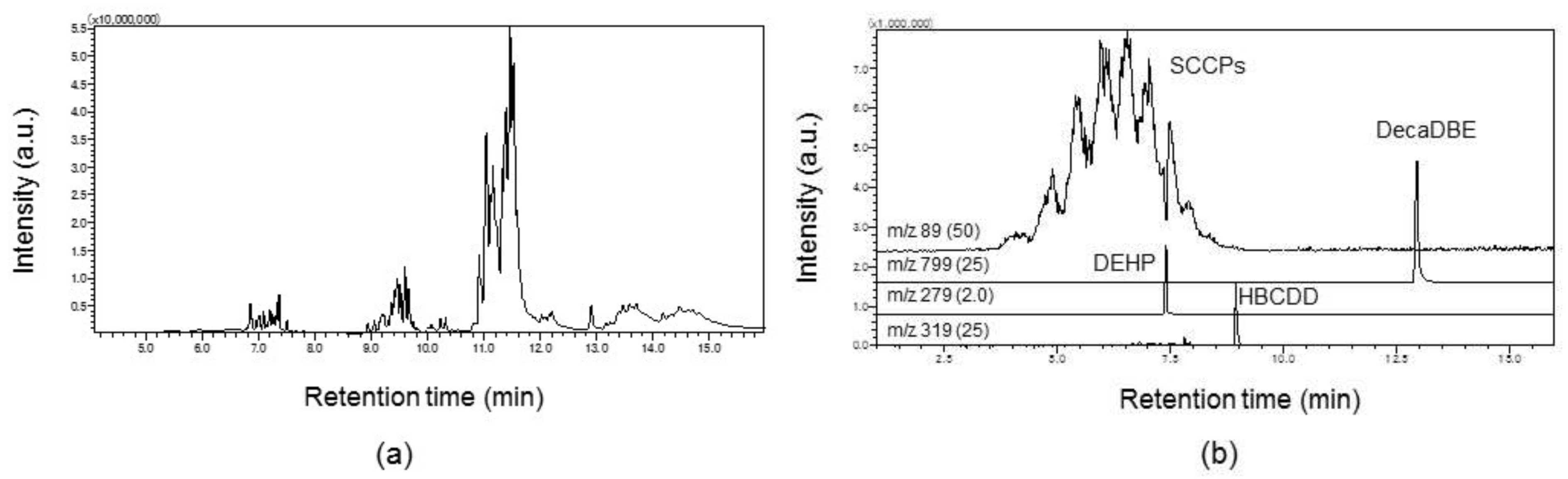
| Additives | Target Ion (m/z) | Confirmation Ion (m/z) |
|---|---|---|
| SCCPs | 89 | 115 |
| HBCDD | 319 | 561 |
| DecaBDE | 799 | 959 |
| DEHP | 279 | 149 |
| Additives Name | Sensitivity (area/(mg/kg)) | R2 | RSD at 1000 mg/kg (%) | LOD (mg/kg) |
|---|---|---|---|---|
| DEHP | 8.8 × 102 | 0.998 | 5.0 | 14 |
| SCCPs | 5.9 × 102 | 0.997 | 1.5 | 37 |
| HBCDD | 8.4 × 10 | 0.989 | 5.2 | 46 |
| DecaBDE | 2.6 × 102 | 0.992 | 1.9 | 46 |
| Parameters | Settings |
|---|---|
| Pyrolyzer | |
| Furnace temperature | 200 °C → 20 °C/min → 300 °C → 5 °C/min → 340 °C (1 min) |
| Interface temperature | 300 °C |
| GC | |
| Column | 100% Dimethyl polysiloxane, length 15 m, I.D. 0.25 mm, film, thickness 0.05 μm |
| Injection port temperature | 320 °C |
| Column oven temperature | 80 °C → (20 °C /min) → 300 °C (5 min) |
| Injection mode | Split (split ratio: 1/50) |
| Carrier gas | Helium, 52.1 cm/s, constant linear velocity |
| MS | |
| Ion source temperature | 230 °C |
| Ionization method | EI, 70 eV |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanagisawa, H.; Kudo, Y.; Nakagawa, K.; Miyagawa, H.; Maruyama, F.; Fujimaki, S. Simultaneous Screening of Major Flame Retardants and Plasticizers in Polymer Materials Using Pyrolyzer/Thermal Desorption Gas Chromatography Mass Spectrometry (Py/TD–GC–MS). Molecules 2018, 23, 728. https://doi.org/10.3390/molecules23040728
Yanagisawa H, Kudo Y, Nakagawa K, Miyagawa H, Maruyama F, Fujimaki S. Simultaneous Screening of Major Flame Retardants and Plasticizers in Polymer Materials Using Pyrolyzer/Thermal Desorption Gas Chromatography Mass Spectrometry (Py/TD–GC–MS). Molecules. 2018; 23(4):728. https://doi.org/10.3390/molecules23040728
Chicago/Turabian StyleYanagisawa, Hiroyuki, Yukihiko Kudo, Katsuhiro Nakagawa, Haruhiko Miyagawa, Fumitaka Maruyama, and Shigehiko Fujimaki. 2018. "Simultaneous Screening of Major Flame Retardants and Plasticizers in Polymer Materials Using Pyrolyzer/Thermal Desorption Gas Chromatography Mass Spectrometry (Py/TD–GC–MS)" Molecules 23, no. 4: 728. https://doi.org/10.3390/molecules23040728