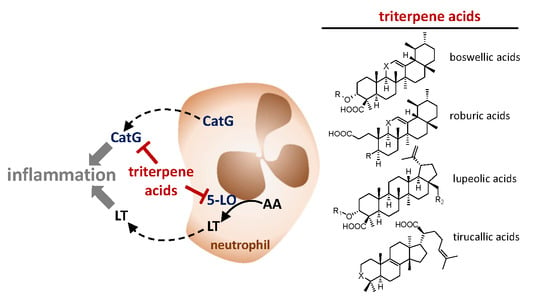
Triterpene Acids from Frankincense and Semi-Synthetic Derivatives That Inhibit 5-Lipoxygenase and Cathepsin G
Abstract
:1. Introduction
2. Results
2.1. Isolation and Semi-Synthesis of the Triterpene Acids
2.2. Inhibition of 5-LO by Natural Occurring Triterpene Acids
2.3. Inhibition of Cathepsin G by Natural Occurring Triterpene Acids
2.4. Effects of Semi-Synthetic BAs against 5-LO and Cathepsin G
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Cells and Cell Isolation
4.3. Expression and Purification of Human Recombinant 5-LO
4.4. Determination of 5-LO Activity in a Cell-Free Assay
4.5. Determination of 5-LO Product Formation in Neutrophils
4.6. Determination of Cathepsin G Activity in a Cell-Free Assay
4.7. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007, 42, 3–27. [Google Scholar] [PubMed]
- Cicero, A.F.; Derosa, G.; Gaddi, A. Combined lipoxygenase/cyclo-oxygenase inhibition in the elderly: The example of licofelone. Drugs Aging 2005, 22, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Buchele, B.; Zugmaier, W.; Simmet, T. Analysis of pentacyclic triterpenic acids from frankincense gum resins and related phytopharmaceuticals by high-performance liquid chromatography. Identification of lupeolic acid, a novel pentacyclic triterpene. J. Chromatogr. B 2003, 791, 21–30. [Google Scholar] [CrossRef]
- Verhoff, M.; Seitz, S.; Northoff, H.; Jauch, J.; Schaible, A.M.; Werz, O. A novel C(28)-hydroxylated lupeolic acid suppresses the biosynthesis of eicosanoids through inhibition of cytosolic phospholipase A(2). Biochem. Pharmacol. 2012, 84, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Verhoff, M.; Seitz, S.; Paul, M.; Noha, S.M.; Jauch, J.; Schuster, D.; Werz, O. Tetra- and pentacyclic triterpene acids from the ancient anti-inflammatory remedy frankincense as inhibitors of microsomal prostaglandin E(2) synthase-1. J. Nat. Prod. 2014, 77, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Jauch, J. Efficient preparation of incensole and incensole acetate, and quantification of these bioactive diterpenes in Boswellia papyrifera by a RP-DAD-HPLC method. Nat. Prod. Commun. 2012, 7, 283–288. [Google Scholar] [PubMed]
- Poeckel, D.; Werz, O. Boswellic acids: Biological actions and molecular targets. Curr. Med. Chem. 2006, 13, 3359–3369. [Google Scholar] [CrossRef] [PubMed]
- Safayhi, H.; Mack, T.; Sabieraj, J.; Anazodo, M.I.; Subramanian, L.R.; Ammon, H.P. Boswellic acids: Novel, specific, nonredox inhibitors of 5-lipoxygenase. J. Pharmacol. Exp. Ther. 1992, 261, 1143–1146. [Google Scholar] [PubMed]
- Siemoneit, U.; Pergola, C.; Jazzar, B.; Northoff, H.; Skarke, C.; Jauch, J.; Werz, O. On the interference of boswellic acids with 5-lipoxygenase: Mechanistic studies in vitro and pharmacological relevance. Eur. J. Pharmacol. 2009, 606, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Siemoneit, U.; Hofmann, B.; Kather, N.; Lamkemeyer, T.; Madlung, J.; Franke, L.; Schneider, G.; Jauch, J.; Poeckel, D.; Werz, O. Identification and functional analysis of cyclooxygenase-1 as a molecular target of boswellic acids. Biochem. Pharmacol. 2008, 75, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Siemoneit, U.; Koeberle, A.; Rossi, A.; Dehm, F.; Verhoff, M.; Reckel, S.; Maier, T.J.; Jauch, J.; Northoff, H.; Bernhard, F.; et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br. J. Pharmacol. 2011, 162, 147–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safayhi, H.; Rall, B.; Sailer, E.R.; Ammon, H.P. Inhibition by boswellic acids of human leukocyte elastase. J. Pharmacol. Exp. Ther. 1997, 281, 460–463. [Google Scholar] [PubMed]
- Tausch, L.; Henkel, A.; Siemoneit, U.; Poeckel, D.; Kather, N.; Franke, L.; Hofmann, B.; Schneider, G.; Angioni, C.; Geisslinger, G.; et al. Identification of Human Cathepsin G as a Functional Target of Boswellic Acids from the Anti-Inflammatory Remedy Frankincense. J. Immunol. 2009, 183, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Henkel, A.; Tausch, L.; Pillong, M.; Jauch, J.; Karas, M.; Schneider, G.; Werz, O. Boswellic acids target the human immune system-modulating antimicrobial peptide LL-37. Pharmacol. Res. 2015, 102, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Syrovets, T.; Gschwend, J.E.; Buchele, B.; Laumonnier, Y.; Zugmaier, W.; Genze, F.; Simmet, T. Inhibition of IkappaB kinase activity by acetyl-boswellic acids promotes apoptosis in androgen-independent PC-3 prostate cancer cells in vitro and in vivo. J. Biol. Chem. 2005, 280, 6170–6180. [Google Scholar] [CrossRef] [PubMed]
- Henkel, A.; Kather, N.; Monch, B.; Northoff, H.; Jauch, J.; Werz, O. Boswellic acids from frankincense inhibit lipopolysaccharide functionality through direct molecular interference. Biochem. Pharmacol. 2012, 83, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Skarke, C.; Kuczka, K.; Tausch, L.; Werz, O.; Rossmanith, T.; Barrett, J.S.; Harder, S.; Holtmeier, W.; Schwarz, J.A. Increased bioavailability of 11-keto-beta-boswellic acid following single oral dose frankincense extract administration after a standardized meal in healthy male volunteers: modeling and simulation considerations for evaluating drug exposures. J. Clin. Pharmacol. 2012, 52, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Jauch, J.; Bergmann, J. An efficient method for the large-scale preparation of 3-O-acetyl-11-oxo-beta-boswellic acid and other boswellic acids. Eur. J. Org. Chem. 2003, 4752–4756. [Google Scholar] [CrossRef]
- Werz, O.; Siemoneit, U.; Henkel, A.; Jauch, J.; Kather, N. Use of Boswellic Acids and Synthetic Acid Derivatives for Inhibiting Microsomal Prostaglandin E2 Synthase and Cathepsin G. Patent Application DE 102008015607 A1, 15 October 2009. [Google Scholar]
- Kather, N. Synthesis and Structure-Activity-Relationships of Boswellic Acids and Derivatives. Doctoral Thesis, University of Saarland, Saarbrücken, Germany, 12 December 2007. [Google Scholar] [CrossRef]
- Werz, O.; Szellas, D.; Henseler, M.; Steinhilber, D. Nonredox 5-lipoxygenase inhibitors require glutathione peroxidase for efficient inhibition of 5-lipoxygenase activity. Mol. Pharmacol. 1998, 54, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Altmann, A.; Poeckel, D.; Fischer, L.; Schubert-Zsilavecz, M.; Steinhilber, D.; Werz, O. Coupling of boswellic acid-induced Ca2+ mobilisation and MAPK activation to lipid metabolism and peroxide formation in human leucocytes. Br. J. Pharmacol. 2004, 141, 223–232. [Google Scholar] [CrossRef] [PubMed]
- De Garavilla, L.; Greco, M.N.; Sukumar, N.; Chen, Z.W.; Pineda, A.O.; Mathews, F.S.; Di Cera, E.; Giardino, E.C.; Wells, G.I.; Haertlein, B.J.; et al. A novel, potent dual inhibitor of the leukocyte proteases cathepsin G and chymase: Molecular mechanisms and anti-inflammatory activity in vivo. J. Biol. Chem. 2005, 280, 18001–18007. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.E.; Schweizer, S.; Bertsche, T.; Dufer, M.; Drews, G.; Safayhi, H. Stimulation of leukotriene synthesis in intact polymorphonuclear cells by the 5-lipoxygenase inhibitor 3-oxo-tirucallic acid. Mol. Pharmacol. 2001, 60, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.C.; Syrovets, T.; Pitterle, K.; Lunov, O.; Buchele, B.; Schimana-Pfeifer, J.; Schmidt, T.; Morad, S.A.; Simmet, T. Tirucallic acids are novel pleckstrin homology domain-dependent Akt inhibitors inducing apoptosis in prostate cancer cells. Mol. Pharmacol. 2010, 77, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.J.; Tausch, L.; Hoernig, M.; Coste, O.; Schmidt, R.; Angioni, C.; Metzner, J.; Groesch, S.; Pergola, C.; Steinhilber, D.; et al. Celecoxib inhibits 5-lipoxygenase. Biochem. Pharmacol. 2008, 76, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Szellas, D.; Radmark, O.; Steinhilber, D.; Werz, O. Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 2003, 17, 949–951. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, T.; Steinhilber, D.; Roth, H.J. Determination of leukotriene B4 by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B 1987, 416, 170–175. [Google Scholar] [CrossRef]
Sample Availability: Only limited samples of the compounds (AKBA 1 and KBA 2) at amounts less than 1 mg are available from the authors. |
| Cmpd. | Structure | 5-LO Activity [% Control] (IC50 in µM) | Cat. G Activity [% Control] (IC50 in µM) | |||
|---|---|---|---|---|---|---|
| cell-based | cell-free | |||||
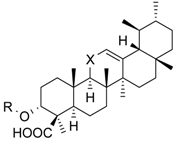 | ||||||
| 1 | R = –CO–CH3 X = C=O | 23.1 ± 9.8 ** (3.0 µM) | 40.7 ± 10.8 * (3.0 µM) | 14.8 ± 0.9 *** (0.6 µM) | ||
| 2 | R = –H X = C=O | 25.7 ± 7.3 ** (3.5 µM) | 31.1 ± 5.0 *** (4.6 µM) | 29.4 ± 3.5 *** (4.1 µM) | ||
| 3 | R = –H X = CH2 | 53.0 ± 7.6 * | 78.7 ± 12.6 | 27.7 ± 1.8 *** (0.5 µM) | ||
| 4 | R = –CO–CH3 X = CH2 | n.i. | 73.5 ± 13.3 | 28.1 ± 4.9 *** (2.0 µM) | ||
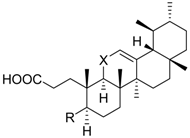 | ||||||
| 5 | R = |  | 74.5 ± 3.7 ** | n.i. | 69.8 ± 8.0 | |
| X = CH2 | ||||||
| 6 | R = | 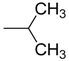 | n.i. | n.i. | 70.4 ± 3.9 ** | |
| X = CH2 | ||||||
| 7 | R = |  | 7.1 ± 3.3 *** (4.3 µM) | 73.0 ± 8.1 | 53.8 ± 8.1 * | |
| X = C=O | ||||||
| 8 | 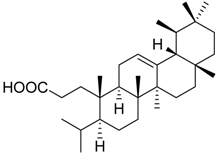 | n.i. | 89.1 ± 7.5 | 59.2 ± 4.0 ** | ||
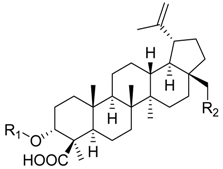 | ||||||
| 9 | R1 = –H R2 = –H | 36.2 ± 5.1 *** (4.0 µM) | 76.6 ± 12.2 | 58.1 ± 3.6 ** | ||
| 10 | R1 = –CO–CH3 R2 = –H | 88.7 ± 6.7 | n.i. | 35.9 ± 2.5 *** (7.5 µM) | ||
| 11 | R1 = –H R2 = –OH | 24.0 ± 7.2 ** (4.6 µM) | 52.7 ± 7.1 ** | 68.1 ± 6.5 * | ||
| 12 | R1 = –CO–CH3 R2 = –OH | 28.4 ± 11.1 * (5.1 µM) | 41.8 ± 3.4 *** (8.3 µM) | 87.2 ± 2.5 * | ||
| 13 | 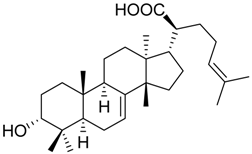 | 8.2 ± 2.2 *** (2.9 µM) | 89.6 ± 8.5 | 72.3 ± 3.6 ** | ||
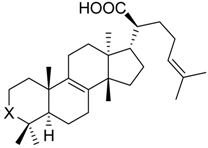 | ||||||
| 14 | X = | 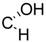 | (S) | 3.1 ± 0.6 *** (1.1 µM) | 66.0 ± 15.1 | 73.0 ± 7.3 |
| 15 | X = | 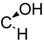 | (R) | 5.2 ± 1.0 *** (3.0 µM) | 64.1 ± 12.4 | 80.1 ± 6.2 |
| 16 | X = C=O | 37.8 ± 2.4 *** (7.1 µM) | 77.1 ± 8.7 | 53.1 ± 5.5 ** | ||
| 17 | X = |  | 75.5 ± 2.1 ** | 75.6 ± 3.0** | 66.7 ± 4.5 ** | |
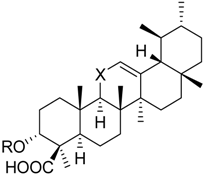 | ||||||
| 18 | X = | 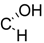 | (R) | 18.3± 2.7 *** (2.8 µM) | 72.8 ± 7.1 | 62.9 ± 6.6 * |
| R = –H | ||||||
| 19 | X = | 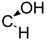 | (S) | 58.2 ± 0.9 *** | 87.2 ± 1.8 ** | 82.7 ± 9.0 |
| R = –H | ||||||
| 20 | X = CH2 R = –CO–COOH | 77.3 ± 5.7 * | n.i. | 38.1 ± 6.4 *** (4.7 µM) | ||
| 21 | X = C=O R = –CO–COOH | 70.3 ± 6.9 * | n.i. | n.i. | ||
| 22 | X = CH2 R = –CO– (CH2)2–COOH | 60.3 ± 8.0 * | 85.9 ± 11.1 | 59.4 ± 7.6 * | ||
| 23 | X = C=O R = –CO– (CH2)2–COOH | 80.8 ± 6.8 | 75.0 ± 4.8* | n.i. | ||
| 24 | X = CH2 R = –CO– (CH2)3–COOH | 65.3 ± 8.0 * | n.i. | 24.5 ± 1.7 *** (3.6 µM) | ||
| 25 | X = C=O R = –CO– (CH2)3–COOH | n.i. | n.i. | 49.1 ± 11.0 * (8.6 µM) | ||
| 26 | X = CH2 R = –CH2–COOH | 57.6 ± 5.3 ** | 78.1 ± 7.4 | 28.8 ± 7.4 ** (3.4 µM) | ||
| 27 | X = C=O R = –CH2–COOH | 68.4 ± 2.1 *** | n.i. | n.i. | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koeberle, A.; Henkel, A.; Verhoff, M.; Tausch, L.; König, S.; Fischer, D.; Kather, N.; Seitz, S.; Paul, M.; Jauch, J.; et al. Triterpene Acids from Frankincense and Semi-Synthetic Derivatives That Inhibit 5-Lipoxygenase and Cathepsin G. Molecules 2018, 23, 506. https://doi.org/10.3390/molecules23020506
Koeberle A, Henkel A, Verhoff M, Tausch L, König S, Fischer D, Kather N, Seitz S, Paul M, Jauch J, et al. Triterpene Acids from Frankincense and Semi-Synthetic Derivatives That Inhibit 5-Lipoxygenase and Cathepsin G. Molecules. 2018; 23(2):506. https://doi.org/10.3390/molecules23020506
Chicago/Turabian StyleKoeberle, Andreas, Arne Henkel, Moritz Verhoff, Lars Tausch, Stefanie König, Dagmar Fischer, Nicole Kather, Stefanie Seitz, Michael Paul, Johann Jauch, and et al. 2018. "Triterpene Acids from Frankincense and Semi-Synthetic Derivatives That Inhibit 5-Lipoxygenase and Cathepsin G" Molecules 23, no. 2: 506. https://doi.org/10.3390/molecules23020506
APA StyleKoeberle, A., Henkel, A., Verhoff, M., Tausch, L., König, S., Fischer, D., Kather, N., Seitz, S., Paul, M., Jauch, J., & Werz, O. (2018). Triterpene Acids from Frankincense and Semi-Synthetic Derivatives That Inhibit 5-Lipoxygenase and Cathepsin G. Molecules, 23(2), 506. https://doi.org/10.3390/molecules23020506