Thermogelling 3D Systems towards Stem Cell-Based Tissue Regeneration Therapies
Abstract
:1. Introduction
The Early Studies of Thermogel 3D Systems in Culture Cell
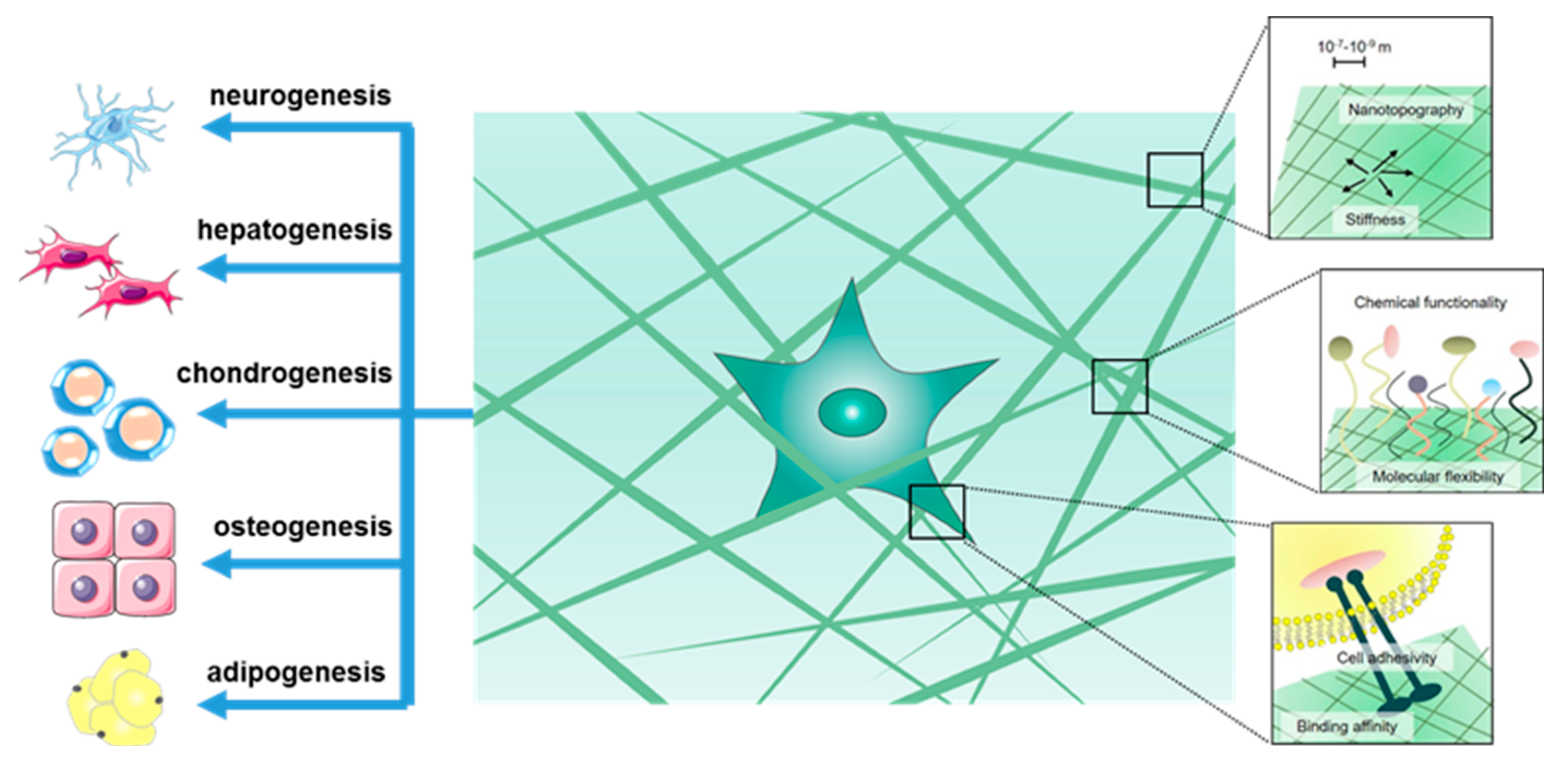
2. Hydrogel-Based 3D Culturing and Differentiation of Stem Cells
3. Stem Cell Specific Differentiation
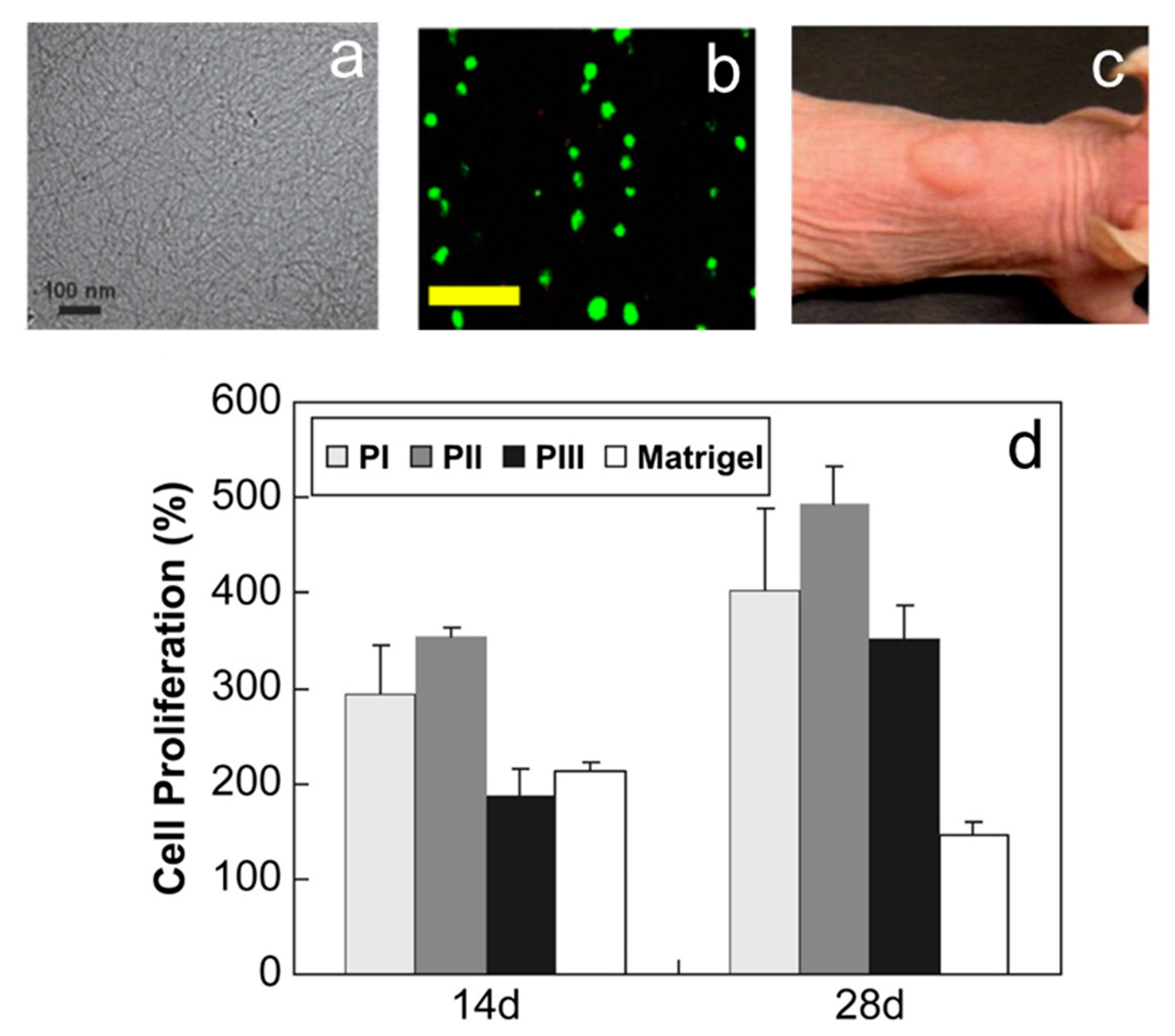
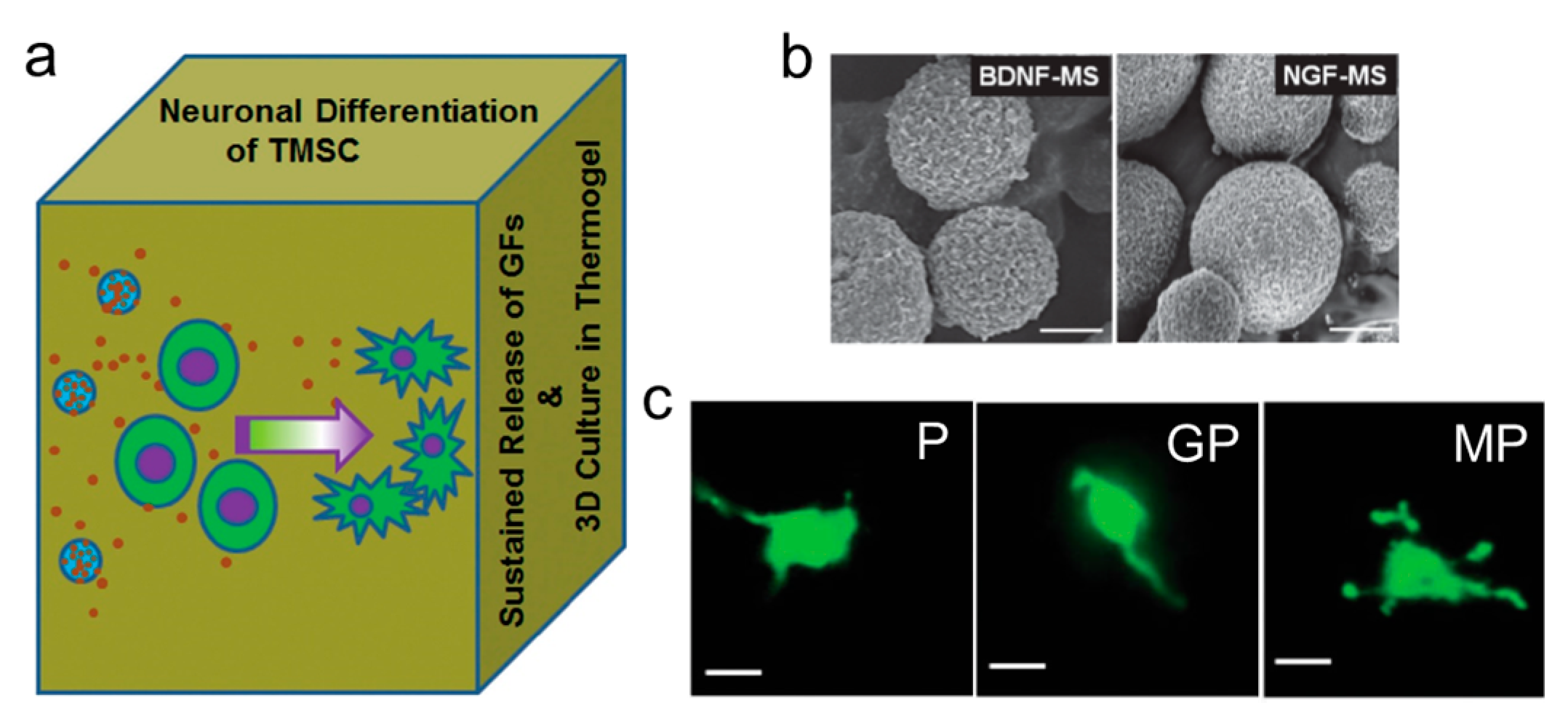
3.1. Scaffold Induced Neuronal Differentiation
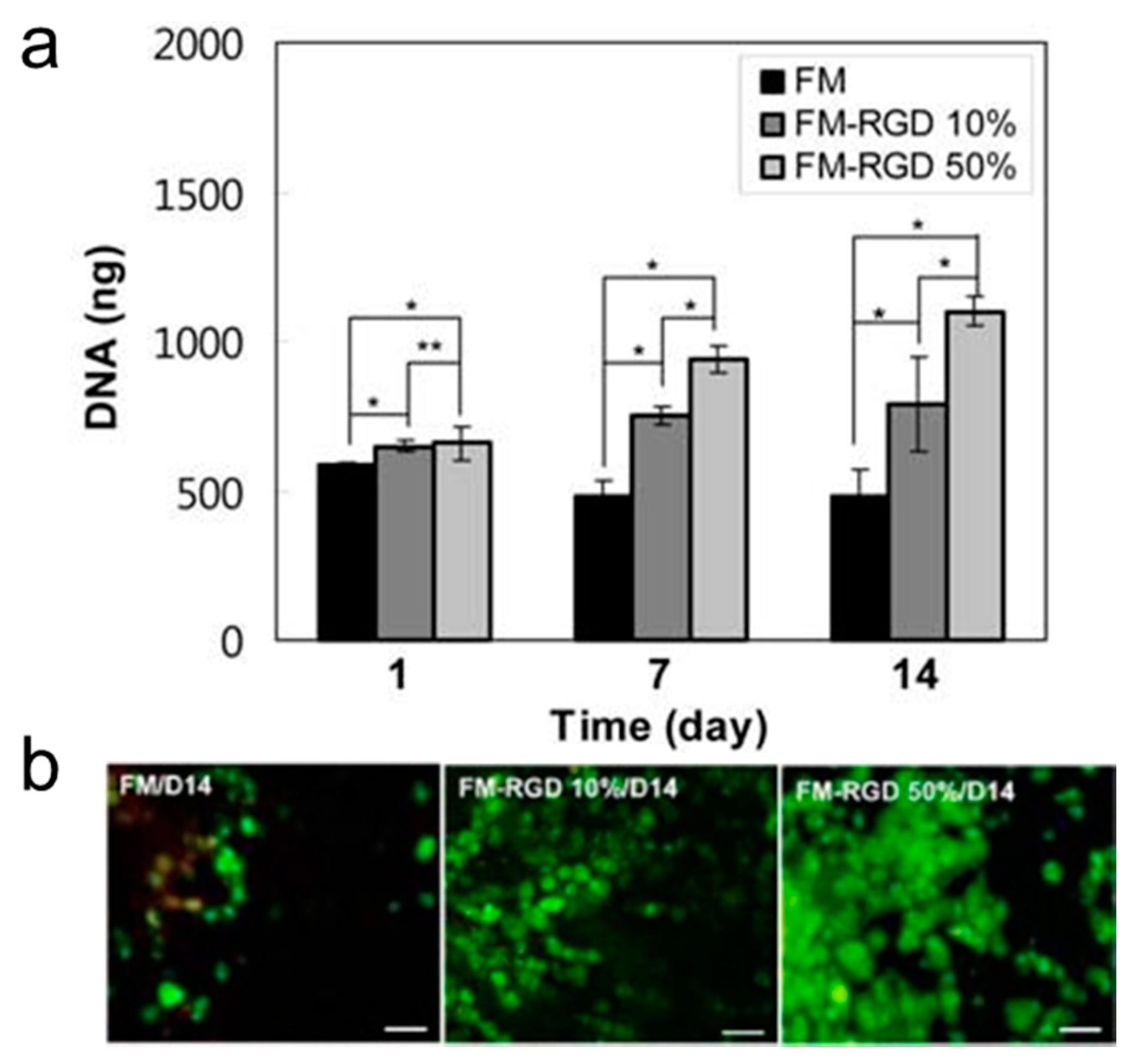
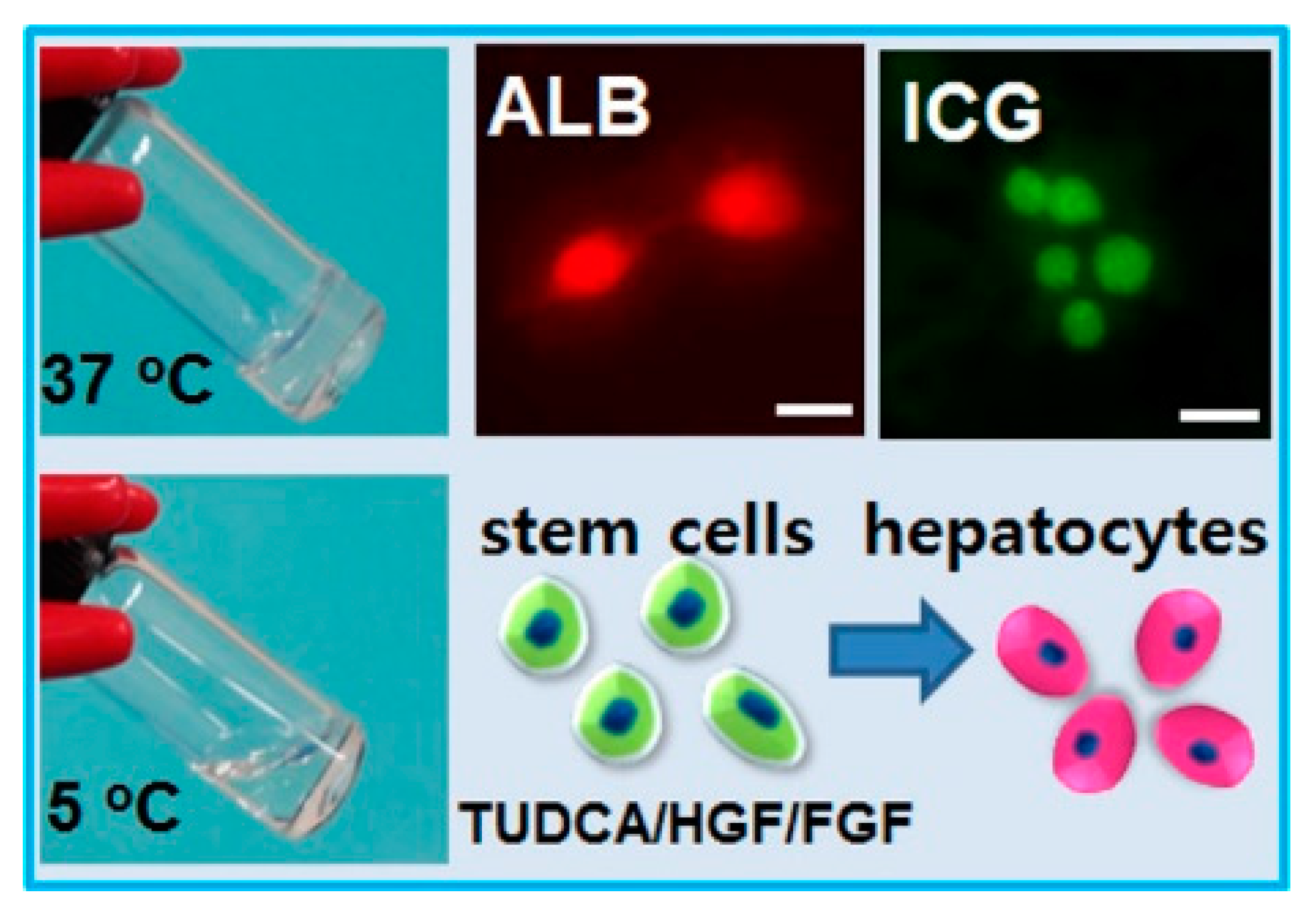
3.2. Scaffold Induced Hepatogenic Differentiation
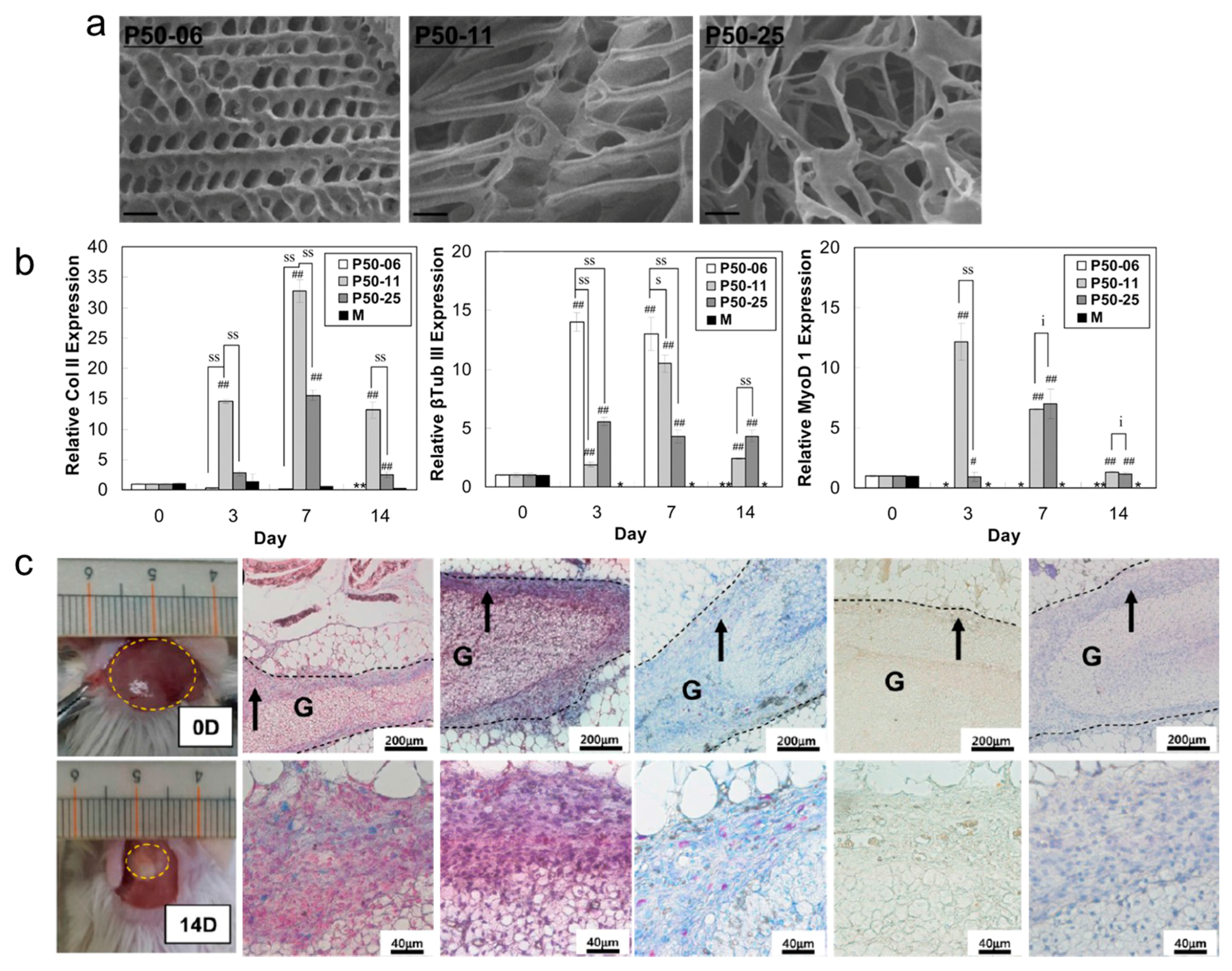
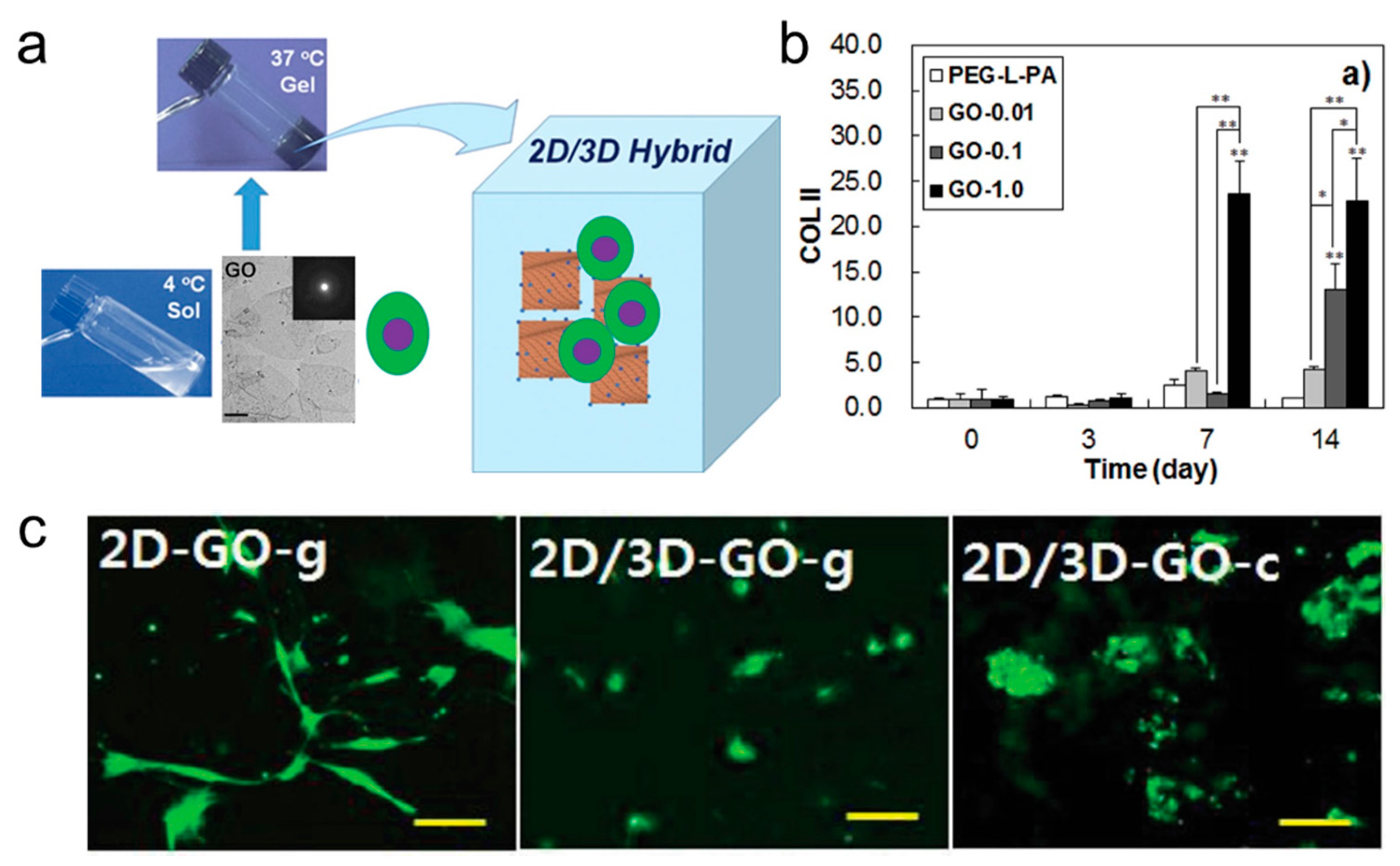
3.3. Scaffold Induced Chondrogenesis Differentiation
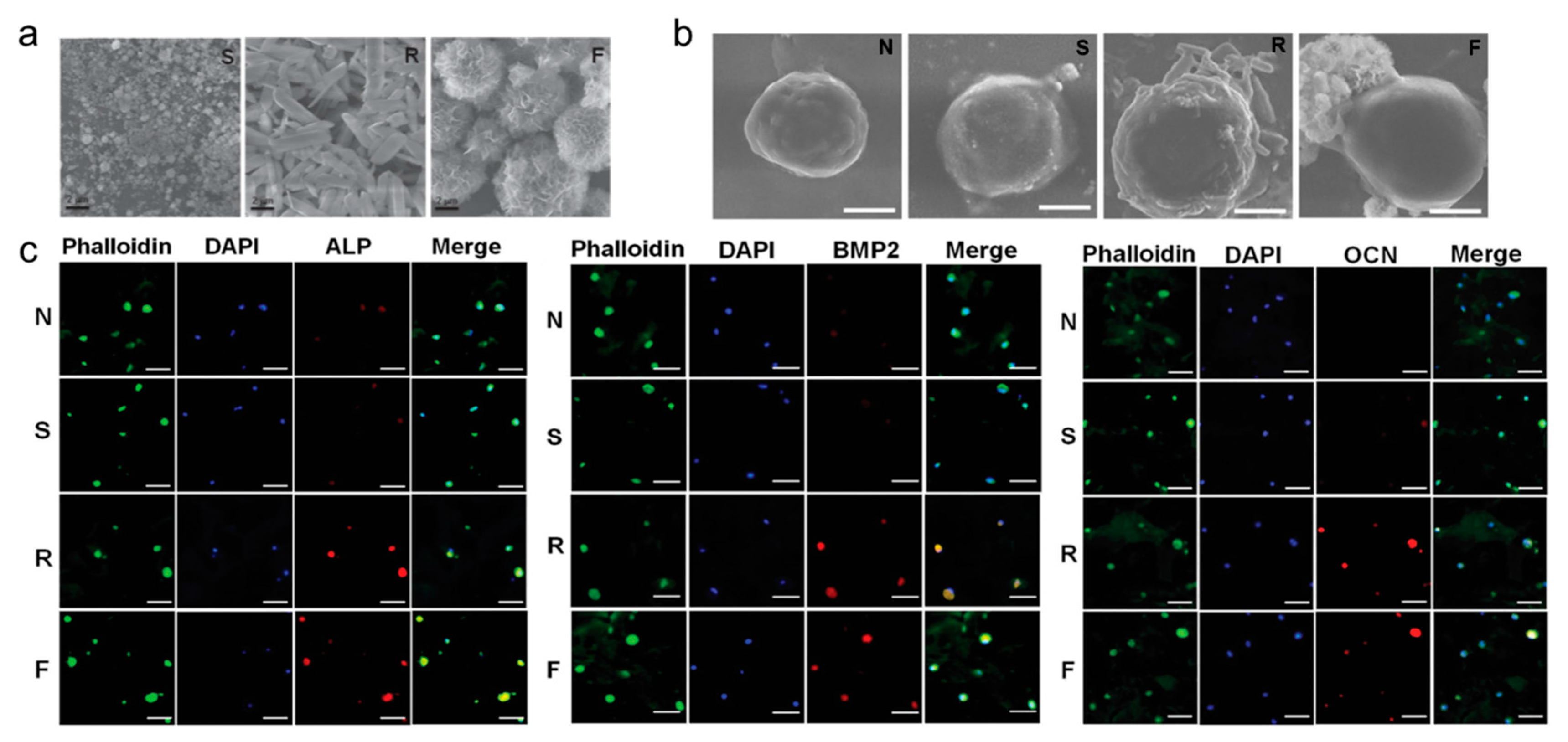
3.4. Scaffold Induced Osteogenic Differentiation
3.5. Scaffold Induced Adipogenic Differentiation
4. Conclusions and Perspectives
Conflicts of Interest
References
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.W.; Rønnov-Jessen, L.; Howlett, A.R.; Bissell, M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA 1992, 89, 9064–9068. [Google Scholar] [CrossRef] [PubMed]
- Bo, G.C.; Min, H.P.; Cho, S.H.; Min, K.J.; Oh, H.J.; Kim, E.H.; Park, K.; Dong, K.H.; Jeong, B. In situ thermal gelling polypeptide for chondrocytes 3D culture. Biomaterials 2010, 31, 9266–9272. [Google Scholar]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.K.; Shinde, U.P.; Yeon, B.; Jeong, B. Recent progress of in situ formed gels for biomedical applications. Prog. Polym. Sci. 2013, 38, 672–701. [Google Scholar]
- Loh, X.J.; Li, J. Biodegradable thermosensitive copolymer hydrogels for drug delivery. Expert Opin. Ther. Pat. 2007, 17, 965–977. [Google Scholar] [CrossRef]
- Moon, H.J.; Ko, D.Y.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Perkins, K.R.; Watson, B.M.; Hacker, M.C.; Bryant, S.J.; Raphael, R.M.; Kasper, F.K.; Mikos, A.G. Thermoresponsive, in situ cross-linkable hydrogels based on N-isopropylacrylamide: Fabrication, characterization and mesenchymal stem cell encapsulation. Acta Biomater. 2011, 7, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Yeon, B.; Park, M.H.; Moon, H.J.; Kim, S.J.; Cheon, Y.W.; Jeong, B. 3D culture of adipose-tissue-derived stem cells mainly leads to chondrogenesis in poly (ethylene glycol)-poly (l-alanine) diblock copolymer thermogel. Biomacromolecules 2013, 14, 3256–3266. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 39, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.L.; Ulery, B.D.; Kan, H.M.; Burks, M.V.; Cui, Z.; Wu, Q.; Nair, L.S.; Laurencin, C.T. A chitosan thermogel for delivery of ropivacaine in regional musculoskeletal anesthesia. Biomaterials 2013, 34, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Shen, W.; Chen, C.; Lei, K.; Yu, L.; Ding, J. Selenium-containing thermogel for controlled drug delivery by coordination competition. RSC Adv. 2015, 5, 97975–97981. [Google Scholar] [CrossRef]
- Wu, Y.L.; Wang, H.; Qiu, Y.; Xian, J.L. Supramolecular cyclodextrin nanocarriers for chemo-and gene therapy towards the effective treatment of drug resistant cancers. RSC Adv. 2016, 6, 44506–44513. [Google Scholar] [CrossRef]
- Vlierberghe, S.V.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Lee, H.J.; Jeong, B. Injectable polypeptide thermogel as a tissue engineering system for hepatogenic differentiation of tonsil-derived mesenchymal stem cells. ACS Appl. Mater. Interfaces 2017, 9, 11568–11576. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Zhang, Z.Z.; Jiang, D.; Qi, Y.S.; Wang, H.J.; Zhang, J.Y.; Ding, J.X.; Yu, J.K. Thermogel-coated poly (ε-caprolactone) composite scaffold for enhanced cartilage tissue engineering. Polymers 2016, 8, 200–213. [Google Scholar] [CrossRef]
- Yu, L.; Hu, H.; Chen, L.; Bao, X.; Li, Y.; Chen, L.; Xu, G.; Ye, X.; Ding, J. Comparative studies of thermogels in preventing post-operative adhesions and corresponding mechanisms. Biomater. Sci. 2014, 2, 1100–1109. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Lei, Y.; Tang, M.; Dai, Z.; Yang, X.; Yu, X.; Yu, L.; Sun, X.; Ding, J. Sustained subconjunctival delivery of cyclosporine A using thermogelling polymers for glaucoma filtration surgery. J. Mater. Chem. B 2017, 5, 6400–6411. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Q.; Liu, M.; Tan, T.; Cao, H. Synthesis and characterization of an injectable hyaluronic acid-polyaspartylhydrazide hydrogel. Bio-Med. Mater. Eng. 2016, 27, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Song, S.; Wang, H.; Zhang, W.; Lin, C.; Ma, S.; Ye, T.; Zhang, L.; Yang, X.; Qin, X. Injectable chitosan thermogels for sustained and localized delivery of pingyangmycin in vascular malformations. Int. J. Pharm. 2014, 476, 232. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Park, M.H.; Cho, S.; Joo, M.K.; Oh, H.J.; Kim, E.H.; Park, K.; Han, D.K.; Jeong, B. Thermal gelling polyalanine-poloxamine-polyalanine aqueous solution for chondrocytes 3D culture: Initial concentration effect. Soft Matter 2011, 7, 456–462. [Google Scholar] [CrossRef]
- Lee, H.; Bo, G.C.; Moon, H.J.; Choi, J.; Park, K.; Jeong, B.; Dong, K.H. Chondrocyte 3D-culture in RGD-modified crosslinked hydrogel with temperature-controllable modulus. Macromol. Res. 2012, 20, 106–111. [Google Scholar] [CrossRef]
- Tabar, V.; Studer, L. Pluripotent stem cells in regenerative medicine: Challenges and recent progress. Nat. Rev. Genet. 2014, 15, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125–1247135. [Google Scholar] [CrossRef] [PubMed]
- Emmert, M.Y.; Hitchcock, R.W.; Hoerstrup, S.P. Cell therapy, 3D culture systems and tissue engineering for cardiac regeneration. Adv. Drug Deliv. Rev. 2014, 69–70, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñezmorán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Ling, Q.D.; Kumar, S.S.; Chang, Y.; Kao, T.C.; Munusamy, M.A.; Alarfaj, A.A.; Hsu, S.T.; Umezawa, A. External stimulus-responsive biomaterials designed for the culture and differentiation of ES, iPS, and adult stem cells. Prog. Polym. Sci. 2014, 39, 1585–1613. [Google Scholar] [CrossRef]
- Park, M.H.; Yu, Y.; Moon, H.J.; Ko, D.Y.; Kim, H.S.; Lee, H.; Ryu, K.H.; Jeong, B. 3D culture of tonsil-derived mesenchymal stem cells in poly(ethylene glycol)-poly(l-alanine-co-l-phenyl alanine) thermogel. Adv. Healthc. Mater. 2014, 3, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Kye, E.J.; Kim, S.J.; Park, M.H.; Moon, H.J.; Ryu, K.H.; Jeong, B. Differentiation of tonsil-tissue-derived mesenchymal stem cells controlled by surface-functionalized microspheres in PEG-polypeptide thermogels. Biomacromolecules 2014, 15, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.Z.; Khademhosseini, A.; Langer, R.; Peppas, N.A. Bioinspired materials for controlling stem cell fate. Acc. Chem. Res. 2010, 43, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Knight, M.M.; Campbell, J.J.; Bader, D.L. Stem cell mechanobiology. J. Cell. Biochem. 2011, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.H.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Hribar, K.C.; Taylor-Weiner, H.; Chen, S.; Engler, A. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014, 13, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; He, D.; Kleiner, G.; Kuluz, J. Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J. Spinal Cord Med. 2007, 30, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.S.; Trojanowski, J.Q.; Lee, V.M. Neurodegenerative diseases: A decade of discoveries paves the way for therapeutic breakthroughs. Nat. Med. 2004, 10, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Moon, H.J.; Jung, B.K.; Jeong, B. Microsphere-incorporated hybrid thermogel for neuronal differentiation of tonsil derived mesenchymal stem cells. Adv. Healthc. Mater. 2015, 4, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jung, J.; Cho, K.J.; Lee, C.K.; Hwang, S.G.; Kim, G.J. Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differentiation 2012, 84, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, E.B. Liver transplantation: Current status and novel approaches to liver replacement. Gastroenterology 2001, 120, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Nussler, A.; Konig, S.; Ott, M.; Sokal, E.; Christ, B.; Thasler, W.; Brulport, M.; Gabelein, G.; Schormann, W.; Schulze, M. Present status and perspectives of cell-based therapies for liver diseases. J. Hepatol. 2006, 45, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Vosough, M.; Moslem, M.; Pournasr, B.; Baharvand, H. Cell-based therapeutics for liver disorders. Br. Med. Bull. 2011, 100, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, M.H.; Moon, H.J.; Park, J.H.; Ko, D.Y.; Jeong, B. Polypeptide thermogels as a three dimensional culture scaffold for hepatogenic differentiation of human tonsil-derived mesenchymal stem cells. ACS Appl. Mater. Interfaces 2014, 6, 17034–17043. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Moon, H.J.; Park, J.H.; Shinde, U.P.; Ko, D.Y.; Jeong, B. PEG-poly (l-alanine) thermogel as a 3D scaffold of bone-marrow-derived mesenchymal stem cells. Macromol. Biosci. 2015, 15, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, I.Y.; Patel, M.; Moon, H.J.; Hwang, S.J.; Jeong, B. 2D and 3D hybrid systems for enhancement of chondrogenic differentiation of tonsil-derived mesenchymal stem cells. Adv. Funct. Mater. 2015, 25, 2573–2582. [Google Scholar] [CrossRef]
- Gamblin, A.L.; Brennan, M.A.; Renaud, A.; Yagita, H.; Lézot, F.; Heymann, D.; Trichet, V.; Layrolle, P. Bone tissue formation with human mesenchymal stem cells and biphasic calcium phosphate ceramics: The local implication of osteoclasts and macrophages. Biomaterials 2014, 35, 9660–9667. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.R.V.; Hwang, Y.; Phadke, A.; Kang, H.; Hwang, N.S.; Caro, E.J.; Nguyen, S.; Siu, M.; Theodorakis, E.A.; Gianneschi, N.C.; et al. Calcium phosphate-bearing matrices induce osteogenic differentiation of stem cells through adenosine signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.M.; Cunniffe, G.M.; Lyons, F.G.; Bessho, K.; Dickson, G.R.; Duffy, G.P.; O’Brien, F.J. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv. Mater. 2012, 24, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Patel, M.; Chung, H.; Jeong, B. Nanocomposite versus mesocomposite for osteogenic differentiation of tonsil-derived mesenchymal stem cells. Adv. Healthc. Mater. 2016, 5, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Moon, H.J.; Ko, D.Y.; Jeong, B. Composite system of graphene oxide and polypeptide thermogel as an injectable 3D scaffold for adipogenic differentiation of tonsil-derived mesenchymal stem cells. ACS Appl. Mater. Interfaces 2016, 8, 5160–5169. [Google Scholar] [CrossRef] [PubMed]
- Comolli, N.; Neuhuber, B.; Fischer, I.; Lowman, A. In vitro analysis of PNIPAAm–PEG, a novel, injectable scaffold for spinal cord repair. Acta Biomater. 2009, 5, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Cha, B.H.; Park, H.; Park, K.S.; Lee, K.Y.; Lee, S.H. The effect of conjugating RGD into 3D alginate hydrogels on adipogenic differentiation of human adipose-derived stromal cells. Macromol. Biosci. 2011, 11, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Hughes, N.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Human mesenchymal stem cell differentiation to NP-like cells in chitosan–glycerophosphate hydrogels. Biomaterials 2008, 29, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, T.P.; Langer, R.; Ferreira, L.S. Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat. Methods 2011, 8, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Saha, K.; Bogatyrev, S.R.; Yang, J.; Hook, A.L.; Kalcioglu, Z.I.; Cho, S.W.; Mitalipova, M.; Pyzocha, N.; Rojas, F. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010, 9, 768–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Cho, S.W.; Son, S.M.; Hudson, S.P.; Bogatyrev, S.; Keung, L.; Kohane, D.S.; Langer, R.; Anderson, D.G. Combinatorial extracellular matrices for human embryonic stem cell differentiation in 3D. Biomacromolecules 2010, 11, 1909–1914. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Young, D.J.; Wu, Y.-L.; Loh, X.J. Thermogelling 3D Systems towards Stem Cell-Based Tissue Regeneration Therapies. Molecules 2018, 23, 553. https://doi.org/10.3390/molecules23030553
Wang X, Young DJ, Wu Y-L, Loh XJ. Thermogelling 3D Systems towards Stem Cell-Based Tissue Regeneration Therapies. Molecules. 2018; 23(3):553. https://doi.org/10.3390/molecules23030553
Chicago/Turabian StyleWang, Xiaoyuan, David James Young, Yun-Long Wu, and Xian Jun Loh. 2018. "Thermogelling 3D Systems towards Stem Cell-Based Tissue Regeneration Therapies" Molecules 23, no. 3: 553. https://doi.org/10.3390/molecules23030553





